Abstract
Background
Cardiac rehabilitation after acute myocardial infarction permits recovery of the heart function and enables secondary prevention programs in which changes in lifestyle habits are crucial. Cardiac rehabilitation often takes place in hospitals without coordination with primary healthcare and is not focused on individual patient preferences and goals, which is the core of the motivational interview. The objective of this study was to evaluate the efficacy of a cardiac rehabilitation program with a motivational interview in patients discharged from hospital after acute myocardial infarction.
Methods/design
A randomized, non-pharmacological clinical trial in six primary healthcare centers in Barcelona (Spain) will assess whether a tailored cardiac rehabilitation program consisting of four motivational interviews and visits with family physicians, primary healthcare nurses and a cardiologist, coordinated with the reference hospital, results in better cardiac rehabilitation than standard care. A minimum sample of 284 participants requiring cardiac rehabilitation after acute myocardial infarction will be randomized to a cardiac rehabilitation group with a motivational interview program or to standard primary healthcare. The main outcome will be physical function measured by the six-minute walk test, and the secondary outcome will be the effectiveness of secondary prevention: a composite outcome comprising control of blood pressure, cholesterol, diabetes mellitus, smoking and body weight. Results will be evaluated at 1,3 and 6 months.
Discussion
This is the first clinical trial to study the impact of a new primary healthcare cardiac rehabilitation program with motivational interviews for patients discharged from hospital after myocardial infarction. Changes in lifestyles and habits after myocardial infarction are a core element of secondary prevention and require patient-centered care strategies such as motivational interviews. Therefore, this study could clarify the impact of this approach on health indicators, such as functional capacity.
Trial registration
ClinicalTriasl.gov NCT05285969 registered on March 18, 2022.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Recovery of heart function after acute myocardial infarction (AMI) requires cardiac rehabilitation (CR) to ensure the best possible physical, mental, and social conditions to regain an active life [1, 2]. CR programs have been shown to reduce morbidity and mortality after AMI, and improve the quality of life and psychological wellbeing [3]. However, CR programs are not widely implemented in EU countries: after AMI, not all patients undergo CR, which varies from 3% in Spain to 90% in Lithuania [4].
CR programs include physical training, health education, psychological interventions and control and follow up of risk factors: smoking, hypertension, cholesterol levels, diabetes mellitus, obesity and sedentary lifestyles (physical activity) [4, 5]. CR programs are often divided into three phases, starting after stabilization of AMI: a) in-hospital, b) early outpatient phase, and c) maintenance [6, 7]. The European Society of Cardiology has focused on hospital-based CR programs, but they may also be outpatient led. Delivering hospital CR programs after AMI to all patients has two drawbacks: poor accessibility and delays in starting due to a lack of rooms and healthcare professionals. While the effectiveness of CR is greater if begun early, currently, most patients do not start CR programs one year after AMI, thus increasing the risk of a worse outcome [8]. A European study showed that only 45% of patients discharged from hospital after AMI with or without revascularization, were referred for CR and only 34% participated [9]. A possible solution to overcoming the barriers to CR programs would be to integrate CR into primary healthcare (PHC).
PHC centers facilitate health care in the community, and citizens have an assigned family physician and PHC nurse [10]. PHC health professionals coordinate with other healthcare professionals, such as cardiologists, and other healthcare settings, including hospitals [11, 12]. PHC services also include home-care programs for patients unable to attend the PHC center due to health problems or disability. PHC physicians and nurses are well positioned to care for patients requiring CR after AMI, because the main objectives are to control risk factors, improve patient self-management and decision-making in diet, exercise routines and weight control, etc. A review and a meta-analysis concluded there were no differences between CR at home or in hospitals with respect to mortality, reinfarction, revascularization, hospitalization, and exercise capacity [13, 14].
In CR, person-centered care is essential, including consideration of patients’ goals, values, previous routines, and environment [15, 16]. This approach requires communication skills, such as motivational interviews (MI). MI is a collaborative, goal-oriented style of communication with particular attention paid to the language of change [17]. In PHC, MI make it possible to establish common objectives that can be monitored agreed between patients and health professionals and which encourages motivation to change by exploring and solving patients’ ambivalence [18]. MI and CR are more effective in the early stages of the disease, for example, after AMI, when the patient is more likely to initiate lifestyle changes [19, 20]. Two systematic reviews showed that MI improved self-care in patients [21, 22] with heart failure. However, the effect of MI combined with a CR program after AMI is unclear. Therefore, in this protocol we plan to study the efficacy of CR using MI compared with the current PHC standard of care after hospital discharge for AMI.
