Abstract
Background
Research about the decision to participate in a clinical study has tended to be limited to single indications and has focused on narrow sets of study and participant characteristics. This study applied stated preference methods to understand the clinical trial design attributes that most influence willingness to participate and how this varied with participant characteristics.
Methods
Adults residing in the US, China, or Poland with a self-reported diagnosis of cancer, heart disease, migraine, rheumatoid arthritis, or multiple sclerosis completed an online survey. Participants were asked whether they would participate in clinical studies defined by seventeen attributes within five categories (payment/support, administration/procedures, treatment-related, study location/time commitment, and data collection/feedback). Participants saw six different hypothetical clinical study profiles. Depending on their participation decision to an initial clinical study profile, the subsequent five questions had one design attribute (randomly selected per question) consecutively improved or deteriorated to elicit preferences. A logistic regression was used to determine which participant characteristics influenced participation decisions. A latent class logit model was used to identify how the influence of study design features varied between participants and whether groups of participants with similar preferences could be identified.
Results
The survey was completed by 487 participants (32% China, 35% Poland, 33% US; 8%–19% per indication). Willingness to participate was found to be a function of participant age, certain elements of quality of life, and previous treatment experience, in particular number of lines of treatment received and experience of adverse events. Willingness to participate was influenced by study design features such as payment, study duration, and time commitment – both the overall time and whether the time was at home or away from home, with the latter being particularly relevant to participants experiencing fatigue due to their disease.
Conclusions
This study quantifies how study designs influence willingness to participate and how this varies with participant types. These findings suggest that it is how an indication influences quality of life and treatment experience, rather than the indication alone, that impacts participation rates, opening the way for insights that are transferrable across indications, which may be particularly useful when considering rare diseases.
Similar content being viewed by others
Background
A successful clinical trial requires efficiently recruiting a sample of necessary size that is representative of the indication being assessed. However, over 80% of trials do not successfully enroll adequate samples within target timelines [1,2,3] and as many as 19% of clinical trials are terminated due to insufficient enrolment [4]. These shortcomings often necessitate adjustments such as extended timelines and additional sites to increase enrolment, which have important financial implications [2]. Studies also face challenges enrolling representative samples, resulting in greater proportions of younger, White, and male participants relative to target populations [5, 6]. Because treatment pharmacokinetics, efficacy, and safety can vary in racial and ethnic subpopulations [7, 8], representative and diverse participation is necessary to minimize outcome disparities between populations [9].
Few studies have investigated how willingness to participate varies with study design and participant characteristics; as such, little is known on how varying study design might improve enrolment rates and representativeness. Studies of willingness to participate in clinical research have largely been limited to single indications and have tended to focus on the influence of relatively narrow sets of participant characteristics, such as socio-demographic and attitudinal characteristics. The most commonly assessed characteristics include gender [10,11,12,13,14], age [10,11,12,13,14,15,16], and education level [11, 15, 17]. The lack of multi-indication studies also limits our understanding of how clinical characteristics influence participation. Research into the impact of disease on participation rates has been limited to proxies for treatment experience and stage of disease (e.g., initial treatment vs. retreatment and palliative vs. curative [13]). In addition, research has not considered how the impact of disease on quality and length of life, or how the type and availability of treatments for a disease influence willingness to participate. Trust in researchers, prior knowledge of clinical trials, and altruism have been associated with willingness to participate [14], although the impact of these factors on participation rates has, to the best of our knowledge, only been quantitatively assessed in a small number of cases [11, 17].
Some studies have quantified how trial design features impact willingness to participate, most commonly finding that participation increases with higher remuneration for participating [12, 18], lower risk of adverse events [10, 12], smaller time commitment associated with participating [18, 19], and involvement of a clinician in the trial or sharing of reports back with a clinician [18, 19]. However, this research does not help inform some of the key design questions currently being asked by study designers, such as how willingness to participate varies with strategies to decentralize trials (i.e., involving fewer hours at a clinical site and more hours at home), additional support for patients, and different methods of participant-completed data collection.
