Abstract
Background
Although previous studies have reported general inexperience with the Epley manoeuvre (EM) among general physicians, no report has evaluated the effect of EM on benign paroxysmal positional vertigo (BPPV) in primary care by using point estimates or certainty of evidence. We conducted this systematic review and meta-analysis and clarified the efficacy of EM for BPPV, regardless of primary-care and subspecialty settings.
Methods
Systematic review and meta-analysis of randomised sham-controlled trials of EM for the treatment of posterior canal BPPV in primary-care and subspecialty settings. A primary-care setting was defined as a practice setting by general practitioners, primary-care doctors, or family doctors. A systematic search was conducted in January 2022 across databases, including Cochrane Central Resister of Controlled Trial, MEDLINE, Embase, Cumulative Index of Nursing and Allied Health Literature, World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov. Primary outcomes were the disappearance of subjective symptoms (vertigo), negative findings (Dix–Hallpike test), and all adverse events. We evaluated the certainty of evidence using the Grading of Recommendations, Assessment, Development and Evaluation approach.
Results
Twenty-seven randomised controlled trials were identified. In primary-care settings, EM reduced the subjective symptoms [risk ratio (RR), 3.14; 95% confidence interval (CI), 1.96–5.02]; however, there was no applicable article for all adverse events. In the subspeciality setting, EM reduced the subjective symptoms (RR, 2.42; 95% CI, 1.64–3.56), resulting in an increase in negative findings (RR, 1.81; 95% CI, 1.40–2.34). The evidence exhibited uncertainty about the effect of EM on negative findings in primary-care settings and all adverse events in subspecialty settings.
Conclusions
Regardless of primary-care and subspecialty settings, EM for BPPV was effective. This study has shown the significance of performing EM for BPPV in primary-care settings. EM for BPPV in a primary-care setting may aid in preventing referrals to higher tertiary care facilities and hospitalisation for follow-up.
Trial registration
The study was registered in protocols.io (PROTOCOL INTEGER ID: 51,464) on July 11, 2021.
Similar content being viewed by others
Background
Benign paroxysmal positional vertigo (BPPV) is a common inner ear disorder. It’s characterized by repeated episodes of vertigo, which are triggered by rapid changes in head position [1]. The most common form of BPPV is the posterior semicircular canals, which account for 85% of cases [2]. However, horizontal canal BPPV is probably much more common than previously recognised [3]. The Dix–Hallpike (DH) manoeuvre is considered the gold standard test for the diagnosis of posterior canal BPPV [4]. Horizontal canal BPPV should be considered when horizontal nystagmus is seen rather than upbeat torsional nystagmus in the DH manoeuvre [3]. There is high-quality and compelling evidence that patients diagnosed with posterior canal BPPV should be offered expeditious treatment with canalith repositioning procedures, commonly referred to as the Epley manoeuvre (EM) [1]. Regarding patients with BPPV, long-term follow-up studies have indicated that vestibular suppressants may not affect symptom resolution; moreover, there is evidence that canalith repositioning procedures are superior to these drugs [5]. EM was first described by Epley in 1992 [6], and systematic review and meta-analysis with small-size randomised controlled trials (RCTs) in the 2014 Cochrane Review [7], including primary-care settings, showed the efficacy of EM for posterior canal BPPV.
Several RCTs on the efficacy of EM on BPPV in primary-care settings [8, 9] have been published since 2014; however, the integrated results of these trials are not yet clear. Furthermore, studies regarding the efficacy of EM in primary-care settings are rare. In the 2014 Cochrane Review [7] of EM for BPPV, 2 of the 11 trials were in primary-care settings [10, 11], and the remainder were conducted in secondary or tertiary care in the otolaryngology departments. It is uncertain whether EM contributes adequately to the treatment of BPPV in the primary-care setting. Therefore, this study aimed to clarify the efficacy of EM for BPPV in primary-care and subspecialty settings.
