Abstract
Background
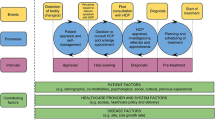
People with early-onset colorectal cancer, under the age of 50, are more likely to experience diagnostic delay and to be diagnosed at later stages of the disease than older people. Advanced stage diagnosis potentially requires invasive therapeutic management at a time of life when these patients are establishing intimate relationships, raising families, building careers and laying foundations for financial stability. Barriers to timely diagnosis at primary care level have been identified but the patient perspective has not been investigated.
Methods
Personal accounts of cancer care are increasingly accessed as rich sources of patient experience data. This study uses mixed methods, incorporating quantitative content analysis and qualitative thematic analysis, to investigate patients’ accounts of early-onset colorectal cancer diagnosis published on prominent bowel cancer support websites in the United Kingdom, Australia and New Zealand.
Results
Patients’ perceptions (n = 273) of diagnostic barriers at primary care level were thematically similar across the three countries. Patients perceived that GPs’ low suspicion of cancer due to age under 50 contributed to delays. Patients reported that their GPs seemed unaware of early-onset colorectal cancer and that they were not offered screening for colorectal cancer even when ‘red flag’ symptoms were present. Patients described experiences of inadequate information continuity within GP practices and across primary, specialist and tertiary levels of care, which they perceived contributed to diagnostic delay. Patients also reported tensions with GPs over the patient-centredness of care, describing discord related to symptom seriousness and lack of shared decision-making.
Conclusions
Wider dissemination of information about early-onset colorectal cancer at primary care level is imperative given the increasing incidence of the disease, the frequency of diagnostic delay, the rates of late-stage diagnosis and the dissatisfaction with patient experience reported by patients whose diagnosis is delayed. Patient education about diagnostic protocols may help to pre-empt or resolve tensions between GPs’ enactment of value-based care and patients’ concerns about cancer. The challenges of diagnosing early-onset colorectal cancer are significant and will become more pressing for GPs, who will usually be the first point of access to a health system for this growing patient population.
Similar content being viewed by others
Introduction
“If I hadn’t ignored my symptoms, if I had gone to my GP when things changed and had symptoms that were unusual for me, maybe it would’ve been caught earlier …. Maybe it wouldn’t have though, because who gets bowel cancer at age 33?”
The global incidence of colorectal cancer (CRC) in people aged under 50 is rising rapidly, in contrast to stable or declining rates of CRC in older adults [1,2,3,4,5,6]. CRC occurring before the age of 50, known as early-onset colorectal cancer (EoCRC), currently accounts for just over 6% of all CRC cases annually in the United Kingdom (UK), [7] some 10% of annual CRC incidence in Australia [8] and just over 11% of all CRC cases annually in New Zealand (NZ) [9]. The average increase in early-onset diagnoses (aged 20–49 years) over a ten year period was 4% per annum in NZ (2007–2016) and 2.8% per annum in both Australia (2006–2015) and the UK (2003–2012); over the same periods, the incidence in CRC diagnoses in those aged 50 and above was decreasing in NZ and Australia, and unchanged in the UK [4]. As a result of increasing EoCRC and static or decreasing CRC in older patients, people under the age of 50 are projected to become a larger proportion of all CRC patients, especially in high-income countries [1, 4].
EoCRC takes longer to diagnose than traditional CRC [10, 11] and features a greater likelihood of diagnosis in late stages of the disease [1, 3, 10, 12]. Late stage diagnosis in EoCRC does not appear to impact mortality but escalates the prospect of invasive therapeutic management [13] at a time of life when these patients are establishing intimate relationships, raising families, building careers and laying foundations for financial stability [14]. Timely diagnosis is crucial to this growing patient population [10, 15].
Data on healthcare-led barriers to timely EoCRC diagnosis [15,16,17] indicate that people eventually diagnosed with EoCRC are likely to spend three months to five years seeking a diagnosis and may have between three and eleven visits to their general practitioners (GPs) [10, 18, 19] and may also see up to four GPs in pursuit of a diagnosis for their symptoms [19]. Patients report also, to a lesser extent, multiple visits to specialists and to emergency services as part of the trajectory for EoCRC diagnosis [17].
Current knowledge of diagnostic delay in EoCRC relies on surveys of EoCRC patient populations and quantitative analysis of patient records. As yet we have little understanding of how EoCRC patients perceive the quality of care they receive during their diagnostic phase and what they think about the actions of their GPs. This information will be of value in understanding the needs of this newly emerging patient population.
