Abstract
Background
Adherence to guidelines for back pain continues to be a challenge, prompting strategies focused on improving education around biopsychosocial frameworks.
Objective
Assess the influence of an interactive educational mobile app for patients on initial care decisions made for low back pain by the primary care provider. The secondary aim was to compare changes in self-reported pain and function between groups.
Methods
This was a randomized controlled trial involving patients consulting for an initial episode of low back pain. The intervention was a mobile video-based education session (Truth About Low Back Pain) compared to usual care. The app focused on addressing maladaptive beliefs typically associated with higher risk of receiving low-value care options. The primary outcome was initial medical utilization decisions made by primary care practitioners (x-rays, MRIs, opioid prescriptions, injections, procedures) and secondary outcomes included PROMIS pain interference and physical function subscales at 1 and 6 months, and total medical costs.
Results
Of 208 participants (71.2% male; mean age 35.4 years), rates of opioid prescriptions, advanced imaging, analgesic patches, spine injections, and physical therapy use were lower in the education group, but the differences were not significant. Total back-related medical costs for 1 year (mean diff = $132; P = 0.63) and none of the 6-month PROMIS subscales were significantly different between groups. Results were no different in opioid-naïve subjects. Instead, prior opioid use and high-risk of poor prognosis on the STarT Back Screening Tool predicted 1-year back pain-related costs and healthcare utilization, regardless of intervention.
Conclusion
Factors that influence medical treatment decisions and guideline-concordant care are complex. This particular patient education approach directed at patients did not appear to influence healthcare decisions made by primary care providers. Future studies should focus on high-risk populations and/or the impact of including the medical provider as an active part of the educational process.
Trial Registration
clinicaltrials.gov NCT02777983.
Similar content being viewed by others
Background
While clinical practice guidelines exist to help drive high-value care and improve outcomes for patients with low back pain (LBP), there continue to be implementation challenges. Low-value care provides no net health benefit in specific clinical scenarios [1]. For LBP expensive tests (e.g. MRI or CT), procedures (e.g. injections, nerve ablations), and therapies (e.g. opioids) deliver limited benefits in terms of reduced pain and increased function [2], especially when part of the initial treatment strategy. The popularity and abundant use of these low-value care components is one reason that low back and neck pain has the highest amount of health care spending out of 154 medical conditions in the US [3]. Strategies to reduce low-value care for LBP continue to be of utmost importance [4].
Patient expectations have been identified as a potential contributor to low-value care, and many of these expectations are based on a biomedical perspective [5,6,7]. The biomedical approach focuses on biological mechanisms as the primary source of symptoms (e.g. a bulging disc, fracture, malalignment of the spine, etc.), which often places patients in a position of perceived frailty and vulnerability [8]. This has the consequence of ignoring or minimizing psychological, social, and environmental influences [9]. The latter are considered more predictive of long-term disability and chronicity [10]. Most procedures, diagnostic tests, and opioids focus on finding or treating a biomedical problem, but are not considered high-value care when used as initial treatment options. In fact, they can often place patients at higher risk for poor recovery. For example, an MRI is likely to show some variance of abnormality even though imaging findings of any kind are unlikely to change the treatment plan or prognosis [11]. A specific pathoanatomical diagnosis cannot be reliably identified in most cases [11] and is not needed to deliver effective care. Incidental findings may even lead to unnecessary and high-risk interventions [12]. However, many of these high-risk treatment options are associated with higher patient satisfaction [13, 14], further complicating treatment decisions for clinicians. Imaging can reduce anxiety [15] as patients expect a clear and specific diagnosis [16, 17], that some feel is not possible without an MRI [6]. However, because of over-reliance on the biomedical paradigm, patients also interpret a decision to forego medical treatment they think important (imaging, procedures, medication) as being associated with low-value care [18] or even as care that physicians for a variety of reasons are trying to ration [19]. Doctors report ordering unnecessary tests because of pressure from patients [20], and are more likely to prescribe opioids when they have less time to address psychological, environmental, and social variables that are commonly related to poor prognosis with back pain [21].