Enrolment into clinical trials could be improved through further understanding how willingness to participate varies with different clinical trial design attributes [20]. The objective of this study was to quantify the impact on willingness to participate of a comprehensive set of study characteristics and how this varies with participant characteristics.
Methods
Overview
An online stated-preference survey was conducted between March and April, 2021 with adult participants (≥ 18 years) with various indications (oncology specific, heart disease, migraine, rheumatoid arthritis, multiple sclerosis). The survey presented participants with a series of clinical study profiles that had desirable and undesirable study characteristics and asked them whether they would be willing to participate in these studies. The study profile information reflected key study information typically provided in an informed consent form. Participants with a wide range of indications were included to understand how the varied impact of a disease on current and future quality of life, as well as life expectancy influences willingness to participate. These indications also had varying levels of treatment availability, which we hypothesized may influence willingness to participate. At the end of the survey, participants were asked sociodemographic and clinical questions. The survey was programmed and hosted in EU Confirmit (Oslo, Norway) and was designed to take participants 30 min to complete; participants were told in advance the time required to complete the survey. The survey was conducted in the United States (US), Poland, and China to test how different healthcare systems, and potentially varied cultural views, influence willingness to participate. The online survey was translated from English into Mandarin and Polish by certified translators and validated by native-language Evidera employees. The study was approved by Ethical & Independent Review Services (E&I; Lee’s Summit, MO, US; E&I study number: 21038–01) and was conducted in accordance with best practice guidelines on preference-based methods from International Society for Pharmacoeconomics and Outcomes Research [21]. All participants provided online consent to participate. No personal information was collected. Results are reported in line with the recommendations outlined in the CHERRIES checklist for online surveys [22].
Participants
Patients from the US (target: n = 160), Poland (target: n = 150), and China (target: n = 155) were identified and recruited by a third-party vendor (Global Perspectives, Norwich, UK) from patient panels. Patients were invited to participate in the study via emails that were generically worded to prevent biases in responsiveness. The emails contained a weblink to access the survey. To participate, patients had to self-report through a screening questionnaire on the first page of the online survey that they were aged ≥ 18 years; could read and understand the language that the survey was conducted in; and had received a diagnosis of lung cancer, colorectal cancer, liver cancer, stomach cancer, ovarian cancer, blood-related cancer, breast cancer, melanoma, prostate cancer, thyroid cancer, heart disease, migraine, rheumatoid arthritis, or multiple sclerosis. Patients also had to indicate that they were willing to consider participating in a clinical trial (responses: ‘yes’, ‘maybe’, ‘definitely not’); patients were excluded if they responded ‘definitely not’, since these patients would not be influenced by the design of a clinical trial. Eligible patients were then presented with the online consent form and after consenting progressed into the main survey. Target quotas were set for the different indications to ensure the sample was sufficiently diverse to allow differences in preferences between indications to be identified: n = 75 with higher mortality cancer (lung, colorectal, liver, stomach, ovarian, and blood cancers); n = 90 with lower mortality cancer (breast, melanoma, prostate, and thyroid cancers); n = 90 with heart disease; n = 85 with migraine; n = 85 with rheumatoid arthritis; n = 40 with multiple sclerosis. Higher- and lower-mortality cancers were defined based on mortality rates [23, 24].