We present our investigation of online patient reports of EoCRC diagnosis. We asked: What experiences and perceptions of health service barriers to diagnosis are reported by EoCRC patients in their online accounts of diagnosis? How do EoCRC patients who have experienced diagnostic delay characterise their interactions with GP services?
Methods
Approach
Personal accounts of healthcare experiences published online are increasingly accessed as rich sources of patient-reported data [20, 21]. There are methodological challenges related to collecting data from these patients' accounts, including ethical concerns, sampling issues and matters of validity [22]. In this section we describe our mixed methods approach to addressing these challenges in order to ensure rigour and transparency [23, 24].
Researcher reflexivity
The study was designed and led by an experienced qualitative researcher with skills in the analysis of web-based patient accounts of personal experience. The research team comprised highly experienced researchers across clinical practice, social science, health systems research and qualitative and quantitative methods. Team members shared interests in identifying, characterising and responding to real-world complexities in the delivery of timely diagnosis to the newly emerging population of people with EoCRC.
Population and context
Websites hosted by three key charitable support organisations were selected as specific research settings: Bowel Cancer Australia, [8] Bowel Cancer UK [7] and Bowel Cancer New Zealand [8]. Australia, New Zealand (NZ) and the United Kingdom (UK) were selected because CRC care is comparable in being financially integrated at primary care level, whereas other anglophone countries, such as the United States, feature highly varied levels of integrated primary healthcare. With permission from these organisations we accessed the public domain sections of these websites in which bowel cancer survivors ‘post’ accounts of their experiences, under banners such as ‘real life stories’ or ‘your stories’ [25].
The study population was selected by criteria sampling rather than representative sampling. Personal accounts were excluded from the study if they were not written by people who were diagnosed under 50 years of age or if accounts solely comprised feedback on, or criticism of, a named institution or clinician.
Ethics and patient and public involvement (PPI)
The study was granted ethical and scientific approval by the Macquarie University Human Research Ethics Committee (MQ HREC Reference No:52020666115757). The committee was satisfied that the use of the personal accounts as data matched the expectations of the individuals who published their accounts online in public domain sections of prominent cancer support websites.
Data collection
Eligible personal accounts of EoCRC published on the host websites as at February 2021 were collected as data. Each account was processed as a separate research text and downloaded into the NVivo 12 qualitative data analysis software program (QSR International Pty Ltd. Version 12, 2018). Each text was de-identified and attributed a unique participant study number to be used on all study documents.
Data analysis
Our mixed methods analysis of the personal accounts involved: quantitative description of the demographic and health status characteristics of the study population; quantitative content analysis to understand the frequency of topics and events of interest to the study; and qualitative thematic analysis to examine the descriptions and perceptions of the events that people reported in their narrative accounts.
The qualitative analysis focused on three key categories of patient experience that are assessed in patient-reported experience measures (PREMs): clinical assessment; continuity of care; and interpersonal care. Sentence-by-sentence analysis organised parts of each text into these three categories. We then looked for topics and themes of EoCRC patient experience of diagnosis within each of these categories. One researcher led the thematic analysis with iterative review and consensus from co-authors.
Techniques to enhance trustworthiness
Though final decisions on themes were not based on numbers of references, cross-checking against the quantitative results was a useful mechanism for comprehending undue emphasis on isolated experiences or experiences only of interest to individual researchers. In particular, the quantitative results provided an important validating context for understanding the scope of negative and dissatisfying experiences comprised in the texts, given that the personal accounts also comprised reports of positive experiences and timely diagnosis.
Results
Quantitative analysis
Demographic and clinical characteristics
Across the three websites, 273 personal accounts met the inclusion criteria: 136 (50%) from UK, 116 (42%) from Australia and 21 (8%) from NZ. Over two-thirds were female (73%), and almost half were aged 30 to 39 years (48%). Two-thirds reported being married or in a relationship (66%) and over half reported being pregnant or having children under 18 years (55%), with the remainder not reporting.
Overwhelmingly, the narrators reported a diagnosis of bowel or colon cancer (94%), with only 6% indicating rectal cancer. The majority was diagnosed at an advanced stage (III or IV) (66.3% overall). When describing the status of their health, 22% gave no indication, 20% were in treatment, 42% were in complete remission, and 4% reported progression. For the most part comorbidities, family history and genetic mutations were not mentioned in the narratives, but these were recorded by 7, 16 and 9% of narrators, respectively. As shown in Table 1, countries sometimes varied from the overall pattern described above.