Methods/design
Main objective
The main objective of the study is to evaluate the efficacy in functional capacity, lifestyle indicators and psychological wellbeing of a new CR program with MI carried out entirely in primary healthcare in patients discharged from hospital after AMI.
Secondary objectives and hypothesis
(i) To compare improvements in functional capacity and changes in risk factors (secondary prevention). (ii) To evaluate the impact of the CR program according to adherence to therapy (drug treatment and physical activity program) and health service use and (iii) to evaluate the efficacy of the CR program based on psychological factors and quality of life after AMI.
The hypothesis of the study is that a PHC CR program with MI after AMI has a positive impact on functional and psychological wellbeing and quality of life compared to standard care.
Trial design and study setting
This will be a randomized controlled trial with two arms: a PHC CR program including MI (Intervention group) versus PHC standard care (Control group). The study will be carried out in seven primary healthcare areas in Barcelona city with six assigned PHC centers, including family physicians, nurses and social workers. The six PHC provide healthcare to 187, 223 people [23] and coordinate actions with the Hospital Clinic of Barcelona, the public high-complexity reference hospital, with an assigned population of 540,000 [24]. Figure 1 shows a map of the area of influence of the PHCs and the location of the hospital. The trial was prospectively registered (before participant recruitment) on ClinicalTrials.gov (NCT05285969) on March 18, 2022.
Partial map of Barcelona (1:30,000). Area of influence of the six PHC (blue), and the Hospital Clinic of Barcelona. The map was
modified from the Cartographic and Geological Institute of Catalonia, which gave permission to reuse their data and content [36]
Participants and eligibility criteria
Potential participants will be PHC patients admitted to the reference hospital due to acute coronary syndrome (diagnostic codes ICD-10: I20-I22) or post-unscheduled cardiac revascularization surgery (code ICD-10: 021x) and discharged to home in the area of the six PHCs. Inclusion criteria will be age > 18 years, indication for CR and voluntary participation. Exclusion criteria will be: (1) acute aortic disease, severe pulmonary hypertension, uncontrolled arrhythmia, decompensated heart failure or significant valvular or congenital heart disease, (2) heart valve and/or interventricular septum surgery, (3) diseases that prevent exercise, (4) osteoarticular diseases that severely limit exercise, (5) severe mental disorder (i.e. schizophrenia, bipolar disorder, major depression or autism, and severe forms of other disorders), (6) cognitive disability, (7) problems of verbal communication and, (8) inclusion in a hospital CR program.
Intervention group
CR with MI will be structured in four sessions, with an optional fifth session, in the six months after discharge. The methodology of the MI sessions will follow the four-phase logical sequence of MI proposed by Rollnick and Millner 1) engage in collaborative relationships, 2) focus on a particular change, 3) evoke intrinsic motivations for change, and 4) plan an immediate step for change [25]. MI will be administered by nurses trained through a certified MI course, who will be offered additional support and counseling. Collaborators will meet at least once a month to standardize the intervention and follow up of issues regarding MI. Each MI session will have defined contents and objectives to ensure homogeneity. The objectives of CR and secondary prevention will be introduced from the first session, (i.e., to increase participation in activities of daily living and self-care and follow recommendations on safe physical activity). Table 1 describes the MI program and the content of each session. Interviewers will collaborate in the coordination of care, ensuring continuity and communication between PHC family physicians, nurses, and the cardiologist).
Standard care group
To standardize comparison with the MI group, all control group patients will receive a kit with information about the actions and procedures to follow (diet, physical activity, smoking cessation, and other recommendations on secondary prevention) and the home physical activity program. Home exercises will be adapted from cardiology guidelines from the United States, Canada and Europe [26]. The collection of data, analytical samples, and information (questionnaires, scales, and clinical information) will be the same as for the intervention group. A collaborating researcher will contact patients by telephone beforehand. Table 2 shows the SPIRIT chart [27], describing the schedule of enrollment, interventions, and assessments.
Strategies to improve adherence to the protocol
To maximize adherence, study collaborators will send reminders of data collection sessions and visits by phone. If patients do not attend, they will be contacted again to avoid losses. Sessions will take place at the initial product dispensing and each study visit thereafter. The only criteria for discontinuing the intervention will be hospitalization due to worsening status. All concomitant care and interventions for health reasons are permitted during the trial.
