Abstract
Background
Epidemiological studies often rely on self-reported health problems and validation greatly improves study quality. In a study of late effects after childhood cancer, we validated self-reported cardiovascular problems by contacting general practitioners (GPs). This paper describes: (a) the feasibility of this approach; and (b) the agreement between survivor-reports and reports from their GP.
Methods
The Swiss Childhood Cancer Survivor Study (SCCSS) contacts all childhood cancer survivors registered in the Swiss Childhood Cancer Registry since 1976 who survived at least 5 years from cancer diagnosis. We validated answers of all survivors who reported a cardiovascular problem in the questionnaire. Reported cardiovascular problems were hypertension, arrhythmia, congestive heart failure, myocardial infarction, angina pectoris, stroke, thrombosis, and valvular problems. In the questionnaire, we further asked survivors to provide a valid address of their GP and a consent for contact. We sent case-report forms to survivors’ GPs and requested information on cardiovascular diagnoses of their patients. To determine agreement between information reported by survivors and GPs, we calculated Cohen’s kappa (κ) coefficients for each category of cardiovascular problems.
Results
We used questionnaires from 2172 respondents of the SCCSS. Of 290 survivors (13% of 2172) who reported cardiovascular problems, 166 gave consent to contact their GP and provided a valid address. Of those, 135 GPs (81%) replied, and 128 returned the completed case-report form. Survivor-reports were confirmed by 54/128 GPs (42%). Of the 54 GPs, 36 (28% of 128) confirmed the problems as reported by the survivors; 11 (9% of 128) confirmed the reported problem(s) and gave additional information on more cardiovascular outcomes; and seven GPs (5% of 128) confirmed some, but not all cardiovascular problems. Agreement between GPs and survivors was good for stroke (κ = 0.79), moderate for hypertension (κ = 0.51), arrhythmias (κ = 0.41), valvular problems (κ = 0.41) and thrombosis (κ = 0.56), and poor for coronary heart disease (κ = 0.15) and heart failure (κ = 0.32).
Conclusions
Despite excellent GP compliance, it was found unfeasible to validate self-reported cardiovascular problems via GPs because they do not serve as gatekeepers in the Swiss health care system. It is thus necessary to develop other validation methods to improve the quality of patient-reported outcomes.
Similar content being viewed by others
Background
Collecting accurate information about cardiac late effects among childhood cancer survivors is important because cancer treatments may cause late cardiotoxicity, particularly after treatment with anthracyclines and chest irradiation [1]. Cardiovascular diseases (CVD) are therefore important causes of non-malignant late morbidity and mortality after childhood cancer [2, 3]. Several studies described CVDs in childhood cancer survivors (further referred to as survivors) [4,5,6,7,8], but only two contained validated outcomes: one multi-center [9], and one single-center study [10] validated questionnaire self-reports.
To ensure that information on morbidity is accurate, patient-reported health problems must be validated. Several studies have illustrated problems with the validity of patient-reported cardiovascular diseases [11, 12]. Researchers have suggested different validation methods, including medical assessments [11, 13], use of hospital discharge databases [14] and comparison with medical records from general practitioners (GPs) [11, 12] or hospitals [10, 15, 16].
In the framework of the Swiss Childhood Cancer Survivor Study, we tested a simple and cost-effective approach: validating survivors’ self-reported cardiovascular problems by contacting their GPs. This paper describes (a) the feasibility of this approach; and (b) the agreement between survivor- and GP-reported cardiovascular problems.
Methods
Study population
The Swiss Childhood Cancer Survivor Study
The population-based Swiss Childhood Cancer Registry (ChCR; www.childhoodcancerregistry.ch) contains information on all children and adolescents in Switzerland who have been diagnosed with cancer since 1976. Inclusion criteria are being diagnosed with leukemia, lymphoma, central nervous system (CNS) tumors, malignant solid tumors, or Langerhans cell histiocytosis at age 0–20 years. The ChCR prospectively collects information on baseline demographics, cancer diagnosis, treatment, and course of disease [17]. The Swiss Childhood Cancer Survivor Study (SCCSS) is a nationwide population-based cohort study that investigates long-term outcomes after childhood cancer [18] including all childhood cancer patients registered in the ChCR who survived five years or more. All participants diagnosed with cancer before age 16 years, who returned the questionnaire and reported a cardiovascular problem, were eligible for this validation study.