Selection of trial features
The features and levels that were used to define study profiles were identified based on a targeted literature review and two workshops with experts involved in clinical trial design. The targeted literature review was conducted within the search limits January 2000 to December 2020, using Embase and MEDLINE databases and with search strings related to adult patient preferences, motivation, and barriers to participating in clinical trials (Additional File 1). The search yielded 28 eligible articles, of which 20 were selected for extraction after excluding articles with significant overlap in concepts, prioritizing articles covering a wider range of indications, and prioritizing articles that quantitatively assessed impacts on willingness to participate. Several concepts were identified from the review which were grouped as (i) trial design and logistics; (ii) benefits and risks of participating; and (iii) attitude, knowledge, and social context influencing participation (Additional File 1: Figures S1–S3). The two workshops were undertaken in January and February, 2021 with 12 experts involved in clinical trial design at Pharmaceutical Product Development (PPD), Inc., who were recruited internally. The experts were specialized in various domains including oncology (n = 3), neurology (n = 3), diabetes, respiratory medicine, internal medicine, patient advocacy, and enterprise data, and digital and decentralized trial solutions (n = 1 for each). The aim of the workshops was to build on the literature to develop a comprehensive set of trial design features, levels, and patient characteristics that may influence willingness to participate. The final 17 trial design features and levels selected for inclusion in the study are shown in Table 1. Definitions of these features provided to participants are given in Additional File 2: Table S1. Some trial design features identified in the review and workshops were not included in the final list as they are invariably out of the control of the study design teams and were thus presented as fixed in preamble to the choice exercise. These included study funding by a pharmaceutical company, that participation may improve health, that studies are approved by an ethics committee or institutional review board, and that medication and care is provided free throughout the duration of the trial.
Stated preference exercise
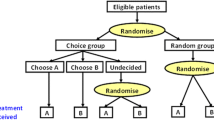
After an introduction to the trial features (Additional File 2: Table S1) and the warm-up exercises, participants were presented with six choice tasks (one choice task per page); in each they were presented with a clinical trial design and asked whether they would be willing to participate in the hypothetical trial. The first choice task is shown in Fig. 1. Where a trial feature had three levels (e.g., “Payment you will receive”: $0, $500, $2,000), the first choice task used the middle level (i.e., $500). Where a feature had two levels, participants were randomized to see different levels of the feature in the first choice task. The survey was adaptive and answers to the initial choice question influenced whether trial features were improved or deteriorated in the five follow-up tasks. If a participant indicated that they were not willing to participate in the trial presented in the first choice task, the survey would improve the level of one randomly selected trial feature (one at a time) in the following five questions, and the participant was again asked whether they would participate (Fig. 2). Likewise, if a participant was willing to participate in the initial choice task, the survey would deteriorate one trial feature at a time in the following five questions. Modified features were highlighted for the participant. A different trial feature was deteriorated or improved in each case and this was randomized to measure preferences for all attributes. The order of the trial features were randomized throughout the study between participants to avoid ordering bias [26]. Participants were not able to review or change their answers. The survey was systematically validated prior to study start, including the screening logic and choice tasks. A researcher familiar with the survey tested the online programming using a practice link to ensure that the screening criteria was appropriately implemented, and ineligible participants were terminated from the screener. The choice task section was validated by reviewing each choice task to ensure that it was presented accurately according to the design.
Measures of participant characteristics
At the end of the survey, participants were required to complete a series of sociodemographic and attitudinal questions, and questions about their illness. Questions adapted from the SF-36 [27], FACIT-FATIGUE [28] and EQ-5D [29] patient reported outcome measures were used to assess quality of life and the impact of illness on fatigue, social activity, mobility, ability to carry out daily activities, pain, and anxiety/depression. To assess trust and altruism, participants were asked to rate their agreement with items q3 and q4 of the Research Attitude Questionnaire [30] (q3: Medical researchers can be trusted to protect the interests of people who take part in their studies; q4: We all have some responsibility to help others by volunteering for medical research). Each were rated on a scale of 1 (strongly disagree) to 5 (strongly agree). Participants were considered to have high trust or high altruism if they rated their agreement as agree or strongly agree for each statement. Participants were also asked about prior clinical trial experience: whether they had been invited to participate in a trial, and whether they had participated in a trial or knew anyone who had participated in a trial.