Patient expectations are often driven by misinformation, often placing clinicians in a conflicted position of choosing to satisfy patients versus following guideline recommendations. In a study of 130 patients, 89% reported learning many of their misconceptions about back pain from previous health care providers, which provides some insight as to why these beliefs are so difficult to unravel [22]. Mass media campaigns that leverage psychosocial paradigms to improve the public’s health literacy related to misconceptions about the biomedical causes of back pain have been called for [23], but the magnitude of their effect is questionable leaving room for improvement [24, 25]. Focusing on the individual patient at the point of care with a more engaging format, right when the problem is most pressing, could provide another influential opportunity for education.
Equipping patients with appropriate and engaging information that shifts the focus from the biomedical perspective to one that addresses psychosocial risk factors has the potential to improve high-value care, and ultimately long-term outcomes. The purpose of this study was to compare initial treatment choices for LBP made by primary care clinicians based on whether the patient was primed with an educational session focused on addressing biomedically-focused misconceptions about the diagnosis, treatment, and prognosis for LBP immediately before seeing the primary care provider. Would a patient receiving this information help influence high-value care decisions for LBP made by the primary care provider? The secondary aim was to compare changes in self-reported pain and function between the two groups from baseline to 6 months, and total back pain-related healthcare costs during the full year after the initial diagnosis.
Methods
Design and trial oversight
This was a parallel group randomized controlled trial with a 1:1 allocation to treatment. Ethics approval was provided by the Institutional Review Board at Army Regional Health Command Central, the trial was registered a priori (clinicaltrials.gov NCT02777983; 05/19/2016), and the CONSORT checklist was used to guide reporting [26].
Setting and participants
Participants were individuals between the ages of 18 and 50 consulting for low back pain in a hospital-based primary care clinic in San Antonio, TX. This age range was chosen as it best aligns with the age range of military personnel. Individuals seeking care in this setting are TRICARE beneficiaries; covered by the health system under the US Defense Health Agency. Participants were excluded if they had prior spine surgery, were currently or recently pregnant within the last 6 months, had any non-musculoskeletal cause for symptoms (severe neurological deficit, fracture, cancer, infection, or other systemic disease), had already sought care for their back pain in the last 3 months, or did not read or write in English. Because the intervention occurred in the Military Health System, and to try and make the findings relevant to a service member population, individuals under the age of 18 or over the age of 50 were also excluded.
Randomization
A randomization sequence was developed by an individual at our partner university that was not participating in the trial in permuted blocks of four. The treatment allocation was written on a 3 × 5 index card, folded in half, and placed in a sealed opaque envelope. This stack of sequentially numbered envelopes was then given to the research team and an envelope was opened by a research coordinator after a participant had enrolled in the study and completed all baseline assessments.
Interventions
The intervention is described in detail according to the Template for Intervention Description and Replication (TIDieR) checklist (Table 1) [27]. Participants were randomized to receive either a guided video-based education session that focused on shifting pain-related attitudes and beliefs from an unhealthy biomedical focus to a more holistic biopsychosocial focus (video from Truth About Low Back Pain) [28] or usual care. The video education also provided a unique and more engaging format to deliver this content (comprehension questions asked at the end, key points reviewed, etc.) compared to traditional books or print media [29]. For individuals randomized to the usual care group, nothing was done differently to influence education or care above and beyond what they would normally receive from the medical staff during their appointment. Participants in the education group were taken into a private room by a credentialed clinician (not their primary care provider), allowed to fully watch the video in the app, answer the related quiz questions, review sections for questions answered incorrectly, and ask additional questions while the clinician reinforced the key messages:
-
1)
Most low back pain resolves in several weeks’ time regardless of pain severity.
-
2)
MRIs and x-rays are not much help in most cases.
-
3)
Over the counter medications should be used sparingly and narcotics should be avoided completely.