Procedures
The Swiss Childhood Cancer Survivor Study (SCCSS) questionnaire survey
Since 2007, the SCCSS sends questionnaires (in German, French or Italian) to all ≥ 5-year survivors. The extensive standardized questionnaire contains questions used in North American and British childhood cancer survivor studies [19,20,21]. It assesses quality of life, fertility, somatic health, current medication and health service use, psychological distress, health behavior, and socioeconomic information. It also includes a section on current and past cardiovascular health. Adolescent survivors aged 16–19 years at study and parents of survivors under the age of 16 received an age-adapted questionnaire. Non-responders were mailed a second copy of the questionnaire and, if they again failed to respond, were contacted by phone.
Survey to general practitioners
All participants in the SCCSS were asked to provide contact details of the GP who knew them best, and to give consent to contact this GP. In Switzerland pediatricians are GPs for all patients under 16 years of age, so we included them in this study. We use the term “GP” for both GPs and primary care pediatricians. In some cases, survivors wrote down hospital-based physicians (mostly pediatric oncologists) instead of GPs. We did not include responses from hospital-based physicians in this analysis.
Validation was done for the questionnaires returned within one data collection wave between 2007 and 2012 (mean time since SCCSS-survey 2.9 years; range 0.3–5.3 years). Each participant contributed with one questionnaire or phone call. All GPs received a study information letter and a case-report form with a pre-paid return envelope (Supplementary File 1) and were asked to transcribe or copy medical records of the survivor’s cardiovascular problems. GPs received no compensation. Those who did not respond within a month were reminded by phone and sent the postal questionnaire again.
Measurements
Demographic data and information on cancer diagnosis and treatment came from the ChCR and included date of birth, sex, current place of residence, type of cancer, age at diagnosis, and time since diagnosis. Type of cancer was classified by the ChCR according to the International Classification of Childhood Cancer (3rd edition) [22].
Cardiovascular problems
Information about cardiovascular problems was collected in the survivor questionnaire, and validated against physician reports.
Questionnaire to childhood cancer survivors (survivor-reports)
One section of the SCCSS questionnaire assessed survivor-reported health problems, including cardiovascular problems (for details see Supplementary File 2). Survivors were asked: “Have you ever been told by a doctor that you had one of the following diseases?” (1) hypertension, requiring medication; (2) arrhythmias; (3) congestive heart failure; (4) myocardial infarction; (5) angina pectoris; (6) stroke; (7) arteriosclerosis; (8) deep vein thrombosis or pulmonary embolism; (9) valvular problems; (10) other cardiovascular problems; as done in previous studies of survivors [19, 20]. We also asked the year of first diagnosis of the disease.
Each response in the cardiovascular section of the SCCSS questionnaire was coded as ‘yes’ for agreement to having a disease and ‘no’ for disagreement. Answers to closed questions were corrected with information given in ‘free-text comment fields’; for instance, the answers of survivors who wrote ‘cerebral hemorrhage’ were corrected to ‘yes’ for the ‘stroke’ diagnosis category.
Survey to general practitioners (validation)
We used an adapted version of the case-report form of the British Childhood Cancer Survivor Study [19] (see Supplementary File 1), and requested information on exact diagnosis, date of diagnosis, diagnostic and therapeutic procedures, and current medication for all cardiovascular problems. We asked the GP for copies of all relevant hospital discharge reports and reports of relevant examinations.
The information from the GP was extracted from the case-report forms and documents received. Cardiovascular problems were then grouped into the same nine categories as in the survivor-reports. We only included GP-reported cardiovascular problems diagnosed before the date of the SCCSS questionnaire completion.
Statistical analysis
First, we determined the proportions of consenting survivors, GPs with contact details and responding GPs. We further determined the proportion of self-reported cardiovascular problems confirmed by the GP (confirmation rate). For this, we separately assessed the number of survivor-reported problems that were confirmed completely, partly, or not at all by the survivor’s GP.
To determine agreement between information reported by survivors and GPs, we calculated Cohen’s kappa (κ) coefficients with 95%-confidence intervals for each category of cardiovascular problem [23, 24]. Kappa measures inter-rater reliability and captures the extent to which the level of agreement exceeds simple chance: κ ≤ 0.4 indicates poor agreement; κ = 0.41–0.60 moderate agreement; κ = 0.61–0.80 good agreement; and κ ≥ 0.8 excellent agreement [23]. Kappa statistics can be strongly influenced by the prevalence of outcomes in the population and asymmetrical imbalances in marginal totals [25]. Thus we also present the percentage of observed total agreement (number of positive and negative answers by survivors and GPs divided by the total) and the separate proportions of positive and negative agreement (number of answers in positive agreement divided by the average number of positive answers; number of answers in negative agreement divided by the average number of negative answers) [26, 27]. For statistical analyses we used Stata version 16.1 (Stata Corporation, Austin, TX, USA).