Diagnostic journey
Table 2 summarises aspects of the diagnostic journey reported by patients in each country. The most common symptom was abdominal pain (49%) followed by stool change (31.5%), rectal bleeding (30%), fatigue (29%) and bowel habit change (29%). The first consult was usually a routine primary care visit (73% of narratives) or an emergency primary care or hospital visit (13%). There was a variety of initial diagnoses; the most common were irritable bowel syndrome (17%), haemorrhoids (9%), anaemia (8%), inflammatory bowel disease (7%) and gynaecological problems (7%). Narratives mentioned a number of diagnostic tests, most commonly colonoscopy (70%), CT scan (45%) and MRI (17%); in 11% of narratives the patient initiated the test request.
While the number of specialist referrals from primary care was not mentioned in 59% of narratives, there was only a single referral in 26% of narratives, while around 15% required two or more specialist referrals. Two-thirds of narratives did not mention the number of outpatient visits (66%), but 14% reported 5 or more such visits. Inpatient visits were mentioned in only 15% of narratives, with diagnosis occurring in the first hospitalisations in more than half of these (8%).
The amount of time from first consultation to diagnosis was not mentioned in 39% of narratives; diagnosis took < 3 months in 21%, 3–12 months in 25%, and 1–5 years in 15%. Among the 40% of accounts that mentioned a duration of three or more months from initial consultation to diagnosis, 24% attributed the delay to missed diagnostic opportunity, while 71% did not report any reason.
Once again, there is some variation in issues mentioned by patients in different countries. Possible differences include, for example, that: Australian narratives mentioned anaemia more frequently; NZ narratives less frequently mentioned CT scans or MRIs; Australian and NZ narratives more frequently mentioned that the patient had initiated a request for a diagnostic test (14–15%) compared to UK narratives (7%); and Australian narratives mentioned less time between first consult to diagnoses than NZ narratives, and more frequently mentioned a single specialist referral.
Qualitative analysis
Unsurprisingly, EoCRC patients’ accounts portrayed help-seeking experiences as satisfactory or positive when diagnosis was straightforward and timely, and characterised care as disappointing or frustrating when barriers to diagnosis were experienced. Our qualitative results suggest that patients experienced similar barriers to timely diagnosis at primary care level in all three countries of interest, despite variations in health systems with regards to clinical practice guidelines (CPGs), referral conventions and diagnostic testing protocols.
In Table 3 we present the key themes that emerged within each of the three categories of patient experience, accompanied by illustrative quotes derived from the qualitative analysis. Additional details on the results in each theme are available in the Additional file 1 Appendix. Low suspicion of cancer given age under 50 was a persistent theme across all stories of healthcare barriers to timely diagnosis, inclusive of GPs, specialists and emergency care services. Patients perceived their age as a factor that shaped the nature of clinical assessments and initial diagnoses, influenced the investigations conducted and referrals given, and created tensions between patients and doctors which obstructed shared decision-making. Eventual referrals for non-urgent colonoscopies and lengthy wait times for non-urgent colonoscopies was also a common theme in descriptions of delayed diagnosis and was reported as a cause of dissatisfaction with GPs.
Discussion
Clinical assessment and barriers to diagnosis
Patients in our study reported that anal bleeding and blood in stools were commonly attributed to haemorrhoids, persistent anaemia was managed as a dietary-related iron deficiency and ongoing symptoms of abdominal pain, bloating, bowel changes and fatigue were investigated and managed for IBS or other common gastrointestinal disorders as well as gynaecological conditions.
A quarter of patients who reported delays of over three months conveyed frustration and dissatisfaction that their GPs had spent so much time focusing on common conditions and described their GP’s low suspicion of cancer as poor-quality care. These findings were largely consistent across the three countries of interest, supporting literature in all three countries that EoCRC patients who experience delays are dissatisfied with the diagnostic actions of their GPs when cancer investigations are not prioritised [2, 10, 15, 17, 26].
This dissatisfaction amongst EoCRC patients points to the need for greater patient education of diagnostic protocols [27]. Though the incidence of CRC is increasing in people aged under 50 years, [4, 13, 28] it is infrequent compared to CRC in older populations. GP inexperience with EoCRC may contribute to diagnostic delay [29] but low suspicion of cancer given age under 50 is also justified. Evidence is emerging of the clinical and molecular features that distinguish EoCRC from traditional CRC, [2, 30, 31] which will drive the evolution of EoCRC-specific CPGs. Until that guidance is made available to GPs, a focus on investigation for common conditions is clinically appropriate in individuals with low or average risk of CRC [31].