Methods
The study was registered in protocols.io (PROTOCOL INTEGER ID: 51,464) [12]. This study was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [13]. We ensured that this study was PRISMA-compliant by consulting the PRISMA 2020 checklist [14] (details provided in Additional file 1).
Inclusion and exclusion criteria
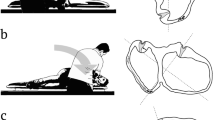
We included RCTs that assessed the efficacy of EM. Cluster randomised and crossover trials were not included. We did not apply language or country restrictions. We included all articles, including published, unpublished, conference abstracts, and letters. We did not exclude studies based on the observation period or publication year. Participants should have presented with the symptoms of repeated episodes of vertigo, mostly on change of position along with nausea and vomiting. Age, sex, and race did not matter. Participants with a clinical diagnosis of BPPV using the DH test, which proved positional nystagmus reflecting involvement of the posterior canal [4], were included. For posterior canal BPPV, a positive DH test is defined by the presence of upbeating and torsional nystagmus with the top pole of rotation beating toward the affected (downside) ear [4]. The study also included participants with subjective BPPV, where nystagmus is not induced by the DH test; subjective BPPV is an important concept [15]. Participants were diagnosed with BPPV by physicians educated in using EM. EM was administered at the first visit. Repeated manoeuvres or the combination with other interventions that included drugs and rehabilitative exercise were not a concern. Patients who could not tolerate the procedure or had serious heart disease or cervical spine lesions were excluded. The intervention was defined as EM involving a series of four head and body movements from sitting to lying, rolling over, and back to sitting [7]. Control was defined as medication, untreated controls, and sham manoeuvre. A sham manoeuvre consists of laying the patient with the head tilted on the affected side for 5 min. The primary-care setting was defined as a practice setting by general physicians, primary-care physicians, and family physicians.
Outcomes of interest
The primary outcomes were the disappearance of subjective symptoms (vertigo), negative findings (DH test), and all adverse events. In general, there should be no more than three primary outcomes, including at least one desirable and at least one undesirable outcome [16]. The secondary outcomes were the disappearance of objective symptoms (nystagmus) and Dizziness Handicap Inventory (DHI) score. All outcomes of interest are detailed in Additional file 2.
Literature search
A systematic search was conducted in January 2022 across databases, including the Central, MEDLINE, Embase, and Cumulative Index of Nursing and Allied Health Literature (details provided in Additional file 3). We also searched the World Health Organization International Clinical Trials Platform Search Portal and ClinicalTrials.gov for ongoing or unpublished trials. We checked the reference lists of studies, including international guidelines [1], reference lists, and articles citing eligible studies. We asked the authors of the original studies for unpublished or Additional data.
Screening, data extraction, and appraisal
Two independent reviewers (YS, HM) screened titles and abstracts and assessed eligibility based on the full texts. We contacted original authors if relevant data was missing. Disagreements between the two reviewers were resolved by discussion, and if this failed, a third reviewer acted as an arbiter (NY). Two reviewers (YS, HM) performed independent data extraction of the included studies using standardized data collection forms. We used a pre-checked form using 10 randomly selected studies. The form included the information on study design, study population, interventions, and outcomes. Any disagreements were resolved by discussion, and if this failed, a third reviewer acted as an arbiter (NY). Two reviewers (YS, HM) evaluated the risk of bias (ROB) independently using the Risk of Bias 2 [17]. Disagreements between the two reviewers were discussed, and if this failed, a third reviewer (ST) acted as an arbiter, if necessary.
Data analysis
We pooled the relative risk ratios (RRs) and 95% confidence intervals (CIs) for the following binary variables: disappearance of subjective symptoms (vertigo), negative findings (Dix–Hallpike test), all adverse events, and disappearance of objective symptoms (nystagmus). We pooled the mean differences and 95% CIs for the following continuous variable: Dizziness Handicap Inventory. We summarised adverse events based on the definition in the original article, but we did not perform a meta-analysis. We requested the original authors for the not-presented data.