Results
Prevalence of self-reported cardiovascular problems in childhood cancer survivors
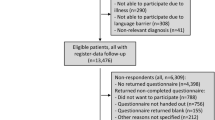
By 2012, we had sent questionnaires to 2933 ≥ 5-year survivors. Of those, 2157 returned the questionnaire (Fig. 1). Of those who returned the questionnaire, 290 reported at least one cardiovascular problem and were eligible for the validation study. Reported cardiovascular problems were: (1) hypertension, requiring medication (n = 96; 33%); (2) arrhythmias (n = 73; 25%); (3) congestive heart failure (n = 41; 14%); (4) myocardial infarction (n = 2; 1%); (5) angina pectoris (n = 23; 8%); (6) stroke (n = 7; 2%); (7) deep vein thrombosis or pulmonary embolism (n = 23; 8%); (8) valvular problems (n = 38; 13%); (9) other cardiovascular problems (n = 57; 20%).
Participants reporting a cardiovascular problem were significantly older at study and at cancer diagnosis and had visited more often a GP during the last 12 months (Table 1).
Feasibility of obtaining GP reports
For various reasons, we could contact only 166 GPs (57%) for the 290 eligible survivors (Fig. 1). Forty-six survivors did not list a GP (16%), either because they reported that a hospital physician was their caregiver (n = 36), or because they did not mention a GP (n = 10). Addresses of four GPs (1%) were incorrect or missing. A quarter of the survivors (n = 74, 26%) did not give consent, either by saying no (n = 43; 15%) to our question about contacting their GP, or by not answering the question (n = 31; 11%).
Of the 166 GPs whom we contacted, 135 (46% of 290 survivors) replied, and 31 (11% of 290) did not (Fig. 1). Of the 135 responders, 128 (44% of 290) returned a case-report form, and seven answered but did not return a case-report form. This was because the GP had retired (n = 4), the GP did not know the survivor (n = 2), or medical records were unavailable (n = 1).
Agreement between survivor- and GP-reported cardiovascular problems
Comparison per survivor
Of 128 GPs who returned a case-report form, 54 (42%) confirmed that the survivor had a cardiovascular problem (Fig. 2). Of these 54 GPs, 36 (28% of 128) reported the same problems as the survivors; 11 (9% of 128) reported the same problem(s) and gave information on additional problems not reported by the survivor; and seven GPs (5% of 128) confirmed some, but not all cardiovascular problems reported by the survivor.
Confirmation of cardiovascular problems by general practitioners. Abbreviations: GP, general practitioner; CVP, cardiovascular problem (one or more). 1 Survivor reported a cardiovascular problem, but GP was not aware of any problem. 2 GP reported other problems than survivor. 3 GP confirmed some, but not all cardiovascular problems reported by the survivor
The remaining 74 of 128 (58%) GPs who returned a case-report form did not confirm the survivor’s self-report (Fig. 2). Of these 74 GPs, 58 (45%) were not aware the survivor had any cardiovascular problem, while 16 (13%) reported that survivors had completely different problems than those indicated in the survey.
Comparison per cardiovascular problem
We compared the agreement between survivor-reported problems and GP reports. In total, 152 cardiovascular problems were reported by 128 survivors for whom GP-reports were available (Table 2). Agreement differed between diagnoses and was highest for stroke (98.4%) and lowest for arrhythmias (78.9%). Inter-rater reliability was good for stroke (κ = 0.79; 95% CI, 0.51 to 1.0), moderate for hypertension requiring medication (κ = 0.51; 95% CI 0.33 to 0.69), arrhythmias (κ = 0.41; 95% CI, 0.18 to 0.64), deep vein thrombosis or pulmonary embolism (κ = 0.56; 95% CI, 0.27 to 0.85) and valvular problems (κ = 0.41; 95% CI, 0.18 to 0.64), and poor for angina pectoris (κ = 0.15; 95% CI, -0.08-0.38) and congestive heart failure (κ 0.32; 95% CI, 0.07–0.57). The number of myocardial infarctions was too small (n = 2) to be evaluated in our young study population. The discordance in reports of cardiovascular problems was mainly explained by more frequent reporting of problems by survivors – e.g., hypertension was reported by survivors in 17 cases, while it was reported by GP only in 3 cases. Similar patterns were observed across conditions.