A confounding aspect of our data was a seeming gender disbalance in clinical assessment; only female patients eventually diagnosed with EoCRC reported that their GPs diagnosed IBS. This finding may be influenced by the gender disproportion in our study population; while approximately the same number of women as men contract CRC under the age of 50, [28] three quarters of our narrators were female. Nevertheless, the literature indicates that the ratio of women to men with IBS across all patient populations is estimated as 3:1, [32, 33] suggesting that IBS would be diagnosed in at least some of the men in the study sample.
Similarly, only female patients reported that GPs discussed a psycho-emotional diagnosis such as stress, anxiety, and disordered eating. Women may be more likely than men to ask questions and discuss concerns in detail, [34] which may lead GPs to explore the psychosocial implications of ongoing symptoms with women more than with men. Our study, however, is not the first to identify possible gender disparity as a factor in delayed diagnosis of CRC. Siminoff et al. [34] found that women may be more likely to experience a missed opportunity for CRC diagnosis than men [34] and Rogers et al. [35] found that men were more likely than women to experience appropriate clinical outcomes in CRC diagnosis.
Continuity of care and barriers to diagnosis
Seeing one GP over time has been associated with a marginally higher likelihood of early cancer diagnosis, [36] particularly with cancers featuring generalised symptoms [37]. Our findings support evidence to the contrary; repeated visits to the same GP increased delays in referral of patients for CRC investigations [38,39,40]. The referral reluctance perceived by patients in our study may indicate that GPs are protecting patients from unnecessary invasive testing and avoiding low-value use of medical resources. Additionally, the referral delay events identifiable in our data may be exaggerated by the demographics of our study population; half the personal accounts were written by people aged 30–39 for whom there is generally less risk of CRC [41, 42].
Aspects of our data, however, challenge this explanation for GPs’ referral decisions. Patients with relevant family history of CRC and persistent symptoms identified as ‘red flags’ [43] for CRC referral at any age [44] reported being given non-urgent referrals when presenting repeatedly to the one GP. These patients described these actions as a result of age bias that delayed diagnosis.
Patients also reported inadequate continuity of care across multiple providers as a barrier to timely diagnosis. Patients described locum GPs, rotating medical staff in large primary care practices, specialist appointments, emergency care for pain management and changing GPs as contexts involving multiple providers.
Poor information-continuity across medical providers is a well-established diagnostic liability, even when changing doctors within one practice [45, 46]. Many patients reported being surprised and frustrated by how little information about their diagnostic trajectory to date was contained in their medical records and in GP referral letters. This points to the broader issue of patients’ expectations that records of their assessments and treatments are adequately shared between health care professionals [46]. Patients in our study voiced concerns that in retelling their histories they would miss vital information and, importantly, would not be able to convey the futility of prior efforts towards diagnosis.
Interpersonal care and barriers to diagnosis
Issues with interpersonal communication and shared decision-making were prominent in the personal accounts of delayed diagnosis across the three countries. In particular, patients’ concerns about symptom seriousness were sources of tension in interactions and relationships with GPs, but also with providers at all levels of care. The refrain, “I wasn’t taken seriously,” which resounds across these stories, is evident also in surveys of EoCRC patient populations [10, 15].
Symptom seriousness has been identified as a crucial point of patient-doctor divergence in medical consultations [47]. Misalignment between a patient’s concerns about cancer and clinical suspicion of cancer at primary care level is widespread [48]. Patients reported that GPs who were responsive to this tension offered a non-urgent referral for colonoscopy, or for other cancer screening, as an explicit act of discord resolution and reassurance. ‘Reassurance referrals’ have been identified as an appropriate means of satisfying the criteria for patient-centred care [44]. In other responses to this tension over symptom seriousness, patients described turning to avenues of care such as emergency services for pain management, or changing GPs.
Our data are notable for the absence of documented communication between patients and GPs about diagnostic protocols for CRC detection in people under the age of 50. GPs may pre-empt tensions over symptom seriousness through discussion about the rationale for referral decisions [49]; this may be especially relevant for patients with low risk of CRC and non-specific symptoms.