We performed the intention-to-treat analysis for all dichotomous data. For continuous data, we did not impute missing data based on the recommendation by the Cochrane Handbook [18]. When original studies only reported standard error or a P-value, we calculated the standard deviation based on the method reported by Altman [19]. If these values were unknown when we contacted the authors, the standard deviation was calculated using confidence interval and t-value based on the method indicated in the Cochrane Handbook [18] or validated method [19]. The validity of these methods was analysed using sensitivity analysis.
We evaluated the statistical heterogeneity by visual inspection of the forest plots and calculating the I2 statistic (I2 values of 0–40%: might not be important; 30–60%: may represent moderate heterogeneity; 50–90%: may represent substantial heterogeneity; and 75–100%: considerable heterogeneity). When there was substantial heterogeneity (I2 > 50%), we assessed the reason for the heterogeneity. The Cochrane chi-squared test (Q-test) was performed for I2 statistic, and a P-value less than 0.10 was defined as statistically significant.
We searched the clinical trial registry system (ClinicalTrials.gov and International Clinical Trials Platform Search Portal) and performed an extensive literature search for unpublished trials. We assessed the potential publication bias by visual inspection of the funnel plot. The Egger test was also performed; we did not conduct the test when we found fewer than 10 trials or trials with similar sample size.
Meta-analysis was performed using Review Manager software (RevMan 5.4). We used a random-effects model. To elucidate the influence of effect modifiers on results, we evaluated the subgroup analyses of the primary outcomes based on age (≥ 65 years), vertigo severity (above average if using a scale), duration (< 30 days or longer), number of BPPV episodes (first or recurrent episode), number of EM sessions (only once vs. more than once), and EM skills in primary-care settings (whether or not they are educated practitioners) when sufficient data were available. The definition of an educated practitioner is one who has been educated in EM methods by an otolaryngologist or neurologist and observed in practice.
We performed sensitivity analysis for the primary outcomes to assess whether the results of the review were robust to the decisions made during the review process by excluding studies using imputed statistics or excluding studies with high or some concern in the overall assessment of the ROB. We created a summary-of-findings table that included an overall grading of the certainty of the evidence for each primary and secondary outcome, evaluated using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach [20].
Results
Study identification
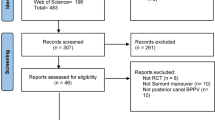
After removing duplicates, we identified 3,236 records during the search conducted in January 2022. We identified 27 RCTs that fulfilled all eligibility criteria and were included in the qualitative synthesis (Fig. 1; details provided in Additional files 4 and 5]). The 27 RCTs provided a pooled sample of 1,629 patients undergoing EM for BPPV. Only 1 RCT [21] did not have valid outcome data.
Characteristics of the included studies
In total, 4 studies were performed in the primary-care setting [8,9,10,11], 15 in the otolaryngology setting [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35], 4 in the neurology setting [36,37,38,39], and 4 in the emergency room setting [40,41,42,43]. The duration of the intervention (if measurements were taken at multiple time points, we integrated them using the shortest period) ranged from the first visit to 1 month. We searched for ongoing studies but could not find them. Of the 27 trials analysis, 19 evaluated the outcome of the disappearance of subjective symptoms [8, 10, 11, 22,23,24,25,26, 28, 29, 32, 34, 36,37,38,39, 41,42,43], 16 evaluated the outcome of negative findings [8, 10, 22, 24, 26,27,28, 30, 31, 33, 35,36,37,38, 41, 42], 2 evaluated the outcome of the disappearance of objective symptoms [8, 23], 1 evaluated the outcome of all adverse events [42], 6 evaluated the outcome of DHI-S (the screening version of DHI) [9, 25, 30, 31, 33, 40], and 1 evaluated the outcome of DHI [35]. For three of the studies that evaluated DHI-S [25, 30, 31], we were unable to retrieve outcome data because no reply was received from the authors. In addition, for one study that evaluated DHI [35] results, we were unable to retrieve outcome data. For studies excluded using the full-text screening, bibliographic information was presented in Additional file 6.