Discussion
In this questionnaire survey of childhood cancer survivors in Switzerland, 13% of survivors reported a cardiovascular problem. This study describes the attempt to validate these survivor-reported problems by contacting survivors’ GPs. We were able to contact only 57% of GPs and only 44% of GPs provided information on survivor’s cardiovascular conditions. Overall, the survivor-reported problem was confirmed in only 19% of cases. In many cases, the obstacle to confirmation was the survivor, who either failed to report a GP or denied consent to contact his GP. In contrast, most of the GPs we could contact responded (81%). When they did reply, we found that inter-rater reliability between survivors and GPs varied by diagnosis: it was good for stroke; moderate for hypertension, arrhythmias, valvular problems, and thrombosis; and poor for coronary artery disease, and heart failure.
Strengths and limitations
To our knowledge this is one of the first studies to evaluate the possibility of validating questionnaire self-reports by contacting GPs in the Swiss health care setting. It is also the first study in Switzerland to focus on validating self-reported cardiovascular problems in childhood cancer survivors. The study was population-based and representative for all childhood cancer survivors in Switzerland.
There are some limitations of this study. First, we did not primarily validate negative reports (instances in which no cardiovascular problem was indicated). However, Olsson et al. found in a population-based study that the number of false negative answers was very small and concluded that validation can be limited to participants with positive replies [16]. Among the negative reports we could validate in our study, the number of “false negative” answers—i.e., survivors did not report problems which were reported by their GP—was also very small. Much more represented were the “false positive” answers—i.e., survivors reported problems which were not confirmed by their GP (Table 2). Second, the questionnaire for survivors relied mainly on closed questions, while the case report forms for GPs used open questions; this may have affected the results. Another reason for non-agreement may be that patients and GPs have different hierarchies concerning what they see as a relevant problem when being asked to answer a question as “What is your problem?“.
Comparison with other studies
Feasibility of obtaining medical reports
Earlier studies were done in countries with different health care systems, so comparison is not straightforward. Most researchers obtained medical records from hospitals or medical registries [28,29,30,31,32,33,34,35,36]. A few studies validated self-reported cardiovascular diseases directly via GPs as we did [11, 37,38,39,40,41,42]. Some investigators recruited participants directly from GPs, making identification and contacting GPs not necessary [11, 38,39,40,41]. One cataract case-control study in the USA used the same validation procedure as we did and reported a similar physician response rate (86%; ours was 81%) [37]. Mulrooney and colleagues assessed cardiac late effects in the Childhood Cancer Survivor Study (CCSS) in the USA and tried to validate self-reports with medical records from hospitals and GPs [9, 43]. They concluded that this approach was not feasible, since they could not obtain all relevant documents [9, 43].
Agreement between survivor- and GP-reports
In our study, agreement was highest for stroke. This is in line with six previous cohort studies showing moderate to good agreement for stroke (κ = 0.43–0.71) [11, 29, 39,40,41,42]. Only moderate inter-rater reliability was found in our population for hypertension, thrombosis, valvular problems, and arrhythmias. This is similar to four previous studies, which found moderate agreement between self-reports and physician reports (κ = 0.44–0.56) [40,41,42, 44]. However, studies combining hospital records and GP-reports for validation found accurate self-reports of hypertension, with good agreement [11, 29]. We found no data on validity of self-reported thrombosis. The German MultiCare Cohort Study found also moderate agreement between self-reported arrhythmias and GP-reports [41]. Finally, we found poor agreement for heart failure and angina pectoris/coronary heart disease. A German hypercholesterinemia cohort study also reported low agreement for angina pectoris (κ = 0.04) and heart failure (κ = 0.29) [11]. Lampe et al. reported good agreement (κ = 0.72) between self-reported angina pectoris and GP-reports [38]. Kehoe described low sensitivity (64%) but relatively high specificity (96%) for self-reported coronary heart diseases [37]. A study on heart failure among > 45 year old Minnesota residents found moderate agreement between self-report and medical record (κ = 0.46) [29].
Validation of self-reported cardiovascular problems in childhood cancer survivors
To our knowledge, only two studies published validated questionnaire self-reports on cardiovascular problems in childhood cancer survivors [9, 10]. Among participants for whom medical report validation was successful, the CCSS has successfully confirmed congestive heart failure in 67% [9]. However, medical report validation could be performed in only 35% of survivors who self-reported the outcome, hence the authors concluded it was not feasible to utilize outcomes validated this way [9]. A study of bone marrow transplanted survivors in the USA validated survivor-reports and found good to excellent agreement between self-reports of cardiovascular diseases and medical records (κ = 0.7–0.8) [10]. However, they validated only myocardial infarction, hypertension, and overall cardiac events. The discrepancy between our findings and theirs might be explained by differences in study populations and methods. They assessed agreement between self-reported complications and medical records for only the first 100 respondents. It is possible that early respondents are more motivated survivors (with special interest in late effects) and answer differently than late responders. Second, their study participants all came from the same highly specialized pediatric oncology center, and all had a bone marrow transplantation. This might have influenced their results since these survivors are usually involved in strict follow-up programs and might thereby be better informed.