Conclusions
Primary care barriers to timely diagnosis are reported by EoCRC patients in three key areas of patient experience: clinical assessment; continuity of care; and patient-centredness of interpersonal interactions. Perceptions of barriers to timely diagnosis across all three areas may stem in part from patients’ limited understanding of the guidelines that steer diagnostic decision-making. Patients perceive their GPs’ low suspicion of cancer given age under 50 as an age bias that contributes to delays. There may be value for GPs in addressing this perception in discussion with patients and providing patients with knowledge about diagnostic protocols.
We conclude that with the rising global incidence of CRC in people aged under 50, there is a mounting imperative for GPs to receive information and clinical guidance on EoCRC diagnosis. In the absence of EoCRC-specific diagnostic protocols, some sources of diagnostic delay may be unavoidable, especially for low-risk individuals presenting with broad symptoms. Other sources of GP-led delay described by patients, such as failure to respond to red flags and to take note of repetitive help-seeking, are clearly addressable. The challenges of diagnosing CRC in young people are significant and will become more pressing for GPs, who will usually be the first point of access to a health system for this growing patient population.
Limitations
In providing the demographics represented in the personal accounts, and by presenting the percentages of personal accounts that identify specific barriers in the nominated categories, we have strived for transparency about the limitations of our data. We note, for example, that our data over-represent experiences of younger EoCRC patients and women, and under-represent patients with rectal cancers.
Participants self-selected to write their narratives, a known source of bias, and thus our study is not generalisable. Notably, there is an absence of data specific to culturally and linguistically diverse (CALD) EoCRC populations, given the significant Indigenous populations in two of the settings of interest to the study: Australia and NZ. As a consequence, the results do not reflect evidence in the literature that racial and ethnic factors impact timely CRC diagnosis [50,51,52]. Language barriers as well as socio-cultural norms and conventions related to personal privacy and autobiographical narration, amongst other factors, may preclude members of migrant and Indigenous populations from publishing online accounts of patient experience or from taking part in interviews and surveys [53]. Developing opportunities for CALD patients to self-report their experiences of EoCRC diagnosis, for example through social media, [53, 54] may enhance the capacity of patient experience research to inform and inspire equitable primary care diagnostic policy and practice [55].
Availability of data and materials
The datasets analysed during the current study are publicly available on the websites of Bowel Cancer UK, Bowel Cancer Australia and Bowel Cancer NZ. The datasets analysed during the current study are also available from the corresponding author on reasonable request. Information/data related to the study is stored on password-protected computers and archived on electronic databases on a secure server. The records of this research study will be retained for a minimum of 5 years post study completion or last publication, in accordance with the Australian National Health and Medical Research Council guidelines for management of data and information in research.
References
Bailey CE, Hu C-Y, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of Colon and Rectal cancers in the United States, 1975-2010. JAMA Surgery. 2015;150(1):17–22. https://doi.org/10.1001/jamasurg.2014.1756.
Dozois EJ, Boardman LA, Suwanthanma W, Limburg PJ, Cima RR, Bakken JL, et al. Young-onset colorectal cancer in patients with no known genetic predisposition: can we increase early recognition and improve outcome? Medicine (Baltimore). 2008;87(5):259–63. https://doi.org/10.1097/MD.0b013e3181881354 PubMed PMID: 18794708.
Abualkhair WH, Zhou M, Ahnen D, Yu Q, Wu X-C, Karlitz JJ. Trends in incidence of early-onset colorectal Cancer in the United States among those approaching screening age. JAMA Netw Open. 2020;3(1):e1920407-e. https://doi.org/10.1001/jamanetworkopen.2019.20407.
Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179. https://doi.org/10.1136/gutjnl-2019-319511.
Araghi M, Soerjomataram I, Bardot A, Ferlay J, Cabasag CJ, Morrison DS, et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol. 2019;6(7):511–8.
Gandhi J, Davidson C, Hall C, Pearson J, Eglinton T, Wakeman C, et al. Population-based study demonstrating an increase in colorectal cancer in young patients. Br J Surg. 2017;104(8):1063–8. https://doi.org/10.1002/bjs.10518.
Bowel Cancer UK. www.bowelcanceruk.org.uk.
Bowel Cancer Australia. www.bowelcanceraustralia.org.
Bowel Cancer NZ. www.bowelcancernz.org.nz.