Efficacy of the intervention
Forest plots for each outcome are described in Additional file 7. The summary of findings provides the certainty of the evidence for the outcome in each setting and is listed in Tables 1 and 2.
Primary outcomes in the primary-care setting
The evidence suggested that EM reduced subjective symptoms (3 studies, 309 participants): RR 3.14; 95% CI 1.96–5.02, I2 = 84%; low certainty evidence. The evidence was uncertain about the effect of EM on the negative findings using the DH test (2 studies, 206 participants): RR 1.46; 95% CI 0.72–2.97, I2 = 63%; very low certainty evidence.
Secondary outcomes in the primary-care setting
The disappearance of objective symptoms and DHI-S were measured in one RCT [9]. The evidence suggested that EM reduced objective symptoms slightly (1 study, 127 participants): RR 0.84; 95% CI 0.73–0.97; low certainty evidence. The evidence suggested that EM resulted in little to no difference in DHI-S (1 study, 134 participants): mean difference − 2; 95% CI -5.51 to 1.51; low certainty evidence.
Primary outcomes in the otolaryngology or subspecialty settings
The evidence suggested that EM reduced subjective symptoms (16 studies, 829 participants): RR 2.42; 95% CI 1.64–3.56, I2 = 84%; low certainty evidence. The evidence suggested that EM resulted in an increase in negative findings using the DH test (16 studies, 912 participants): RR 1.81; 95% CI 1.40–2.34, I2 = 79%; low certainty evidence. The evidence was uncertain about the effect of EM on all adverse events: one study [42] (50 participants) reported all adverse events.
Secondary outcomes in the otolaryngology or subspecialty settings
The disappearance of objective symptoms was measured in one RCT [23], and DHI-S was measured in two RCTs [33, 40]. The evidence suggested that EM reduced objective symptoms slightly (1 study, 58 participants): RR 1.69; 95% CI 1.08–2.66; low certainty evidence. The evidence was uncertain about the effect of EM on DHI-S (2 studies, 70 participants): mean difference − 8.24; 95% CI -28 to 11.51; very low certainty evidence.
Quality assessment
Risk of Bias 2 was used for the evaluation of the ROB. Most studies were at high or some concern ROB, as per the Cochrane ROB assessment tool (details provided in Additional file 8). In the primary-care and otolaryngology or subspecialty settings, the disappearance of subjective symptoms was a subjective assessment and resulted in a high ROB. Two studies of negative findings in the primary-care setting [8, 10] had some concern ROB because one study demonstrated a low ROB except for the randomisation process, and the other demonstrated a low ROB except for deviations from the intended intervention. There was no study about all adverse events in primary-care settings.
Two studies of negative findings in the otolaryngology or subspecialty settings [24, 36] had a low ROB, and six studies [8, 10, 35, 37, 41, 42] had some concern ROB. The other studies demonstrated a high ROB. One study [42] of all adverse events in the otolaryngology or subspecialty settings had a high ROB because the outcome was measured. There was evidence of publication bias using Egger’s test (P < 0.001) in a reduction of subjective symptoms and an increase in negative findings using the DH test in the otolaryngology or subspecialty settings.
Subgroup analysis and sensitivity analysis
The prespecified subgroup analyses for the primary outcomes revealed no significant differences among subgroups (details provided in Additional file 9). We were unable to perform subgroup analyses for items ‘vertigo severity’, ‘number of BPPV episodes (first or recurrent episode)’, and number of EM sessions (only once vs more than once)’ because there were no applicable studies.
The prespecified sensitivity analysis for the primary outcomes was carried out because there was no study that used imputed statistics, but two studies [24, 36] were a low ROB in the outcome of negative findings using the DH test in the otolaryngology or subspecialty settings. Similar results were obtained in the sensitivity analysis (details provided in Additional file 10).