Main outcome
Physical functional capacity
Improvement in aerobic capacity and resistance, measured by the six-minute walk test [28]
Secondary outcomes
Effectiveness of secondary prevention
A composite variable that groups secondary prevention measures: BP (values < 140/90 mmHg), cholesterol (c-LDL < 70 mg/dL), diabetes mellitus (plasma glycosylated hemoglobin < 7%), absolute cessation or no initiation of smoking and weight (body mass index in the range of 18.5-25 kg/m2).
Psychological status and quality of life
Measured by the Psychological General Well-Being Index (PGWBI) [29] and the generic SF-12 [30].
Other variables and factors
Variables are described in Table 3 and tests or instruments and their characteristics in Table 4, including sociodemographic and household characteristics, clinical status, use of health services, disease management, and lifestyle habits and psychological and emotional status.
Participant timeline
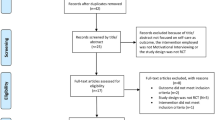
Patients meeting the inclusion criteria will be asked to participate during hospital admission. Potential participants will be given written and verbal information about the study and, if they agree to participate, will be asked to complete the informed consent document. The study coordinator will assign patients to the study groups using a previously-generated blinded random sequence. Participants will be contacted to specify the date of the baseline data collection visit. Sociodemographic and baseline characteristics will be evaluated at the beginning of the study. Follow-up evaluations will be made at 1, 3 and 6 months. Baseline and follow-up evaluations will be made by researchers unaware of the group to which each patient is assigned. Figure 2 shows the study flowchart and timeline.
Sample size calculation
The six-minute walk test is sensitive and specific in measuring changes in functional capacity. Evidence shows the minimum clinically-relevant difference is 30 m [31, 32]. Accepting an alpha risk of 0.05 and a beta risk of 0.2 in a bilateral contrast, with a standard deviation of 80 m in the six-minute walk test, 284 participants (142 in the intervention group and 142 in the control group) will be required to detect differences of ≥ 30 m. To achieve adequate participant enrollment and reach the minimum sample size, we analyzed the incidence of new AMI in the six PHC areas and foresee that three years will be sufficient to reach the minimum sample size.
Randomization
A randomization sequence will be generated and participants will be assigned using a centralized method with hidden assignation. Only the nurses who carry out the MI will be aware of the participants in the MI group. The assessments at 1, 3 and 6 months will be made by an assessor blinded to the group assignment. The study coordinator will record whether they were informed of the assignment of participants.
Data management and monitoring
All study information will be saved securely, and all participant information will be stored using an electronic secure server system with limited access. The information will be identified by a coded identification number to ensure confidentiality. All records with names or other personal identifiers (like locator forms and informed consent forms) will be stored separately from study registers and identified by a coded number. The main database will be protected with a password-protected system.
Questionnaires will be stored after informed consent. Follow-up questionnaires will be collected at 3 and 6 months after the baseline questionnaire. For follow-up visits (control blood tests and administration of questionnaires), a researcher will make an appointment with patients by phone and provide the dates of interviews.
Data management and monitoring will follow the pre-planning foreseen in the monitoring plan. A data monitoring committee (principal investigator, statistician and a collaborator from each PHC) will ensure the integrity of data recording.
Statistical methods
Outcomes will be evaluated at 1, 3 and 6 months. Participant characteristics will be described using central tendency measures: mean or median and variability: standard deviation or interquartile range for continuous variables, and frequencies and percentages for categorical variables. The results of the physical functional capacity and the six-minute walk test will be transformed into units of metabolic equivalent of task (MET) using the equation and conversion table of the American College of Sports Medicine [33]. Between-group differences will be studied using the Student’s t-test for two samples. The magnitude of the effect will be calculated using Cohen's D. The effect of the intervention or standard care will be calculated using the Student’s t-test or Mcnemar’s test for paired samples. A mixed linear regression model will be used to evaluate trends in each arm, adjusting for variables of interest. For the inference analysis, co-variables that correlate (sociodemographic factors) will be used and adjusted analyzes made. In the comparison of multiple hypotheses, adjustment of the level of statistical significance (α = 0.05) will be used to avoid type I errors. All analyzes will made per protocol and intention-to-treat. The analysis will be made using R v3.5.2 [34].
Discussion
This protocol aims to study the effectiveness of a post-AMI CR program with MI in PHC. CR will be carried out in PHC centers and may be innovative in allowing patients to access CR.