Newcomer KL, Porter LD. A delayed path to diagnosis: findings from young-onset colorectal cancer patients and survivors. J Clin Oncol. 2021;39(3_suppl):5. https://doi.org/10.1200/JCO.2021.39.3_suppl.5.
Di Leo M, Zuppardo RA, Puzzono M, Ditonno I, Mannucci A, Antoci G, et al. Risk factors and clinical characteristics of early-onset colorectal cancer vs. late-onset colorectal cancer: a case-case study. Eur J Gastroenterol Hepatol. 2021;33(9):1153–60.
Chen FW, Sundaram V, Chew TA, Ladabaum U. Advanced-stage colorectal cancer in persons younger than 50 years not associated with longer duration of symptoms or time to diagnosis. Clin Gastroenterol Hepatol. 2017;15(5):728–37.e3. https://doi.org/10.1016/j.cgh.2016.10.038.
Abdelsattar ZM, Wong SL, Regenbogen SE, Jomaa DM, Hardiman KM, Hendren S. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer. 2016;122(6):929–34. https://doi.org/10.1002/cncr.29716.
Blum-Barnett E, Madrid S, Burnett-Hartman A, Mueller SR, McMullen CK, Dwyer A, et al. Financial burden and quality of life among early-onset colorectal cancer survivors: a qualitative analysis. Health Expect. 2019;22(5):1050–7. https://doi.org/10.1111/hex.12919.
Araujo L, Breau G, George M, Dau H, Gastonguay L, Brown EH, et al. Shared experiences of diagnosis and treatment of young-onset colorectal cancer: a patient-oriented qualitative study. J Psychosoc Oncol Res Prac. 2020;2(1):e17. https://doi.org/10.1097/or9.0000000000000017.
CCA. Young-onset colorectal Cancer survey report. Washington: Colorectal Cancer Alliance; 2020.
BCUK. Never too young. UK: Bowel Cancer UK; 2020.
Yarden RI, Newcomer KL. Young onset colorectal cancer patients are diagnosed with advanced disease after multiple misdiagnoses. American Association for Cancer Research (AACR) Annual Meeting 2019. Session PO. SHP01.01 - Science and Health Policy 13347. 2019.
Yarden R, Newcomer KL, Peterson D. A population-based study of young-onset colorectal cancer patients: effect of knowledge gaps among patients and providers on stage at diagnosis and quality of life. J Clin Oncol. 2020;38(15_suppl):4095. https://doi.org/10.1200/JCO.2020.38.15_suppl.4095.
Robinson KM. Unsolicited narratives from the internet: a rich source of qualitative data. Qual Health Res. 2001;11(5):706–14. https://doi.org/10.1177/104973201129119398.
O’Brien M, Clark D. Use of unsolicited first-person written illness narratives in research: systematic review. J Adv Nurs. 2010;66(8):1671–82. https://doi.org/10.1111/j.1365-2648.2010.05349.x.
Shannon-Baker P. Making paradigms meaningful in mixed methods research. J Mixed Methods Res. 2015;10(4):319–34. https://doi.org/10.1177/1558689815575861.
Carter N, Bryant-Lukosius D, DiCenso A, Blythe J, Neville AJ. The use of triangulation in qualitative research. Oncol Nurs Forum. 2014;41(5):545–7. https://doi.org/10.1188/14.onf.545-547.
Flick U. Mantras and myths: the disenchantment of mixed-methods research and revisiting triangulation as a perspective. Qual Inq. 2016;23(1):46–57. https://doi.org/10.1177/1077800416655827.
Fielding NG, Lee RM, Blank G. The SAGE handbook of online research Methods: SAGE Publications; 2016.
Colorectal Cancer Alliance. Young-onset colorectal Cancer survey report. Washington: 2018.
Campos FG. Colorectal cancer in young adults: a difficult challenge. World J Gastroenterol. 2017;23(28):5041–4. https://doi.org/10.3748/wjg.v23.i28.5041.
Connell LCMJ, Braghiroli MI, Hoff PM. The rising incidence of younger patients with colorectal Cancer: questions about screening, biology, and treatment. Curr Treat Options in Oncol. 2017;18(4):23. https://doi.org/10.1007/s11864-017-0463-3.
Hamilton W, Round A, Sharp D, Peters TJ. Clinical features of colorectal cancer before diagnosis: a population-based case–control study. Br J Cancer. 2005;93(4):399–405. https://doi.org/10.1038/sj.bjc.6602714.