Discussion
This study suggests that regardless of primary-care and subspecialty settings, EM for BPPV was effective. The primary-care setting has fewer studies and a smaller sample size than that in the subspecialty setting and showed very low to low evidence for improvement in the subjective and objective endpoints. In the primary-care setting, it has been pointed out that EM is not adequately performed due to the level of skill and lack of confidence using the DH test [44]. EM is often not performed, and the patient is treated with oral medications [45]. However, EM can be learned through video-based training [46] and can be performed within a 10-minute consultation [44], making it valuable for the primary-care setting. Not only is the treatment of BPPV with EM effective, being able to address the problem definitively in the office is satisfying for both the patient and doctor.
Previous studies have not presented point estimates and CoE separately for primary-care and other settings. This study assessed the efficacy of EM for BPPV in the primary-care setting. Although not specific to the primary-care setting, the Cochrane Review examining the efficacy of EM for BPPV included two trials of the primary-care setting [7] and showed that complete resolution of vertigo occurred significantly more often in the EM group compared with the sham manoeuvre or control group, and conversion from a positive to a negative DH test significantly favoured the EM group compared with the sham manoeuvre or control group. The efficacy of EM was demonstrated in the primary-care setting and Cochrane Review.
Furthermore, subgroup analyses of age and duration were performed to search for causes of heterogeneity, but no significant differences were found. Studies evaluated in all settings have reported no significant difference in efficacy between older and younger patients. However, many older patients with BPPV may have difficulty performing canalith repositioning procedures due to various orthopaedic and vascular problems, such as limited range of motion of the cervical spine, kyphosis, or a history of vertebrobasilar insufficiency or stroke, and thus require careful enforcement [47]. Regarding the symptom duration, a BPPV vertigo attack lasts approximately 30 s; there is no obvious reason why EM should be more or less effective at different times between onset and spontaneous resolution if the mechanism of onset is similar between cases [7]. This study showed no significant differences in the subgroup analysis of the symptom duration persistence divided by 30 days, consistent with this hypothesis.
This study has several strengths. First, we registered the protocol according to the PRISMA guidelines and employed a robust methodology with comprehensive evidence searching. Second, we used the GRADE approach for assessing the certainty of evidence (CoE) and referred to the Cochrane Handbook [20]. Third, this is the first study to report point estimation and CoE separately for primary-care and subspecialty settings. The 2014 Cochrane Review [7] included a section for EM in the primary-care setting but not for CoE. In addition, we were able to add and analyse the literature on the primary-care setting since 2014, when the Cochrane Review was published.
This study has several potential limitations. First, long-term effects could not be evaluated because the shortest timing was used as the timing for evaluating outcomes. BPPV is a spontaneously resolving disease with an average symptom duration of 39 days [48]. Therefore, the follow-up duration in the included studies was enough for clinical assessment. Second, we were unable to perform subgroup analysis for dizziness severity, presence or absence of recurrence, and repeated EM. Third, experienced physicians performed EM for BPPV in the included studies. A previous study reported the general lack of experience using the DH test among general physicians [8]. As the procedure is simple, the effect size may differ depending on the therapist’s experience. This study evaluated the efficacy of the intervention based on the assumption that EM skills were mastered. Recent related articles have shown that even non-specialists can achieve excellent results if they are trained in the technique [49, 50]. Fourth, we did not perform a sensitivity analysis of studies wherein DH was performed as an objective measure in follow-up; consequently, we may not have been able to assess the true efficacy of the intervention.
This study demonstrated the efficacy of EM for BPPV in the primary-care setting, but it was based on several small-scale studies with a high ROB. We believe that more large-scale, high-quality studies are needed to estimate more accurate efficacy. We were unable to perform subgroup analysis for dizziness severity, presence or absence of recurrence, and repeated EM. Future studies need to assess whether it could be a source of heterogeneity.
Conclusions
Regardless of primary-care and subspecialty settings, EM for BPPV was effective. The results of this study support EM for BPPV in the primary-care setting. EM for BPPV in primary-care settings may aid in preventing referrals to higher tertiary-care facilities and hospitalisation for follow-up. Furthermore, results reported herein are expected to provide further insight into the cost-effectiveness of implementing EM in the primary care setting.