Statistical analysis
The choice task analysis was designed using Ngene software (ChoiceMetrics, Sydney, Australia), as described elsewhere [31,32,33], and other analyses were conducted using R (version 3.6.1). Only completed surveys were analyzed. Unless noted otherwise, all statistical tests were two-sided and used a significance level of 0.05. No adjustments for multiple comparisons were performed unless noted otherwise. A logistic regression was used to identify patient characteristics that influenced the decision to participate in the initial trial presented. Three variations of the logistic regression were conducted, each including different patient characteristic variables, to assess how accurately decisions could be predicted if different variations of patient characteristics were known: Model 1 – all patient characteristic variables included (i.e., disease-related variables, treatment-related variables, sociodemographic variables, and attitudinal variables); Model 2 – only disease-related variables and country of residence included; Model 3 – all variables excluding disease-related variables and country of residence. The three variants were manually defined to represent cases where the trial designer would have incomplete information about individuals’ characteristics. For the logistic regression analysis, we combined participants with 1–2 conditions. Pearson correlations among the selected covariates were computed to avoid potential collinearity issues (covariates with an absolute correlation larger than 0.8 were excluded from the analysis [34]). Several criteria were used to evaluate the statistical performance of the different models: Bayesian Information Criteria (BIC) and out-of-sample predictive validity (i.e., % of unseen choices correctly predicted by the model). The out-of-sample predictive validity was obtained with a tenfold cross-validation procedure.
A latent class logistic (LCL) model [35] was estimated on the five follow-up tasks to analyze the likelihood that participants would change their initial decision to participate as features were varied. The optimal number of latent classes (probabilistic groups with distinct preferences), was determined by estimating LCL models with 2 to 5 classes. Each model was estimated with 20 different sets of starting values to increase the chance of finding the best solution (i.e., global maxima). The final number of classes was selected by balancing goodness-of-fit with the number of included parameters using the BIC and interpretability (i.e., the classes have logical and clinical relevance). Odds ratios (ORs) were used to report the effect of changes in the clinical trial design features on the decision to participate relative to changes in one feature – transport provision. The LCL estimates were then used to compute the class membership probabilities (i.e., probability of each participant falling into the different classes defined in the LCL model). The variability in these probabilities was then analyzed in a Beta regression model as a function of respondents’ personal characteristics.
Results
Participant characteristics
From approximately 150,000 invitation emails, 8,950 individuals visited the first page of the survey (view rate, 0.06%; Additional File 3: Figure S4). Of these, 8,275 individuals (92.5%) were not eligible (i.e., did not consent, were screened out, or quota full); this included 58 of 545 otherwise eligible participants (10.6%) who responded that they would ‘definitely not’ consider participating in a clinical trial. Ultimately, 561 were eligible and provided online consent (participation rate, 0.06%). The survey was completed by 487 participants (completion rate, 86.8%). The mean age was 47 ± 17 years (Table 2). Three-quarters (n = 360, 74%) of participants had a college education or higher, more than half (n = 276, 57%) were employed 30 h or longer per week, and a third (n = 161, 33%) were not working in a paid job. Most participants (n = 412, 85%) said that they trusted medical researchers and most (n = 404, 83%) were altruistic – as indicated by interest in volunteering for medical research. Migraine and rheumatoid arthritis were the most represented indications, followed by heart disease (Table 3). Two hundred participants reported one of the pre-specified medical conditions, 115 reported two, and 172 reported three or more. Most participants (n = 300, 61%) had taken ≥ 3 treatments for their condition and most could afford or easily afford (n = 440, 90%) their current medications. Most participants rated their health as good or fair (n = 366, 75%). There were large correlations (Cohen’s coefficient, 0.5–0.7) identified between country and age group (0.63), employment status and age group (0.61), and employment status and country (0.54) (Additional File 4: Table S2). Additional participant demographics and baseline characteristics are given in Additional File 5: Table S3. The median response time [10th–90th percentile] for the choice task part of the survey did not significantly differ across the three countries (Brown-Mood Median Test: chi-square = 5.3, p = 0.071): USA = 12.7 min [7.6–26.5]; Poland: 12.2 min [6.7–26.5]; China: 11.3 min [5.5–39.8].