-
4)
The two most important things you can do for your back pain are to 1) Stay Active, and 2) Think Positive.
Outcomes
The primary outcome was low-value medical utilization (x-rays, MRIs, opioid prescriptions, injections, procedures, etc.) that took place as the initial treatment option within the first 7 days of care. If no treatment was registered beyond this time point, then patients were classified as having no additional care. Medical utilization was determined from manual review of medical records, as well as extraction from the Military Health System Data Repository by procedural (Current Procedural Terminology - CPT) and diagnostic codes (International Classification of Diseases, 9th and 10th revisions - ICD9 and ICD10). We identified all common pharmacological and non-pharmacological interventions utilized for LBP, to include pharmaceutical analgesics (NSAIDs, acetaminophen, ketorolac injections, opioid-based pain relievers to include tramadol), acupuncture, dry-needling, manual therapy and spinal manipulation, therapeutic exercise, as well as referrals to specialty care (physical therapy, sports medicine, orthopaedics, etc.) and diagnostic procedures (e.g., x-rays, MRI, CT-scan). The supplementary online appendix has the list of procedure and diagnosis codes that were utilized. Outcome assessors were blinded to group allocation at all time points. Medical utilization data was fully extracted from the medical records and from the MDR database by an independent data analyst working for the hospital, blinded to the treatment allocation of each participant.
The secondary outcomes included the change in Patient Reported Outcomes Measurement Information System (PROMIS) scores (pain intensity, pain interference, and physical function subscales) at 1 and 6 months and 1-year total LBP-related medical costs. As psychosocial risk factors can drive healthcare utilization for low back pain, we also captured the risk of poor prognosis using the STarT Back Screening Tool (SBST) [30] and the Optimal Screening for Prediction of Referral and Outcomes Yellow Flag (OSPRO-YF) tool [31] at baseline. The SBST tool is a 9-item tool that classified patients into low, medium, and high risk for poor long-term outcome. The OSPRO-YF is a 10-item tool that identifies the presence of 11 “yellow flags” that represent psychosocial risk factors within three distinct domains: negative mood, positive affect and coping, and fear avoidance [31].
Patient satisfaction
Patient satisfaction with the care each received for LBP was measured for each participant using a 17-item instrument that has been validated and found capable of distinguishing among three different dimensions of satisfaction (caring, information and treatment effectiveness) among patients with LBP attending primary care [32]. Each item asks about satisfaction on a 5-point Likert scale from 1 = strongly agree to 5 = strongly disagree.
Data source
Data were sourced from the Military Health System Data Repository (MDR), which captured data from 260 sources worldwide for any individual covered by the TRICARE insurance program. This includes data from all outpatient and inpatient encounters, in civilian and military hospitals and clinics, pharmacy and radiology data, for military service members, their dependents and family members, and retired service members and their families all around the world. Data is continuously validated for 90 days, with updates from many sources, before variables are converted from ‘raw’ to ‘final’. Data for this study was extracted after 90 days from the last date of interest to ensure maximum validity of data.
Statistical approach
There were various healthcare utilization outcomes of interest, captured as dichotomous measures of occurrence (YES or NO). We calculated that a sample size of 206 patients would provide the trial with 80% power, at a two-sided alpha level 0.05 and a Cohen’s W of 0.25 for a small effect size, resulting in a critical χ2 of 11.07. The sample-size calculation was performed with the use of G*Power software, version 3.1.9.6 [33]. We added 14 additional patients to account for potential loss to follow-up for a total recruitment goal of 220.
For the primary outcome, we calculated frequency of each type of initial treatment with a chi-square analysis and reported odds ratios with 95% confidence intervals for each care option based on the treatment group of initial randomization. If events happened multiple times (e.g., 2+ physical therapy visits, 2 different opioid prescriptions, etc.) the frequency was counted once. For assessment of change in PROMIS subscales from baseline to 6 months between groups, we utilized a linear mixed effects model (LMM), with Bonferroni adjustment for multiple comparisons (baseline, 1 month, 6 months). We chose the LMM because it is flexible and appropriate in accounting for unbalanced and missing data in repeated measures mixed model designs [34]. We report estimated marginal means with 95% confidence intervals. Participants were analyzed according to the group of initial assignment.