Yeo HBD, Abelson JS, Zheng XE, Yantiss R, Shah MA. Early-onset colorectal cancer is distinct from traditional colorectal cancer. Clin Colorectal Cancer. 2017;16(4):293–9.e6. https://doi.org/10.1016/j.clcc.2017.06.002.
Hull MA, Rees CJ, Sharp L, Koo S. A risk-stratified approach to colorectal cancer prevention and diagnosis. Nat Rev Gastroenterol Hepatol. 2020;17(12):773–80. https://doi.org/10.1038/s41575-020-00368-3.
Kim YS, Kim N. Sex-gender differences in irritable bowel syndrome. J Neurogastroenterol Motil. 2018;24(4):544–58. https://doi.org/10.5056/jnm18082.
Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clinical Epidemiology. 2014;6:71–80. https://doi.org/10.2147/CLEP.S40245.
Siminoff LA, Rogers HL, Harris-Haywood S. Missed Opportunities for the Diagnosis of Colorectal Cancer. Biomed Res Int. 2015;2015:285096. https://doi.org/10.1155/2015/285096.
Rogers HL, Dumenci L, Epstein RM, Siminoff LA. Impact of Patient Gender and Race and Physician Communication on Colorectal Cancer Diagnostic Visits in Primary Care. J Womens Health (Larchmt). 2019;28(5):612–20. https://doi.org/10.1089/jwh.2018.6961.
Hamilton WWF, Rubin G, Neal RD. Improving early diagnosis of symptomatic cancer. Nat Rev Clin Oncol. 2016;13(12):740–9. https://doi.org/10.1038/nrclinonc.2016.109.
Ridd MJ, Ferreira DLS, Montgomery AA, Salisbury C, Hamilton W. Patient-doctor continuity and diagnosis of cancer: electronic medical records study in general practice. Br J Gen Pract. 2015;65(634):e305–e11. https://doi.org/10.3399/bjgp15X684829.
Macleod U, Mitchell ED, Burgess C, Macdonald S, Ramirez AJ. Risk factors for delayed presentation and referral of symptomatic cancer: evidence for common cancers. Br J Cancer. 2009;101(2):S92–S101. https://doi.org/10.1038/sj.bjc.6605398.
Parsonage RK, Hiscock J, Law R-J, Neal RD. Patient perspectives on delays in diagnosis and treatment of cancer: a qualitative analysis of free-text data. Br J Gen Pract. 2017;67(654):e49. https://doi.org/10.3399/bjgp16X688357.
Rotar AM, Van Den Berg MJ, Schäfer W, Kringos DS, Klazinga NS. Shared decision making between patient and GP about referrals from primary care: does gatekeeping make a difference? Plos one. 2018;13(6):e0198729-e. https://doi.org/10.1371/journal.pone.0198729.
Skinner CS, Ahn C, Halm EA, Bishop WP, McCallister K, Sanders JM, et al. Recommendation of colorectal cancer testing among primary care patients younger than 50 with elevated risk. Prev Med. 2017;102:20–3. https://doi.org/10.1016/j.ypmed.2017.06.014.
Mitchell E, Macdonald S, Campbell NC, Weller D, Macleod U. Influences on pre-hospital delay in the diagnosis of colorectal cancer: a systematic review. Br J Cancer. 2008;98(1):60–70. https://doi.org/10.1038/sj.bjc.6604096.
Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6(11):99. https://doi.org/10.3390/jcm6110099.
Cancer Council Australia. Clinical practice guidelines for the prevention, early detection and management of colorectal cancer. 2017.
Romano MJ, Segal JB, Pollack CE. The association between continuity of care and the overuse of medical procedures. JAMA Intern Med. 2015;175(7):1148–54. https://doi.org/10.1001/jamainternmed.2015.1340.
Gordon P. If I’ve told you once....People’s views on record sharing between the health and care professionals involved in their care in Surrey. Surrey: Healthwatch Surrey; 2015.
Iedema R, Manidis M. Patient-clinician communication: an overview of relevant research and policy literatures. Sydney: Australian Commission on Safety and Quality in Health Care and UTS Centre for Health Communication; 2013.
Virgilsen LF, Pedersen AF, Vedsted P, Petersen GS, Jensen H. Alignment between the patient’s cancer worry and the GP’s cancer suspicion and the association with the interval between first symptom presentation and referral: a cross-sectional study in Denmark. BMC Fam Pract. 2021;22(1):129. https://doi.org/10.1186/s12875-021-01480-2.