Participant characteristics influencing willingness to participate
In the initial choice task, most participants (n = 344, 71%) indicated that they were willing to participate in the clinical study presented to them (i.e., ‘Study A’) (Fig. 1). Table 4 reports the logistic regression results of the initial participation decision. When all participant characteristics were considered (Model 1), participants were less likely to participate in Study A if they were diagnosed over 6 years ago or had depression, and were more likely to participate if they had previously experienced treatment side effects, had a lower self-perceived life expectancy, experienced disease impacts on their social activities, or were more altruistic (p < 0.1 for all). Participants’ country of residence also affected willingness to participate, with those in China more likely to participate than those in the US (p < 0.01), although there was no significant difference in willingness to participate between participants in Poland and those in the US.
The modelling performance, measured with BIC and predictive accuracy, remained similar when the model was run with only disease-related characteristics and country (Model 2) or with all characteristics excluding disease-related characteristics and country (Model 3) (Table 4).
In Model 2, the participant characteristics that influenced the initial decision to participate were time since diagnosis (p < 0.05) and country (p < 0.01), similar to Model 1, but also the number of medical conditions (p < 0.1); those with three or more medical conditions were more likely to be willing to participate.
In Model 3, the participant characteristics that influenced the initial decision to participate were the number of previous lines of treatment (p < 0.05), experience of treatment side effects (p < 0.05), feeling anxious or depressed (p < 0.1), experience of disease impacts on social activities (p < 0.05), age (p < 0.01), and altruism (p < 0.01). With the exception of experience of side effects and altruism, these participant characteristics emerged either as being relevant to understanding willingness to participate or their effect was found to be significant once indication was no longer controlled for in the model.
Trial design features influencing willingness to participate
When the levels of trial design features were altered (i.e., one feature changed at a time in the five follow-up tasks), participants changed their initial decision to participate in 25% of questions. Less than half (42%) of participants always maintained their initial participation decision and 3% changed their decision in all five follow-up questions.
When assessing which trial design features influenced willingness to participate, the latent class model identified two groups (classes) of participants, which provided the best balance between model fit and interpretability (Fig. 3 and Additional File 6: Table S4). Class 1 (36.1%) were influenced by several trial design features, whereas Class 2 (63.9%) were more concerned with treatment characteristics and less influenced by changes in trial design other than payment for participating. When trial design features were altered, Class 2 changed their initial decision to participate on fewer occasions than Class 1 (8% vs. 55%).
Relative to changes in transport provision, Class 1 were more likely to change their participation decision than Class 2 when the following design features were varied: time commitment away from home (OR, 11.83 vs. 0.90), having an invasive procedure (OR, 7.13 vs. 2.92), time commitment at home (OR, 3.40 vs. 0.58), and level of results-sharing with participants (OR, 2.29 vs. 1.32). These attributes did not have a significant impact on the participation decisions of Class 2. For Class 1, time commitment away from home was of greater concern than time commitment at home (OR, 11.83 vs. 3.40).
Relative to changes in transport provision, Class 2 cared more than Class 1 about the requirement to stop existing medication for their condition (OR, 11.56 vs. 1.66).
Two characteristics had a high impact on participation decisions of both classes relative to the impact of changing transport provision: payment (Class 1 OR, 7.37; Class 2 OR, 7.81) and risk of serious side effects (Class 1 OR, 9.04; Class 2 OR, 13.89).
For both classes, relative to transport provision, participation decisions were relatively less influenced by variation in childcare provision, having a concierge service, the ability to continue treatment after trial, likelihood of placebo, the requirement to wear a device, or the requirement to self-report data electronically vs. on paper.