Sensitivity analysis
Because prior opioid use is one of the strongest predictors of future opioid use, we conducted a sensitivity analysis excluding all patients with any opioid prescription fills in the prior year.
Exploratory analysis
We also conducted an exploratory analysis to assess the influence of psychosocial risk factors on the primary outcome (initial healthcare utilization choices). We utilized the same analysis employed for the primary outcome (initial treatment received) but based on high versus medium/low STarT Back risk or presence of any yellow flags in any of the 3 OSPRO-YF domains regardless of initial treatment randomization assignment.
Missing data
For our primary outcome, we didn’t expect any missing values because it was based on healthcare utilization data. This is a closed, single-payer system where all care is covered (essentially a government-sponsored socialized medical system). As many as 3% have other health insurance (most often through spouses), but there is no evidence the other insurance is used and not likely if they initially sought care in this system, especially where there is no co-pay for care. If a healthcare event was not present in the MDR, we assumed it did not happen. For the secondary outcomes, we prespecified the use of our statistical model as the primary plan for handling missing data.
Results
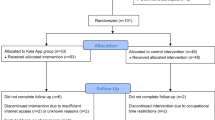
There were 220 participants that enrolled between March and October 2016, but 12 were found to have evidence of back-related care in the 3 months prior to enrollment and were excluded from further analyses (Fig. 1). For the 208 participants included (71.2% male; mean age 35.3 years; Table 2), the rates of medication prescription, diagnostic tests, and specialty referrals put into place after the initial consultation were no different between groups (Table 3). The app education session did not influence decisions made by primary care providers compared to usual care alone. Many patients received a combination of non-pharmacological (Fig. 2) and pharmacological treatment (Fig. 3), and only 15 patients received no treatment at all (procedures, pharmacological agents, or referrals). In the sensitivity analyses, the utilization of low-value care was lower for opioid-naïve patients that received the app education, but the differences were not significant.
The changes in PROMIS pain and physical function were also no different between groups at 6 months (physical function mean difference = 0.35, 95CI -2.46, 1.76; pain interference mean difference = 1.05, 95CI -1.27, 3.37; pain intensity mean difference = 0.003, 95CI -0.58, 0.59).
In the exploratory analysis of initial treatment options based on the presence of various psychosocial and prognostic risk factors, most treatment options were given in higher proportion to patients with yellow flags or prognostic risk factors of pain-associated distress; however, the differences were not statistically significant (Table 4). Opioid prescriptions and pharmacological treatments in general (driven primarily by NSAID prescriptions) were significantly higher in patients that were high-risk on the STarT Back screening tool and had fear avoidance beliefs, respectively (Table 4). This study was not powered to assess differences based on these domains, and no definitive inferences can be made. No harm or unintended effect of treatment was reported by any participant in this study.
Patient satisfaction with the care they received was not different between groups (Table 5). The mean overall score and standard deviation for all 17 questions was 54.0 (6.0) for the usual care group and 54.6 (3.7) for the education group. All three of the subscale scores were also similar between both groups.
Discussion
Equipping patients with proper education about best care practice and expectations for low back pain within the context of a biopsychosocial framework had no influence on treatment decisions made by primary care providers compared to patients not getting the education. Any actual or perceived pressure from patients to make care decisions that contradict guideline-adherent care was unchanged with this educational approach.