Interpretation of the results
Feasibility of obtaining medical reports
Although the response rate of GPs was excellent (81%), our validation method failed for several reasons. First, many survivors did not consent to contact GPs or did not provide sufficient contact details. Second, many survivors did not have a GP, but listed their former pediatric oncologist as the person who knew their current health problems best. This indicates that the survivor might not have been referred back to primary care or might not have gone there. Third, some GPs had retired, or did not have records available.
Agreement between survivors and GPs
The agreement was low for two probable reasons: inaccurate reporting by the survivor or incorrect reporting by the GP. Inaccurate reporting by survivors could be caused by poor understanding of their health problem. Self-reports are most accurate for well-defined diseases with clear symptomatology like thrombosis, hypertension, myocardial infarction and stroke, which is easy to understand for patients [37, 45, 46]. Our findings underline this: we found lower agreement for poorly defined diseases with a wide spectrum of symptoms and causes, like arrhythmias, heart failure and coronary heart disease. Single high-impact events such as strokes are well reported since they result in hospitalization and patients do not forget them. Moreover, the neurological symptoms of a stroke often persist long after initial hospitalization and patients are reminded of this event in their everyday life. As for CVD with moderate agreement (e.g., hypertension, arrhythmia), previous studies found that awareness of these conditions depends on length of treatment duration, employment, and education status [47, 48]. Coronary artery disease and heart failure—CVD with poor agreement—can vary dramatically in their presentation over time, which determines the patients’ evaluation of their illness, their self-care and thus their GP’s knowledge of the diagnosis. Inaccurate reporting by GPs can result if the GP is unaware of the cardiovascular problem. In the Swiss health care system, survivors can see several doctors in parallel and GPs are usually not gate-keepers to specialized healthcare [49]. Therefore, survivors may have consulted different doctors for their cardiovascular problems, and their GP may not have been informed.
Follow-up care in the Swiss health care system
In Switzerland, children diagnosed with cancer are treated in one of nine pediatric oncology clinics. After completion of treatment, survivors are followed-up at the treatment center for up to 10 years or until the age of 18 years. Many are then transferred back to the primary care system or specialists such as endocrinologists or oncologists depending on the individual’s outcome. This transfer is often complicated [50, 51]. Even if survivors choose a GP for follow-up care, their GP might not be aware of all their health problems. A recent survey among Swiss GPs showed, that 74% of respondents were not aware of survivors needing follow-up, and 28% also stated that they do not have enough experience to provide follow-up care [51]. Survivors transitioning to a GP may therefore receive insufficient follow-up care due to the current state of awareness among GPs.
Implications for practice
In Switzerland, it is not feasible to validate patient-reported cardiovascular events via GPs since the information flow between primary care providers and specialists or tertiary care centers seems to be inadequate, so alternative methods must be attempted. To validate self-reported events, medical examinations of survivors might be performed, or their data linked with data from diagnostic registries or hospital databases. For clinical purposes, information flow between health care providers might also be improved by the electronic patient dossier of the Swiss Federal Office of Public Health. Its introduction is currently being revised [52]. Another way of improving the flow of information between primary care providers and specialists or tertiary care centers would be to increase the proportion of those registered with a GP. This could be achieved by introducing incentives for patients (e.g., lowering health insurance charges) or providers (e.g., capitation payments), or by mandatory registration [53]. As a result, the gatekeeping function of GPs would be strengthened.
Long-term follow-up (LTFU) programs for childhood cancer survivors in Switzerland may provide easier access to information on late effects. In such a program (e.g., using Passport for Care®), follow-up clinics serve as gatekeepers and maintain information from multiple disciplines [54]. Until now, only a small proportion of adult survivors is served by a LTFU clinic in Switzerland [55].
Conclusions
Despite excellent GP compliance, it was not feasible to validate self-reported cardiovascular problems via GPs because they do not serve as gatekeepers in the Swiss health care system. It is thus necessary to develop other validation methods to improve the quality of patient-reported outcomes, and to introduce policies improving the flow of information between health care providers. This would be beneficial not only for research purposes, but more importantly for a better clinical care of patients.
Data availability
The data generated and analyzed during the current study are not publicly available to maintain the privacy of participants. Data is available from the corresponding author upon reasonable request.