Availability of data and materials
The datasets generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- BPPV:
-
Benign Paroxysmal Positional Vertigo
- EM:
-
Epley Manoeuvre
- RR:
-
Risk Ratio
- CI:
-
Confidence Interval
- DH:
-
Dix–Hallpike
- RCTs:
-
Randomised Controlled Trials
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis
- DHI:
-
Dizziness Handicap Inventory
- ROB:
-
Risk of Bias
- GRADE:
-
Grading of Recommendations, Assessment, Development and Evaluation
- DHI-S:
-
Screening version of DHI
- CoE:
-
Certainty of Evidence
References
Bhattacharyya N, Gubbels SP, Schwartz SR, Edlow JA, El-Kashlan H, Fife T, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156:1–S47.
Hornibrook J. Benign paroxysmal positional vertigo (BPPV): history, pathophysiology, office treatment and future directions. Int J Otolaryngol. 2011;2011:835671.
Bhandari R, Bhandari A, Hsieh YH, Edlow J, Omron R. Prevalence of horizontal canal variant in 3,975 patients with Benign Paroxysmal positional Vertigo: a cross-sectional study. Neurol Clin Pract. 2023;13(5): e200191.
Fife TD, Iverson DJ, Lempert T, Furman JM, Baloh RW, Tusa RJ, et al. Practice parameter: therapies for benign paroxysmal positional vertigo (an evidence-based review): report of the Quality standards Subcommittee of the American Academy of Neurology. Neurology. 2008;70:2067–74.
Sharif S, Khoujah D, Greer A, Naples JG, Upadhye S, Edlow JA. Vestibular suppressants for benign paroxysmal positional vertigo: a systematic review and meta-analysis of randomized controlled trials. Acad Emerg Med. 2023;30(5):541–51.
Epley JM. The canalith repositioning procedure: for treatment of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 1992;107:399–404.
Hilton MP, Pinder DK. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2014;12:Cd003162.
Ballvé JL, Carrillo-Muñoz R, Rando-Matos Y, Villar I, Cunillera O, Almeda J, et al. Effectiveness of the Epley manoeuvre in posterior canal benign paroxysmal positional vertigo: a randomised clinical trial in primary care. Br J Gen Pract. 2019;69:e52–60.
Carrillo Muñoz R, Ballve Moreno JL, Villar Balboa I, Rando Matos Y, Cunillera Puertolas O, Almeda Ortega J, et al. A single Epley manoeuvre can improve self-perceptions of disability (quality of life) in patients with pc-BPPV: a randomised controlled trial in primary care. Aten Primaria. 2021;53: 102077.
Munoz JE, Miklea JT, Howard M, Springate R, Kaczorowski J. Canalith repositioning maneuver for benign paroxysmal positional vertigo: randomized controlled trial in family practice. Can Fam Physician. 2007;53:1049–53.
Xie K, Du S-W, Gao J-J, Shou G-I, Jian H-Y, Li Y-Z. Clinical efficacy of Epley procedure for treatment of benign paroxysmal positional vertigo of posterior semicircular canal. Chin J Gen Pract. 2012;2:20.
Saishoji Y, Yamamoto N, Fujiwara T, Mori H, Taito S. The efficacy of the Epley maneuver for benign paroxysmal positional vertigo (BPPV) in primary care setting- a systematic review and meta-analysis. Protocols.io. 2021;15(1):1–0.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med. 2009;6: e1000100.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Moreno JLB, Munoz RC, Matos YR, Balboa IV, Puertolas OC, Ortega JA. Responses to the Dix-Hallpike test in primary care: a comparison between subjective and objective benign paroxysmal positional vertigo. Aten Primaria. 2021;53(8): 102023.
Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. p. 2011; updated March 2011. Available from: http://www.training.cochrane.org.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0. Cochrane. p. 2019; updated July 2019. Available from: http://www.training.cochrane.org/handbook.
Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3. Cochrane. p. 2022; updated February 2022. Available from: http://www.training.cochrane.org/handbook.