However, psychosocial beliefs associated with greater risk for poor prognosis were common in this cohort of patients seeking initial care for LBP in primary care settings. Over half of the patients had at least 1 yellow flag on the OSPRO-YF and approximately 1 in 5 were considered high-risk on the SBST. Regardless of beliefs, there was large variability in the care delivered, much of which would not be considered high-value or concordant with current clinical practice guidelines [35, 36]. Initial treatments were not significantly different for patients that received the biopsychosocial risk-focused app interaction prior to their visit with the primary care clinician compared to those that did not receive the app. Education on self-management is key as an early intervention, with a strong focus on addressing maladaptive beliefs, while routine imaging and opioid-based pain medications are not recommended as initial treatment strategies [11, 35]. In this study, initial care for a new episode of back pain consisted of 134 (64.4%) receiving a specialty care referral, 58 (27.9%) having an x-ray ordered, and 24 (11.5%) having an MRI ordered (Fig. 2). For pharmacological interventions, 109 (52.4%) received NSAIDs, 75 (36.1%) received muscle relaxers, and 11 (5.2%) received opioids (Fig. 3). Patients also received analgesic patches, vitamin D supplementation, and transcutaneous electrical stimulation (TENS) units for home use (Figs. 2 and 3), all treatments with unknown or little efficacy.
Despite a high proportion of individuals with maladaptive psychosocial beliefs at baseline, patient-focused education did not result in any changes in treatment decisions made by primary care providers. A prior study in military soldiers found that a brief educational session focused on addressing psychosocial beliefs, and delivered in group format, resulted in decreased healthcare seeking for low back pain in the following year [37]. However, all were healthy without LBP at the time of the education, and therefore the decisions related to whether they should seek care in the first place were different than those about what intervention should be received after the decision has been made to seek care. They also would have had additional time to digest the information before seeing a medical provider. Perhaps more time to mentally process the information (instead of immediately before their consultation) would lead to greater advocacy for high-value care. While education has been targeted at clinicians with mixed results in the past [25], the clinicians in our trial were blinded to the treatment arm and entire educational component for the duration of the study. It is possible that some providers adapt to routines for managing specific conditions, where passive patient personalities are less likely to challenge any decisions made by clinicians. Patients generally want to be actively involved in management decisions for LBP [38], but without the clinician being aware of the education the patient received, they would be limited in their ability to engage and continue the conversations that were started. One study found that focusing psychosocial education and training on medical students and general practitioner trainees can likely influence treatment decisions [39]. For our study, revealing the group allocation would have biased providers and likely confounded the impact of the educational app session. Bringing the clinician into the education process earlier would likely have great merit and should be considered in future studies. Receiving the education from their general practitioner, with whom they may already have a stronger rapport, could also influence its impact. It could also be that the clinicians themselves are providing conflicting or predominately biomedically-focused information, and the patient must now balance what they learned in the video education app versus what they are hearing from the provider.
Similar educational programs focused on targeting the psychosocial component behind treatment decisions have had varying levels of success. Mass media campaigns have been shown to positively influence both patient and provider beliefs about LBP [24], but it is unclear if the change in beliefs results in changes in clinical practice. In addition, while decisions appear to be improved when dealing with vignettes [39], real-life scenarios may have different outcomes. In emergency medicine settings, Choosing Wisely campaigns were able to significantly improve knowledge and awareness of guideline recommendations for LBP imaging, however it did not translate into any actual reduction in imaging rates [25]. In 6 large primary care clinics where clinicians committed ahead of time to follow guidelines, there were only minor changes (1.2 to 1.9%) in low-value care decisions that were not sustained in the long-term [40].
The psychosocial profiles of patients and their relation to higher utilization of treatment needs further investigation. Targeting only patients at high risk for poor prognosis (psychosocial risk factors present) may be necessary to truly understand the value of these patient or provider-focused education interventions. The effect may be diluted with the inclusion of all patients (low and high risk). This study was not powered to fully detect those differences, which could explain why medical utilization was higher in patients presenting with psychosocial risk factors, but the differences were not significant. Other studies have shown that high rates of fear avoidance [41] beliefs and pain catastrophizing result in higher healthcare utilization [42, 43]. Identifying high-risk individuals to prioritize for this type of education could be beneficial. At the same time, the minimal cost and effort to deliver this care to everyone is likely sustainable and could help create a better culture of information within specific clinical settings.