Abbreviations
- CCSS:
-
Childhood Cancer Survivor Study
- CVD:
-
Cardiovascular diseases
- CNS:
-
Central nervous system
- GP:
-
General practitioners
- LTFU:
-
Long-term follow-up
- ChCR:
-
Swiss Childhood Cancer Registry
- SCCSS:
-
Swiss Childhood Cancer Survivor Study
- USA:
-
United States of America
References
Lenneman CG, Sawyer DB. Cardio-Oncology: an update on cardiotoxicity of Cancer-Related treatment. Circ Res. 2016;118(6):1008–20.
Schindler M, Spycher BD, Ammann RA, Ansari M, Michel G, Kuehni CE, et al. Cause-specific long-term mortality in survivors of childhood cancer in Switzerland: a population-based study. Int J Cancer. 2016;139(2):322–33.
Armenian SH, Armstrong GT, Aune G, Chow EJ, Ehrhardt MJ, Ky B, et al. Cardiovascular Disease in survivors of Childhood Cancer: insights into epidemiology, pathophysiology, and Prevention. J Clin Oncol. 2018;36(21):2135–44.
de Baat EC, Feijen EAM, Reulen RC, Allodji RS, Bagnasco F, Bardi E, et al. Risk factors for heart failure among Pan-european Childhood Cancer survivors: a PanCareSurFup and ProCardio Cohort and Nested Case-Control Study. J Clin Oncol. 2023;41(1):96–106.
Kooijmans ECM, van der Pal HJH, Pluijm SMF, Bresters D, van Dulmen-den Broeder E, et al. Hypertension in long-term childhood cancer survivors after treatment with potentially nephrotoxic therapy; DCCSS-LATER 2: renal study. Eur J Cancer. 2022;172:287–99.
Mulrooney DA, Hyun G, Ness KK, Ehrhardt MJ, Yasui Y, Duprez D, et al. Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: report from the Childhood Cancer Survivor Study cohort. BMJ. 2020;368:l6794.
Arnold N, Merzenich H, Wingerter A, Schulz A, Schneider A, Prochaska JH, et al. Promotion of arterial stiffness by Childhood Cancer and its characteristics in adult long-term survivors. J Am Heart Assoc. 2021;10(5):e015609.
Feijen EAM, van Dalen EC, van der Pal HJH, Reulen RC, Winter DL, Keuhni CE, et al. Increased risk of cardiac ischaemia in a pan-european cohort of 36 205 childhood cancer survivors: a PanCareSurFup study. Heart. 2021;107(1):33–40.
Leisenring WM, Mertens AC, Armstrong GT, Stovall MA, Neglia JP, Lanctot JQ, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2319–27.
Louie A, Robison L, Bogue M, Hyde S, Forman S, Bhatia S. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25(11):1191.
Englert H, Müller-Nordhorn J, Seewald S, Sonntag F, Völler H, Meyer-Sabellek W, et al. Is patient self-report an adequate tool for monitoring cardiovascular conditions in patients with hypercholesterolemia? J Public Health. 2010;32(3):387.
Merzenich H, Blettner M, Niehoff D, Schwentner L, Schmidt M, Schmitt M, et al. Cardiac late events in German breast cancer patients: a validation study on the agreement between patient self-reports and information from physicians. BMC Cardiovasc Disord. 2018;18(1):218.
Bowlin SJ, Morrill BD, Nafziger AN, Lewis C, Pearson TA. Reliability and changes in validity of self-reported cardiovascular disease risk factors using dual response: the behavioral risk factor survey. J Clin Epidemiol. 1996;49(5):511–7.
Reedijk M, Huss A, Verheij RA, Peeters PH, Vermeulen RCH. Parkinson’s disease case ascertainment in prospective cohort studies through combining multiple health information resources. PLoS ONE. 2020;15(7):e0234845.
Weiss A, Sommer G, Kuonen R, Scheinemann K, Grotzer M, Kompis M, et al. Validation of questionnaire-reported hearing with medical records: a report from the Swiss Childhood Cancer Survivor Study. PLoS ONE. 2017;12(3):e0174479.
Olsson L, Svärdsudd K, Nilsson G, Ringqvist I, Tibblin G. Validity of a postal questionnaire with regard to the prevalence of myocardial infarction in a general population sample. Eur Heart J. 1989;10(11):1011.
Sommer G, Schindler M, Redmond S, Pfeiffer V, Konstantinoudis G, Ammann RA, et al. Temporal trends in incidence of childhood cancer in Switzerland, 1985–2014. Cancer Epidemiol. 2019;61:157–64.
Kuehni CE, Rueegg CS, Michel G, Rebholz CE, Strippoli MP, Niggli FK, et al. Cohort profile: the Swiss childhood cancer survivor study. Int J Epidemiol. 2012;41(6):1553–64.