Class allocation was influenced by participant characteristics (Table 5). Those who were older (≥ 66 years old relative to 18–50 years old) and those whose condition impacted their ability to engage in social activities were more likely to be in Class 2 – the class that was more concerned by having to stop their existing medication, and were also more concerned by avoiding side effects. Those who had received more lines of treatment, those who struggle to afford their medications, and those who experienced fatigue from their condition were more likely to be in Class 1 – the class that was more concerned by how the study design would impact the location of study activities (e.g., at or away from home).
Discussion
The objective of this study was to quantify the impact of differing study characteristics on willingness to participate and how this varies with participant characteristics. The results of this study suggest that participation rates are a function of how an indication influences quality of life as well as a patient's treatment experience, not the indication itself. Further, participation rates can be influenced by study design features such as payment, study duration, and amount and location of time commitment, with the latter being particularly relevant to participants who are experiencing fatigue due to their disease.
In line with earlier studies, participants who were more altruistic or who had received more treatments were more willing to participate in clinical studies [13, 14, 17] and participants who were older were less willing to participate [10,11,12, 15]. It is unclear from prior quantitative studies whether willingness to participate is influenced by gender [10, 11, 14], education [10, 15], affordability of medications [15], or trust in medical researchers [12] due to variability in results and a lack of evidence for these characteristics. In our study, these characteristics were less predictive of willingness to participate. Several participant characteristics not considered in previous studies were found to influence willingness to participate, including the impact of disease on elements of quality of life, especially their social life and whether they were anxious or depressed; and whether patients had experienced adverse events with their existing treatments. Several other characteristics that we hypothesized might predict willingness to participate were not found to influence participation, such life expectancy (self-perceived), indication, and employment status.
Willingness to participate differed between countries. Specifically, Chinese participants had higher willingness to participate than US participants. Such country effects have also been found in other studies; for example, those with a Swiss nationality were found to have higher willingness to participate than those from European and other countries [10]. However, it may be that such effects reflect differences in the samples between countries, rather than being a genuine country effect. In our study, country was correlated with other participant characteristics such as age and employment status. Further, the predictive validity of the models were not reduced when country was removed from the model, with other participant characteristics then proving more relevant, such as the number of prior lines of therapy, experience of treatment side effects, impact of disease on quality of life (social activities and anxiety/depression), age, and altruism. This suggests that it is variation between countries in these characteristics, rather than the country itself, that drive participation decisions.
All participants were influenced by a number of study design features, including: study duration and, mirroring other studies [10, 12, 18, 36], the amount of payment and the likelihood of experiencing adverse events. The influence of other study design features varied between participants. Participants who were older and whose disease impacted their social activities were more concerned with having to stop existing medication to participate, as well as being more concerned about side effects. Other participants, particularly those whose disease caused them to feel fatigued, were concerned with the time commitment associated with being in a study, especially if that was away from home. This cohort may be more likely to participate in de-centralized studies, implementation of which has accelerated in recent years – largely due to the COVID-19 pandemic [37]. Clinical trial decentralization is expected to be a long-term trend that may ease the burden of participating and potentially improve recruitment and retention of diverse participants [38]. Our results help to understand which populations this may be most effective in. In contrast to other studies [10, 18], willingness to participate was not significantly influenced by the likelihood of participants being in the placebo group or whether the trial involved an open label extension, which may be due to our study controlling for a greater number of study characteristics.