No differences in mortality, reinfarction, revascularization, hospitalization, and exercise between CR at home or in hospitals have been shown [13]. However, there is a lack of programs and research specifying CR programs in PHC, and no indicators have assessed standard care. As no program has been designed specifically in Spain, if the results of this trial are as expected, this PHC-based program could increase participation in CR post-AMI programs. Research on non-hospital-based CR programs has a poor level of methodological reporting, with details of interventions often poorly reported. Therefore, to achieve sufficient quality, we have followed the seven core recommendations of the UK CR standards [35]. The fourth component refers to the assessment of patient needs which, in the PHC context, refers directly to a patient-centered approach. In our CR program, the patient-centered approach and the patient’s self-perceived objectives will be covered by the MI component which, at the same time is the core component of the whole program. Our perspective is that MI rehabilitation will be effective only when placing the patient as the individual at the center of the whole CR program.
Limitations of the study
The study will be carried out in patients from a single, urban hospital. This may limit the generalizability of the results to semi-urban or rural areas. Second, the complete blinding of participants to their group assignment may not be guaranteed because patients may discover they have been assigned to the control group. However, this issue is common in MI research. Finally, the duration of the follow-up will not allow study of the long-term effects, although a subsequent study with a cohort design is possible. In addition, the proposed study is restricted to the improvement in the first six months of outpatient treatment of CR.
Availability of data and materials
The study steering committee will accept requests for data sharing once the study is completed. The steering committee will evaluate the scientific soundness of each proposed project and will grant access whenever projects seem scientifically sound. All data sharing will apply to national and international legislation, rules, and other regulations by regional or national authorities. Applications for data require a formal application and will be decided upon by the board of the scientific group.
Abbreviations
- AMI:
-
Acute myocardial infarction
- BP:
-
Blood pressure
- CR:
-
Cardiac rehabilitation
- CRP:
-
Cardiac rehabilitation programs
- CVD:
-
Cerebrovascular disease
- DM:
-
Diabetes mellitus
- HF:
-
Heart failure
- LDL:
-
Low-density lipids
- MI:
-
Motivational interviewing
- PHC:
-
Primary healthcare
- RCT:
-
Randomized clinical trial
References
Bjarnason-Wehrensa B, McGeeb H, Zwislerc A-D, Piepolid M, Benzere W, Jean-Paul Schmidf PD, et al. Cardiac rehabilitation in Europe: results from the European Cardiac Rehabilitation Inventory Survey. Eur J Cardiovasc Prev Rehabil. 2010;17:410–8.
Abreu A, Pesah E, Supervia M, Turk-Adawi K, Bjarnason-Wehrens B, Lopez-Jimenez F, et al. Cardiac rehabilitation availability and delivery in Europe: How does it differ by region and compare with other high-income countries?: Endorsed by the European Association of Preventive Cardiology. Eur J Prev Cardiol. 2019;26(11):1131–46.
Shields GE, Wells A, Doherty P, Heagerty A, Buck D, Davies LM. Cost-effectiveness of cardiac rehabilitation: a systematic review. Heart. 2018;104:1403–10.
Piepoli M, Corrà U, Adamopoulos S, Benzer W, Bjarnason-Wehrens B, Cupples M, et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention & Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. Eur J Prev Cardiol. 2014;21(6):664–81.
Martín R. Efectividad de la rehabilitación cardíaca en un grupo de pacientes de alto riesgo. Rev Enferm Cardiol. 2018;25(75):34–9.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology.(ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Hear J. 2016;18(8):891–975.
Kotseva K, Wood D, De BD, De BG, Rydén L, Jennings C, et al. EUROASPIRE IV: A European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardio. 2016;23(6):636–48.
Haykowsky M, Scott J, Esch B, Schopflocher D, Myers J, Paterson I, et al. A Meta-analysis of the effects of Exercise Training on Left Ventricular Remodeling Following Myocardial Infarction: Start early and go longer for greatest exercise benefits on remodeling. Trials. 2011;12(1):92.
Bohplian S, Bronas UG. Motivational Strategies and Concepts to Increase Participation and Adherence in Cardiac Rehabilitation: AN INTEGRATIVE REVIEW. J Cardiopulm Rehabil Prev. 2022;42(2):75–83.
Gené-Badia J, Ascaso C, Escaramis-Babiano G, Sampietro-Colom L, Catalán-Ramos A, Sans-Corrales M, et al. Personalised care, access, quality and team coordination are the main dimensions of family medicine output. Fam Pract. 2007;24:41–7.