Strengths and limitations
The strengths of this study include the moderate sample size and long-term follow-up, as well as the closed single-payer system which allows essentially complete capture of all healthcare utilization events. This is also a potential weakness, as a single-payer government setting, these findings from the Military Health System may not be representative of results seen in other settings. It is possible that some patients revealed or shared with the primary care provider the content and nature of the video app, which may have introduced bias (the clinician would now know about the education patients are receiving and ask future patients what they received). There was a larger proportion of patients in the app education group that had prior opioid use (60.2% versus 46.7%) which may have influenced the opioid outcomes as that is a strong predictor of future opioid use. Secondary analyses of subgroups (high risk and presence of yellow risk factors) were all performed with an inappropriately powered sample size, which limits any conclusions.
Conclusion
Factors that influence medical decisions and guideline-concordant care are complex. Despite many patients with LBP having maladaptive psychosocial beliefs at baseline, an attempt to address those beliefs at the point of care with the patient through an innovative software app interaction did not change initial treatment decisions made by the clinician. This particular patient education approach did not appear to influence healthcare decisions. Future studies should consider focusing exclusively on high-risk populations, on a simultaneous focus of both the patient and clinician, and on the impact of including the medical provider in the educational experience from the beginning.
Availability of data and materials
Reasonable requests for data will be considered after meeting proper data sharing agreement requirements from the US Defense Health Agency (DHA) (Data Sharing Agreement Applications [DSAA] and templates can be found at health.mil). After DSA approval by the DHA, please contact the corresponding author for data.
References
Mafi JN, Russell K, Bortz BA, Dachary M, Hazel WA Jr, Fendrick AM. Low-Cost, High-Volume Health Services Contribute The Most To Unnecessary Health Spending. Health Aff. 2017;36:1701–4.
Atlas SJ. Management of Low Back Pain: Getting From Evidence-Based Recommendations to High-Value Care. Ann Internal Med. 2017;166:533–4.
Dieleman JL, Cao J, Chapin A, Chen C, Li Z, Liu A, et al. US Health Care Spending by Payer and Health Condition, 1996-2016. JAMA. 2020;323:863–84.
Buchbinder R, Underwood M, Hartvigsen J, Maher CG. The Lancet Series call to action to reduce low value care for low back pain: an update. Pain. 2020;161:S57.
Main CJ, Foster N, Buchbinder R. How important are back pain beliefs and expectations for satisfactory recovery from back pain? Best Pract Res Clin Rheumatol. 2010;24:205–17.
Petersen L, Birkelund R, Ammentorp J, Schiøttz-Christensen B. “An MRI reveals the truth about my back”: a qualitative study about patients’ expectations and attitudes toward the value of MRI in the assessment of back pain; 2016. https://doi.org/10.5750/ejpch.v4i3.1122.
Hoffmann TC, Del Mar CB, Strong J, Mai J. Patients’ expectations of acute low back pain management: implications for evidence uptake. BMC Fam Pract. 2013;14:7.
Darlow B, Dean S, Perry M, Mathieson F, Baxter GD, Dowell A. Easy to Harm, Hard to Heal: Patient Views About the Back. Spine. 2015;40:842–50.
Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, et al. What low back pain is and why we need to pay attention. Lancet. 2018. https://doi.org/10.1016/S0140-6736(18)30480-X.
Hruschak V, Cochran G. Psychosocial predictors in the transition from acute to chronic pain: a systematic review. Psychol Health Med. 2018;23:1151–67.
Chou R, Qaseem A, Owens DK, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Diagnostic imaging for low back pain: advice for high-value health care from the American College of Physicians. Ann Intern Med. 2011;154:181–9.