Cohen HS, Kimball KT. Effectiveness of treatments for benign paroxysmal positional vertigo of the posterior canal. Otol Neurotol. 2005;26:1034–40.
Lynn S, Pool A, Rose D, Brey R, Suman V. Randomized trial of the canalith repositioning procedure. Otolaryngol Head Neck Surg. 1995;113:712–20.
Yimtae K, Srirompotong S, Srirompotong S, Sae-Seaw P. A randomized trial of the canalith repositioning procedure. Laryngoscope. 2003;113:828–32.
Lee JD, Shim DB, Park HJ, Song CI, Kim MB, Kim CH, et al. A multicenter randomized double-blind study: comparison of the Epley, semont, and sham maneuvers for the treatment of posterior canal benign paroxysmal positional vertigo. Audiol Neurootol. 2014;19:336–41.
Kaur J, Shamanna K. Management of benign paroxysmal positional vertigo: a comparative study between Epleys manouvre and Betahistine. Int Tinnitus J. 2017;21:30–4.
Sherman D, Massoud EAS. Treatment outcomes of benign paroxysmal positional vertigo. J Otolaryngol. 2001;30:295–9.
Wolf M, Hertanu T, Novikov I, Kronenberg J. Epley’s manoeuvre for benign paroxysmal positional vertigo: a prospective study. Clin Otolaryngol Allied Sci. 1999;24:43–6.
Simhadri S, Panda N, Raghunathan M. Efficacy of particle repositioning maneuver in BPPV: a prospective study. Am J Otolaryngol. 2003;24:355–60.
Saeedi M, Khosravi MH, Bayatpoor ME. Comparing the effects of Epley maneuver and cinnarizine on benign positional paroxysmal vertigo: a randomized clinical trial. Galen Med J. 2019;8: e866.
Panuganti A, Loka SR, Tati S, Punga AK. Comparative study of management of BPPV (benign paroxysmal positional vertigo) with only Drugs versus Drugs plus Epley manoeuvre. Indian J Otolaryngol Head Neck Surg. 2019;71(Suppl 2):1183–6.
Maslovara S, Soldo SB, Puksec M, Balaban B, Penavic IP. Benign paroxysmal positional vertigo (BPPV): influence of pharmacotherapy and rehabilitation therapy on patients’ recovery rate and life quality. NeuroRehabilitation. 2012;31:435–41.
Liang SB, Li L, He HY. The efficacy of Epley procedure for treatment of benign paroxysmal positional vertigo of the posterior semicircular canal. J Youjiang Med Univ Natly. 2010;2:7.
Bruintjes TD, Companjen J, van der Zaag-Loonen HJ, van Benthem PP. A randomised sham-controlled trial to assess the long-term effect of the Epley manoeuvre for treatment of posterior canal benign paroxysmal positional vertigo. Clin Otolaryngol. 2014;39:39–44.
Blakley BW. A randomized, controlled assessment of the canalith repositioning maneuver. Otolaryngol Head Neck Surg. 1994;110:391–6.
Celis-Aguilar E, Mayoral-Flores HO, Torrontegui-Zazueta LA, Medina-Cabrera CA, León-Leyva IC, Dehesa-López E. Effectiveness of Brandt Daroff, Semont and Epley maneuvers in the treatment of benign paroxysmal positional vertigo: a randomized controlled clinical trial. Indian J Otolaryngol Head Neck Surg. 2022;74:314–21.
von Brevern M, Seelig T, Radtke A, Tiel-Wilck K, Neuhauser H, Lempert T. Short-term efficacy of Epley’s manoeuvre: a double-blind randomised trial. J Neurol Neurosurg Psychiatry. 2006;77:980–2.
Ebadi H, Borgheie A, Mali M, Talebi M, Rabiei M. Comparison between the effectiveness of physical maneuver and medicinal therapy in the treatment of benign paroxysmal positional vertigo. J Mazandaran Univ Med Sci. 2007;17:1–8.