Palomo L, Gené-Badia J, Rodríguez-Sendín JJ. The reform of primary care, between the last refuge of adventure and innovation. SESPAS report Gac Sanit. 2012;26(Suppl 1):14–9.
Santesmases-Masana R, González-de Paz L, Hernández-Martínez-Esparza E, Kostov B, Navarro-Rubio MD. Self-Care Practices of Primary Health Care Patients Diagnosed with Chronic Heart Failure: A Cross-Sectional Survey. Int J Environ Res Public Heal. 2019;16(1625):1–16.
Buckingham SA, Taylor RS, Jolly K, Zawada A, Dean SG, Cowie A, et al. Home-based versus centre-based cardiac rehabilitation: Abridged Cochrane systematic review and meta-analysis. Open Hear. 2016;3(2): e000463.
Dalal HM, Doherty P, Taylor RS. Cardiac rehabilitation. Vol. 351, BMJ. 2015. p. h5000.
Arena R, Williams M, Forman DE, Cahalin LP, Coke L, Myers J, et al. Increasing referral and participation rates to outpatient cardiac rehabilitation: The valuable role of healthcare professionals in the inpatient and home health settings: A science advisory from the american heart association. Circulation. 2012;125(10):1321–9.
Fariba Jokar, Hojatllah Yousefi, Alireza Yousefy MS. Begin Again and Continue With Life: A Qualitative Study on the Experiences of Cardiac Rehabilitation Patients. J Nurs Res. 2017;25(5):344–52.
Rollnick S, Miller W. What is Motivational Interviewing? Behav Cogn Psychother. 1995;23(4):325–34.
Masterson Creber R, Patey M, Lee CS, et al. Motivational interviewing to improve self-care for patients with chronic heart failure: MITI-HF randomized controlled trial. Patient Educ Couns. 2016;99:256–64.
Hardcastle SJ, Taylor AH, Bailey MP, Harley RA, Hagger MS. Effectiveness of a motivational interviewing intervention on weight loss, physical activity and cardiovascular disease risk factors: A randomised controlled trial with a 12-month post-intervention follow-up. Int J Behav Nutr Phys Act. 2013;10:1–16.
Paradis V, Cossette S, Frasure-Smith N, Heppell S, Guertin MC. The efficacy of a motivational nursing intervention based on the stages of change on self-care in heart failure patients. J Cardiovasc Nurs. 2010;25(2):130–41.
Chew HSJ, Cheng HY, Chair SY. The suitability of motivational interviewing versus cognitive behavioural interventions on improving self-care in patients with heart failure: A literature review and discussion paper. Appl Nurs Res. 2019;45:17–22.
Tamara Sokalski, K Alix Hayden, Shelley Raffin Bouchal, Pavneet Singh KK-S. Motivational Interviewing and Self-care Practices in Adult Patients With Heart Failure: A Systematic Review and Narrative Synthesis. J Cardiovasc Nurs. 2020;32(2):107–115.
Generalitat de Catalunya. Població de referència del Servei Català de la Salut per a l’any 2020. Dades per ABS i UP assignada. Available in https://catsalut.gencat.cat/web/.content/minisite/catsalut/proveidors_professionals/registres_catalegs/documents/poblacio-referencia.pdf; 2020. p. 1–29.
Hospital Clínic de Barcelona. Available from: https://www.clinicbarcelona.org/en
Miller WR (William R, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2013. 482 p.
Rehabilitation. BC. Home Exercise Program Guidelines. BRONSON. 2016.
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, SPIRIT, et al. statement: Defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7.
Badia X, Gutiérrez F, Wiklund I, Alonso J. Validity and reliability of the Spanish version of the Psychological General Well-Being Index. Qual Life Res. 1996;5(1):101–8.
Schmidt S, Vilagut G, Garin O, Cunillera O, Tresserras R, Brugulat P, et al. Reference guidelines for the 12-Item Short-Form Health Survey version 2 based on the Catalan general population. Med Clin (Barc). 2012;139(14):613–25.
Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23(2):377–81.
Fulk GD, He Y. Minimal clinically important difference of the 6-minute walk test in people with stroke. J Neurol Phys Ther. 2018;42(4):235–40.
The American College of Sports Medicine (ACSM). ACSM’s guidelines for exercise testing and prescription. 11th ed. Lippincott Williams & Wilkins. Wolters Kluwer; 2021.
R Core Team. R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing. Vienna, Austria; 2021. Available from: http://www.r-project.org/
Cowie A, Buckley J, Doherty P, Furze G, Hayward J, Hinton S, et al. Standards and core components for cardiovascular disease prevention and rehabilitation. Heart. 2019;105(7):510–5.