Webster BS, Bauer AZ, Choi Y, Cifuentes M, Pransky GS. Iatrogenic consequences of early magnetic resonance imaging in acute, work-related, disabling low back pain. Spine. 2013;38:1939–46.
Adams J, Bledsoe GH, Armstrong JH. Are Pain Management Questions in Patient Satisfaction Surveys Driving the Opioid Epidemic? Am J Public Health. 2016;106:985–6.
Carrico JA, Mahoney K, Raymond KM, Mims L, Smith PC, Sakai JT, et al. The Association of Patient Satisfaction-Based Incentives with Primary Care Physician Opioid Prescribing. J Am Board Fam Med. 2018;31:941–3.
Schers H, Wensing M, Huijsmans Z, van Tulder M, Grol R. Implementation barriers for general practice guidelines on low back pain a qualitative study. Spine. 2001;26:E348–53.
Lim YZ, Chou L, Au RT, Seneviwickrama KMD, Cicuttini FM, Briggs AM, et al. People with low back pain want clear, consistent and personalised information on prognosis, treatment options and self-management strategies: a systematic review. J Physiother. 2019. https://doi.org/10.1016/j.jphys.2019.05.010.
Verbeek J, Sengers M-J, Riemens L, Haafkens J. Patient expectations of treatment for back pain: a systematic review of qualitative and quantitative studies. Spine. 2004;29:2309–18.
Carman KL, Maurer M, Yegian JM, Dardess P, McGee J, Evers M, et al. Evidence that consumers are skeptical about evidence-based health care. Health Aff. 2010;29:1400–6.
Lantz PM, Evans WD, Mead H, Alvarez C, Stewart L. Knowledge of and Attitudes Toward Evidence-Based Guidelines for and Against Clinical Preventive Services: Results from a National Survey. Milbank Q. 2016;94:51–76.
Campbell EG, Regan S, Gruen RL, Ferris TG, Rao SR, Cleary PD, et al. Professionalism in medicine: results of a national survey of physicians. Ann Intern Med. 2007;147:795–802.
Neprash HT, Barnett ML. Association of Primary Care Clinic Appointment Time With Opioid Prescribing. JAMA Netw Open. 2019;2:e1910373.
Setchell J, Costa N, Ferreira M, Makovey J, Nielsen M, Hodges PW. Individuals’ explanations for their persistent or recurrent low back pain: a cross-sectional survey. BMC Musculoskelet Disord. 2017;18:466.
O’Keeffe M, Maher CG, Stanton TR, O’Connell NE, Deshpande S, Gross DP, et al. Mass media campaigns are needed to counter misconceptions about back pain and promote higher value care. Br J Sports Med. 2018;:bjsports – 2018–099691.
Suman A, Armijo-Olivo S, Deshpande S, Marietta-Vasquez J, Dennett L, Miciak M, et al. A systematic review of the effectiveness of mass media campaigns for the management of low back pain. Disabil Rehabil. 2020;:1–29.
Chandra K, Atkinson PR, Chatur H, Fraser J, Adams CL. To Choose or Not To Choose: Evaluating the Effect of a Choosing Wisely Knowledge Translation Initiative for Imaging in Low Back Pain by Emergency Physicians. Cureus. 2019;11:e4002.
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687.
Clinically Relevant Technologies. The Truth About Low Back Pain. App Store. 2018. https://apps.apple.com/us/app/the-truth-about-low-back-pain/id1325643930. Accessed 12 Mar 2020.
Burton AK, Waddell G, Tillotson KM, Summerton N. Information and advice to patients with back pain can have a positive effect. A randomized controlled trial of a novel educational booklet in primary care. Spine. 1999;24:2484–91.
Hill JC, Whitehurst DGT, Lewis M, Bryan S, Dunn KM, Foster NE, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;378:1560–71.
Butera KA, George SZ, Lentz TA. Psychometric evaluation of the Optimal Screening for Prediction of Referral and Outcome Yellow Flag (OSPRO-YF) tool: factor structure, reliability, and validity. J Pain. 2019. https://doi.org/10.1016/j.jpain.2019.09.003.