Hawkins M, Lancashire E, Winter D, Frobisher C, Reulen R, Taylor A, et al. The British Childhood Cancer Survivor Study: objectives, methods, population structure, response rates and initial descriptive information. Pediatr Blood Cancer. 2008;50(5):1018–25.
Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the childhood cancer survivor study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–39.
Howell CR, Bjornard KL, Ness KK, Alberts N, Armstrong GT, Bhakta N, et al. Cohort Profile: the St. Jude Lifetime Cohort Study (SJLIFE) for paediatric cancer survivors. Int J Epidemiol. 2021;50(1):39–49.
Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of Childhood Cancer, third edition. Cancer. 2005;103(7):1457–67.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977:159 – 74.
Fleiss JL, Cohen J, Everitt B. Large sample standard errors of kappa and weighted kappa. Psychol Bull. 1969;72(5):323.
Grouven U, Bender R, Ziegler A, Lange S. Der Kappa-Koeffizient. Dtsch Med Wochenschr. 2007;132(1):65–8.
Cicchetti DV, Feinstein AR. High agreement but low kappa: II. Resolving the paradoxes. J Clin Epidemiol. 1990;43(6):551–8.
Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol. 1990;43(6):543–9.
Barr E, Tonkin A, Welborn T, Shaw J. Validity of self-reported cardiovascular disease events in comparison to medical record adjudication and a statewide hospital morbidity database: the AusDiab study. Intern Med J. 2009;39(1):49–53.
Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57(10):1096–103.
Carter K, Barber PA, Shaw C. How does self-reported history of Stroke compare to Hospitalization Data in a Population-based survey in New Zealand? Stroke. 2010;41(11):2678–80.
Haapanen N, Miilunpalo S, Pasanen M, Oja P, Vuori I. Agreement between questionnaire data and medical records of chronic diseases in middle-aged and elderly Finnish men and women. Am J Epidemiol. 1997;145(8):762.
Britton A, Milne B, Butler T, Sanchez-Galvez A, Shipley M, Rudd A, et al. Validating self-reported strokes in a longitudinal UK cohort study (Whitehall II): extracting information from hospital medical records versus the Hospital Episode statistics database. BMC Med Res Methodol. 2012;12:83.
Choe S, Lee J, Lee J, Kang D, Lee JK, Shin A. Validity of self-reported stroke and myocardial infarction in Korea: the Health examinees (HEXA) study. J Prev Med Public Health. 2019;52(6):377–83.
Jackson CA, Mishra GD, Tooth L, Byles J, Dobson A. Moderate agreement between self-reported stroke and hospital-recorded stroke in two cohorts of Australian women: a validation study. BMC Med Res Methodol. 2015;15:7.
Muggah E, Graves E, Bennett C, Manuel DG. Ascertainment of chronic diseases using population health data: a comparison of health administrative data and patient self-report. BMC Public Health. 2013;13:16.
Langballe R, John EM, Malone KE, Bernstein L, Knight JA, Lynch CF, et al. Agreement between self-reported and register-based cardiovascular events among Danish breast cancer survivors. J Cancer Surviv. 2018;12(1):95–100.
Kehoe R, Wu SY, Leske MC, Chylack LT. Comparing self-reported and physician-reported medical history. Am J Epidemiol. 1994;139(8):813.
Lampe FC, Walker M, Lennon LT, Whincup PH, Ebrahim S. Validity of a self-reported history of doctor-diagnosed angina. J Clin Epidemiol. 1999;52(1):73–81.
Walker MK, Whincup PH, Shaper AG, Lennon LT, Thomson AG. Validation of patient recall of doctor-diagnosed heart attack and stroke: a postal questionnaire and record review comparison. Am J Epidemiol. 1998;148(4):355–61.
Teh R, Doughty R, Connolly M, Broad J, Pillai A, Wilkinson T, et al. Agreement between self-reports and medical records of cardiovascular disease in octogenarians. J Clin Epidemiol. 2013;66(10):1135–43.
Hansen H, Schafer I, Schon G, Riedel-Heller S, Gensichen J, Weyerer S, et al. Agreement between self-reported and general practitioner-reported chronic conditions among multimorbid patients in primary care - results of the MultiCare Cohort Study. BMC Fam Pract. 2014;15:39.
Steinkirchner AB, Zimmermann ME, Donhauser FJ, Dietl A, Brandl C, Koller M, et al. Self-report of chronic diseases in old-aged individuals: extent of agreement with general practitioner medical records in the German AugUR study. J Epidemiol Community Health. 2022;76(11):931–8.