Generalitat de Catalunya. Institut Cartogràfic i Geològic de Catalunya [Internet]. 2022. Available from: https://www.icgc.cat/en/
Morisky D, Green L, Levine D. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74.
Rodríguez Chamorro MÁ, García-Jiménez E, Amariles P, Rodríguez Chamorro A, et al. Review of the test used for measuring therapeutic compliance in clinical practice. Aten Primaria. 2008;40(8):413–7.
Gual A, Contel M, Segura L, Ribas A, Colom J. The ISCA (Systematic Interview of Alcohol Consumption), a new instrument to detect risky drinking. Med Clin (Barc). 2001;117(18):685–9.
Huynh T, Kouz S, Yan A, Danchin N, Loughlin JO, Schampaert E, et al. Canada acute coronary syndrome risk score: A new risk score for early prognostication in acute coronary syndromes. Am Heart J. 2013;166(1):58–63.
Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51.
Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med Care. 1996;34(3):220–33.
Broadhead WE, Gehlbach SH, de Gruy FV, Kaplan BH. The Duke-UNC Functional Social Support Questionnaire. Measurement of social support in family medicine patients. Med Care. 1988;26(7):709–23.
Bellón Saameño JA, Delgado Sánchez A, Luna del Castillo JD, Lardelli Claret P. Validez y fiabilidad del cuestionario de apoyo social funcional Duke-UNC-11. Aten Primaria. 1996;18(4):153–6, 158–63..
Nasreddine Z, Phillips N, Bëdirian V. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9.
Ojeda N, Del Pino R, Ibarretxe-Bilbao N, Schretlen D, Peña J. Test de evaluación cognitiva de Montreal: normalización y estandarización de la prueba en población española. Rev Neurol. 2016;63:488–96.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: A New Depression Diagnostic and Severity Measure. J Gen Intern Med. 2001;16(9):606–13.
Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Terol M, López-roig S, Rodríguez-Marín J, Martín-aragón M, Pastor M, Reig M. Propiedades psicométricas de la escala Hospitalaria de ansiedad y estrés (HaD) en población española. Ansiedad y Estrés. 2007;13:163–76.
Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, et al. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sport Exerc. 1997;29(6 Suppl):S1-205.
Roman B, Ribas L, Ngo J, Serra L. Validación en población catalana del cuestionario internacional de actividad física. Gac Sanit. 2013;27(3):254–7.
Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4 Pt 1):1005–26.
Moreno-Chico C, González-de Paz L, Monforte-Royo C, Arrighi E, Navarro-Rubio MD, Fernández-Puebla AG. Adaptation to European Spanish and psychometric properties of the Patient Activation Measure 13 in patients with chronic diseases. Fam Pract. 2017;34(5).
Acknowledgements
We thank David Buss and Gerard Gutiérrez-Gómez for technical advice
Funding
The Carlos III Institute of Health, Ministry of Economy and Competitiveness (Spain), awarded on the 2019 call (reference PI19/00010), co-funded with European Union ERDF funds (European Regional Development Fund). The Department of Health of the Generalitat de Catalunya, in the 2020 call of the Strategic Plan of Research and Innovation in Health (PERIS) 2016–2020, (reference SLT017/20/000205). Instituto de Salud Carlos III,PI19/00010,Luis González de Paz,Departament de Salut,Generalitat de Catalunya,SLT017/20/000205,Luis González de Paz
The funding sources will play no role in the study design, data collection, analysis, interpretation, or writing of the manuscript. The evaluation committee will annually inspect the progress of the study and compliance with the study protocol. The study coordinators will report the results annually and the Sponsor may audit study completion and best practices.
Author information
Authors and Affiliations
Contributions
Concept and design: RRR, LGdP; statistical analysis: BK; critical review of the protocol: all authors. The authors read and approved the final manuscript. .
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of the Hospital Clinic of Barcelona (Ref. HCB/2019/0727). Before any evaluation or intervention, participants will provide written informed consent. Any protocol modifications will seek authorization of the Research Ethics Committee and thereafter will be made public.
Consent to publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rodríguez-Romero, R., Falces, C., Kostov, B. et al. A motivational interview program for cardiac rehabilitation after acute myocardial infarction: study protocol of a randomized controlled trial in primary healthcare. BMC Prim. Care 23, 106 (2022). https://doi.org/10.1186/s12875-022-01721-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12875-022-01721-y