Cherkin D, Deyo RA, Berg AO. Evaluation of a physician education intervention to improve primary care for low-back pain. II. Impact on patients. Spine. 1991;16:1173–8.
Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Fitzmaurice GM, Ravichandran C. A primer in longitudinal data analysis. Circulation. 2008;118:2005–10.
Qaseem A, Wilt TJ, McLean RM, Forciea MA. Clinical Guidelines Committee of the American College of Physicians. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166:514–30.
Pangarkar SS, Kang DG, Sandbrink F, Bevevino A, Tillisch K, Konitzer L, et al. VA/DoD Clinical Practice Guideline: Diagnosis and Treatment of Low Back Pain. J Gen Intern Med. 2019;34:2620–9.
George SZ, Childs JD, Teyhen DS, Wu SS, Wright AC, Dugan JL, et al. Brief psychosocial education, not core stabilization, reduced incidence of low back pain: results from the Prevention of Low Back Pain in the Military (POLM) cluster randomized trial. BMC Med. 2011;9:128.
Chou L, Ranger TA, Peiris W, Cicuttini FM, Urquhart DM, Sullivan K, et al. Patients’ perceived needs of health care providers for low back pain management: a systematic scoping review. Spine J. 2018;18:691–711.
Dwyer CP, MacNeela P, Durand H, O’Connor LL, Main CJ, McKenna-Plumley PE, et al. Effects of Biopsychosocial Education on the Clinical Judgments of Medical Students and GP Trainees Regarding Future Risk of Disability in Chronic Lower Back Pain: A Randomized Control Trial. Pain Med. 2020;21:939–50.
Kullgren JT, Krupka E, Schachter A, Linden A, Miller J, Acharya Y, et al. Precommitting to choose wisely about low-value services: a stepped wedge cluster randomised trial. BMJ Qual Saf. 2018;27:355–64.
Garcia AN, Cook C, Rhon D. Which patients do not seek additional medical care after a self-management class for low back pain? An observational cohort. Clin Rehabil. 2019;33:1831–42.
Rhon DI, Lentz TA, George SZ. Utility of catastrophizing, body symptom diagram score and history of opioid use to predict future health care utilization after a primary care visit for musculoskeletal pain. Fam Pract. 2020;37:81–90.
Lentz TA, Rhon DI, George SZ. Predicting Opioid Use, Increased Health Care Utilization and High Costs for Musculoskeletal Pain: What Factors Mediate Pain Intensity and Disability? J Pain. 2020;21:135–45.
Acknowledgements
We would like to thank the Geneva Foundation as well as Drs Katie Dry and Laurel Proulx with their support and assistance with coordination and data collection of this trial.
Disclaimer
The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, Department of Defense, or the U.S. Government.
Disclosure
None of the authors have any conflicts of interest to disclose.
Funding
This research was supported by the U.S. Department of Defense Military Operational Medicine Research Program (W911QY-15-1-0017).
Author information
Authors and Affiliations
Contributions
DR and JF derived the concept. DR received grant funding. TG and RM managed local recruitment and enrollment of participants. TG created the figures. All authors assisted with the interpretation and deriving the implication of the work. All authors assisted with writing the manuscript, as well as approving the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study received ethics approval by the Institutional Review Board at Brooke Army Medical Center. All participants provided informed consent to participate in the study. All methods were carried out in accordance with the Code of Federal Regulations (45 CFR 46) for the protection of human subjects from the U.S. Department of Health and Human Services.
Consent for publication
N/A
Competing interests
None of the authors have any conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rhon, D.I., Mayhew, R.J., Greenlee, T.A. et al. The influence of a MOBile-based video Instruction for Low back pain (MOBIL) on initial care decisions made by primary care providers: a randomized controlled trial. BMC Fam Pract 22, 200 (2021). https://doi.org/10.1186/s12875-021-01549-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12875-021-01549-y