Pilnick A, Dingwall R. On the remarkable persistence of asymmetry in doctor/patient interaction: a critical review. Soc Sci Med. 2011;72(8):1374–82. https://doi.org/10.1016/j.socscimed.2011.02.033.
Blackmore T, Norman K, Kidd J, Cassim S, Chepulis L, Keenan R, et al. Barriers and facilitators to colorectal cancer diagnosis in New Zealand: a qualitative study. BMC Fam Pract. 2020;21(1):206. https://doi.org/10.1186/s12875-020-01276-w.
Koo JH, Arasaratnam MM, Liu K, Redmond DM, Connor SJ, Sung JJY, et al. Knowledge, perception and practices of colorectal cancer screening in an ethnically diverse population. Cancer Epidemiol. 2010;34(5):604–10. https://doi.org/10.1016/j.canep.2010.05.013.
Hill S, Sarfati D, Robson B, Blakely T. Indigenous inequalities in cancer: what role for health care? ANZ J Surg. 2013;83(1–2):36–41. https://doi.org/10.1111/ans.12041.
Hswen Y, Hawkins JB, Sewalk K, Tuli G, Williams DR, Viswanath K, et al. Racial and ethnic disparities in patient experiences in the United States: 4-year content analysis of twitter. J Med Internet Res. 2020;22(8):e17048. https://doi.org/10.2196/17048.
Rice ES, Haynes E, Royce P, Thompson SC. Social media and digital technology use among indigenous young people in Australia: a literature review. Int J Equity Health. 2016;15(1):81. https://doi.org/10.1186/s12939-016-0366-0.
Greenhalgh T. Cultural contexts of health: the use of narrative research in the health sector. Copenhagen: World Health Organisation; 2016.
National Health and Medical Research Council (NHMRC) Australia. National statement on ethical conduct in human research 2007 (updated 2018). Canberra: Commonwealth of Australia; 2018. Reference number E72. ISBN 1864962755.
Lamprell K, Fajardo Pulido D, Tran Y, Nic Giolla Easpaig B, Liauw W, Arnolda G, et al. Personal accounts of young-onset colorectal cancer organized as patient-reported data: Protocol for a mixed methods study. JMIR Res Proto. 2021;10(2):e25056. https://doi.org/10.2196/25056.
World Medical Association (WMA). Declaration of Helsinki: ethical principles for medical research involving human subjects. Bulletin World Health Organisation; 2001;79(4):373–4.
Acknowledgements
Thank you to the colorectal cancer patient advocacy organisations Bowel Cancer UK, Bowel Cancer Australia and Bowel Cancer NZ for facilitating our access to patients’ stories and to the patients who wrote about their experiences.
Funding
This work was supported by a National Health and Medical Research Council of Australia [NHMRC grant number 1135048 – the Centre of Research Excellence in Implementation Science in Oncology] which is administered by the Australian Institute of Health Innovation, Macquarie University, Sydney. JB reports multiple grants funded by the National Health and Medical Research Council and other providers.
Author information
Authors and Affiliations
Contributions
KL devised the study. KL and DFP undertook the detailed design of the study, in consultation with all authors. KL and DFP prepared the study materials. KL undertook the analysis in consultation with DFP, SSO and GA. KL wrote the first draft of the manuscript. All authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent was obtained from the legal custodians of the published material that was analysed in this study. The selected CRC support organisations consented to the analysis and/or the use of quotes and excerpts from the personal accounts published on their websites and to the dissemination of findings. The Macquarie University Human Research Ethics Committee approved the study (MQ HREC Reference No:52020666115757) granting that it has been conducted in accordance with the Australian National Statement on Ethical Conduct in Human Research (2007-Updated 2018) [56] and poses no risk of privacy infringement to authors of the texts. The study also adheres to the principles for IMR established by the British Psychological Society [57]. Further, all methods were performed in accordance with the relevant guidelines and regulations asserted by the World Medical Association Declaration of Helsinki [58].
Consent for publication
Not applicable.
Competing interests
None declared
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Appendix. Detailed results of qualitative analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lamprell, K., Pulido, D.F., Arnolda, G. et al. People with early-onset colorectal cancer describe primary care barriers to timely diagnosis: a mixed-methods study of web-based patient reports in the United Kingdom, Australia and New Zealand. BMC Prim. Care 24, 12 (2023). https://doi.org/10.1186/s12875-023-01967-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12875-023-01967-0