Asawavichianginda S, Isipradit P, Snidvongs K, Supiyaphun P. Canalith repositioning for benign paroxysmal positional vertigo: a randomized, controlled trial. Ear Nose Throat J. 2000;79:732–7.
Angeli SI, Hawley R, Gomez O. Systematic approach to benign paroxysmal positional vertigo in the elderly. Otolaryngol Head Neck Surg. 2003;128:719–25.
Sacco RR, Burmeister DB, Rupp VA, Greenberg MR. Management of benign paroxysmal positional vertigo: a randomized controlled trial. J Emerg Med. 2014;46:575–81.
Jia H, Lu Y. Effectiveness of treatment of benign paroxysmal positional vertigo by Epley maneuver. J Med Forum. 2005;26(21). https://drive.google.com/file/d/1Kj8Fte-QdqURlYE79LEhC0gsrWHHfPrf/view?usp=sharing.
Froehling DA, Bowen JM, Mohr DN, Brey RH, Beatty CW, Wollan PC, et al. The canalith repositioning procedure for the treatment of benign paroxysmal positional vertigo: a randomized controlled trial. Mayo Clin Proc. 2000;75:695–700.
Chang AK, Schoeman G, Hill M. A randomized clinical trial to assess the efficacy of the Epley maneuver in the treatment of acute benign positional vertigo. Acad Emerg Med. 2004;11:918–24.
Cranfield S, Mackenzie I, Gabbay M. Can GPs diagnose benign paroxysmal positional vertigo and does the Epley manoeuvre work in primary care? Br J Gen Pract. 2010;60:698–9.
Grill E, Strupp M, Müller M, Jahn K. Health services utilization of patients with vertigo in primary care: a retrospective cohort study. J Neurol. 2014;261:1492–8.
Glasziou P, Heneghan CC. Epley and the slow boat from research to practice. Evid Based Med. 2008;13:34–5.
Balatsouras DG, Koukoutsis G, Fassolis A, Moukos A, Apris A. Benign paroxysmal positional vertigo in the elderly: current insights. Clin Interv Aging. 2018;13:2251–66.
Imai T, Ito M, Takeda N, Uno A, Matsunaga T, Sekine K, et al. Natural course of the remission of vertigo in patients with benign paroxysmal positional vertigo. Neurology. 2005;64(5):920–1.
Edlow JA, Carpenter C, Akhter M, Khoujah D, Marcolini E, Meurer WJ, et al. Guidelines for reasonable and appropriate care in the emergency department 3 (GRACE-3): Acute dizziness and vertigo in the emergency department. Acad Emerg Med. 2023;30(5):442–86.
Khoujah D, Naples JG, Silva L, Edlow JA, Gerberi DJ, Carpenter CR, et al. Epley maneuver for benign paroxysmal positional vertigo: evidence synthesis for guidelines for reasonable and appropriate care in the emergency department. Acad Emerg Med. 2023;30:501–16.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors conceptualized the study, analysed the findings, and approved the final version of the manuscript. YS and HM reviewed all retrieved citations and manuscripts. YS drafted the manuscript; NY, TF, HM, and ST provided critical edits.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA 2020 Checklist.
Additional file 2.
Outcomes of interest.
Additional file 3.
Search strategies.
Additional file 4.
Characteristics of the included Studies (primary-care setting) (N = 4).
Additional file 5.
Characteristics of the included studies (otolaryngology or subspecialty settings) (N = 23).
Additional file 6.
Characteristics of studies excluded from qualitative and quantitative synthesis.
Additional file 7.
Forest plots for each outcome.
Additional file 8.
Risk of bias table.
Additional file 9.
Subgroup analysis.
Additional file 10.
Sensitivity analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Saishoji, Y., Yamamoto, N., Fujiwara, T. et al. Epley manoeuvre’s efficacy for benign paroxysmal positional vertigo (BPPV) in primary-care and subspecialty settings: a systematic review and meta-analysis. BMC Prim. Care 24, 262 (2023). https://doi.org/10.1186/s12875-023-02217-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12875-023-02217-z