Mulrooney D, Yeazel M, Kawashima T, Mertens A, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339(dec08 1):b4606.
Muhajarine N, Mustard C, Roos LL, Young TK, Gelskey DE. Comparison of survey and physician claims data for detecting hypertension. J Clin Epidemiol. 1997;50(6):711–8.
Paganini-Hill A, Chao A. Accuracy of recall of hip fracture, heart attack, and cancer: a comparison of postal survey data and medical records. Am J Epidemiol. 1993;138(2):101.
Tretli S, Lund-Larsen PG, Foss OP. Reliability of questionnaire information on cardiovascular disease and diabetes: cardiovascular disease study in Finnmark county. J Epidemiol Commun Health. 1982;36(4):269–73.
Wolde M, Azale T, Debalkie Demissie G, Addis B. Knowledge about hypertension and associated factors among patients with hypertension in public health facilities of Gondar city, Northwest Ethiopia: Ordinal logistic regression analysis. PLoS ONE. 2022;17(6):e0270030.
Frewen J, Finucane C, Cronin H, Rice C, Kearney PM, Harbison J, et al. Factors that influence awareness and treatment of atrial fibrillation in older adults. QJM. 2013;106(5):415–24.
Reinhardt UE. The Swiss Health System. JAMA: J Am Med Association. 2004;292(10):1227–31.
Rebholz CE, von der Weid NX, Michel G, Niggli FK, Kuehni CE. Follow-up care amongst long-term childhood cancer survivors: a report from the Swiss Childhood Cancer Survivor Study. Eur J Cancer. 2010;47(2):221–9.
Michel G, Gianinazzi ME, Vetsch J, Mader L, Lupatsch JE, von der Weid NX, et al. Physicians’ experience with follow-up care of childhood cancer survivors - challenges and needs. Swiss Med Wkly. 2017;147:w14457.
Federal Office of Public Health. Weiterentwicklung elektronisches Patientendossier 2023 [Available from: https://www.bag.admin.ch/bag/de/home/strategie-und-politik/nationale-gesundheitsstrategien/strategie-ehealth-schweiz/umsetzung-vollzug/weiterentwicklung-epd.html.
Marchildon GP, Brammli-Greenberg S, Dayan M, De Belvis AG, Gandre C, Isaksson D, et al. Achieving higher performing primary care through patient registration: a review of twelve high-income countries. Health Policy. 2021;125(12):1507–16.
Poplack DG, Fordis M, Landier W, Bhatia S, Hudson MM, Horowitz ME. Childhood cancer survivor care: development of the Passport for Care. Nat Rev Clin Oncol. 2014;11(12):740–50.
Tinner EM, Gumy Pause F, Diezi M, Bergstraesser E, Hengartner H, Eisenreich B, et al. Long-term follow-up after childhood cancer in Switzerland: a position statement from the pediatric Swiss LTFU working group. Schweizer Krebs-Bulletin. 2019;39(3):212–5.
Acknowledgements
We would like to warmly thank all survivors, families, general practitioners, and pediatricians who participated in this study, Kali Tal for providing editorial assistance with the manuscript, the team of the Swiss Childhood Cancer Registry, and the team of the Swiss Childhood Cancer Survivor Study.
Funding
This work was supported by the European Union (7th Framework Programme, Grant Agreement Number 257505, “PanCareSurFup”), Swiss Cancer Research (Grant no. KFS-5027-02-2020), Stiftung für krebskranke Kinder - Regio Basiliensis, and the University of Basel Research Fund for Excellent Junior Researchers.
Author information
Authors and Affiliations
Contributions
NXvdW and CEK conceptualized and supervised the study. CS, NXvdW, and CEK acquired funding for this study. EMH, SE, GM, and LW collected the data. EMH analyzed the data and drafted the manuscript. EMH, TS, SE, GM, LW, CS, EB, NXvdW, CEK reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations. The Ethics Committee of the Canton of Bern (166/2014; 2021 − 01462) granted ethical approval for ChCR and SCCSS. All participants or their parents provided their informed consent. By returning the questionnaire, cancer survivors agreed that we could use their data for research. Consent to contact GPs was given within the questionnaire. If the participant was under the age of 16 years, the questionnaire was filled in by parents. By returning the questionnaire, the parents consented that we could use this data for research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hau, EM., Sláma, T., Essig, S. et al. Validation of self-reported cardiovascular problems in childhood cancer survivors by contacting general practitioners: feasibility and results. BMC Prim. Care 25, 81 (2024). https://doi.org/10.1186/s12875-024-02322-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12875-024-02322-7