Our study had limitations. First, the study included many trial design features, which could have made the choice tasks cognitively burdensome. To mitigate this, the initial choice task adopted a single profile format asking whether participants would participate in this one trial (yes/no), and follow-up choice tasks varied only one feature at a time (highlighted to the participant). While this design helped to limit complexity, it also limited the amount of information gathered on trade-offs and the ability to test interactions between design characteristics. Second, it was not possible to conduct cognitive interviews to test that the concepts were being interpreted similarly across the three countries studied. Further research could usefully include this step. Third, recruitment quotas focused only on indication. Whilst the sample was diverse by country, gender, and age, it was not diverse in ethnic or racial background within each country; therefore, it was not possible to investigate whether ethnicity or race influenced willingness to participate. Fourth, participants self-reporting their diagnosis rather than it being clinically confirmed, and recall bias could have affected participants’ responses to the clinical and sociodemographic questions. Fifth, participants were sampled from an opt-in panel of individuals who signed up to participate in health care research studies; therefore, willingness to participate may be overestimated. Professional and established access panels were used to mitigate risks that might have resulted from recruitment via self-reported diagnosis. Sixth, hypothetical bias is inherent in stated preference research, since participants are asked to make hypothetical choices that may not reflect their actual decisions if they were invited to a clinical trial [39]. Although external validity of stated preference data has been demonstrated under certain conditions [40], further work is required to test the external validity of stated preference research. Finally, at the screening stage, patients who would ‘definitely not’ participate in any form of clinical trial were excluded – corresponding to 10% of the otherwise eligible participants. This aligns with similar research showing 10.3% would ‘certainly refuse’ to take part in a fictive trial of a new respiratory drug [36], although we expect the rate to vary between indications and countries. While our study did not explore the reasons behind this choice (e.g., with follow-on questions), understanding why some individuals will never participate in clinical trials is an important avenue for future research. This may correspond to attitudinal factors among patients, such as altruism and trust [14, 16, 17].
Our work has generated preliminary insights into which trial design features influence patients’ decisions to participate in clinical trials. However, the stated-preference design was simple (one trial feature varying in each follow-up question) due to the large number of potentially relevant trial features included. While this design provides insight into the relative importance of trial features, alternative designs that simultaneously vary a smaller number of trial features (e.g., discrete choice experiments) would further our understanding of how patients trade-off features of study designs when making participation decisions. Future studies would also benefit from involving larger samples and a broader range of countries and indications. Results generated from these studies should provide insight to sponsors for optimizing study design to aid enrolment.
Conclusions
This study aimed to quantity the impact of study and patient characteristics on clinical study participation rates. The results suggest that those designing studies can improve participation by increasing payments to participants, reducing study duration, and decentralizing studies. These findings may not be so unexpected. However, our research quantifies these effects and how they vary with patient characteristics, raising the possibility of using such insights to simulate how changing designs will influence participation. Moreover, it was possible to identify meta-characteristics that explained why participation rates varied between indications and countries, raising the possibility that such predictions might be generalizable. Realizing these possibilities will require further participation data that allows investigation of the trade-offs patients are willing to make between key study design features.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available as they are proprietary and no consent was sought from participants to allow sharing of data with third parties beyond the current study. Requests for anonymized data can be made to the corresponding author.
Abbreviations
- BIC:
-
Bayesian Information Criterion
- OR:
-
Odds ratio
- US:
-
United States
References
Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrøm M, Johansen M, Taskila TK, Sullivan FM, Wilson S, Jackson C, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360.
Andersen DL. A Guide To Patient Recruitment and Retention. Boston, Massachusetts: Thomson CenterWatch; 2004.
Harrer S, Shah P, Antony B, Hu J. Artificial Intelligence for Clinical Trial Design. Trends Pharmacol Sci. 2019;40(8):577–91.
Carlisle B, Kimmelman J, Ramsay T, MacKinnon N. Unsuccessful trial accrual and human subjects protections: an empirical analysis of recently closed trials. Clin Trials. 2015;12(1):77–83.
Labots G, Jones A, de Visser SJ, Rissmann R, Burggraaf J. Gender differences in clinical registration trials: is there a real problem? Br J Clin Pharmacol. 2018;84(4):700–7.
Stewart JH, Bertoni AG, Staten JL, Levine EA, Gross CP. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Ann Surg Oncol. 2007;14(12):3328–34.
Taylor JS, Ellis GR. Racial differences in responses to drug treatment: implications for pharmacotherapy of heart failure. Am J Cardiovasc Drugs. 2002;2(6):389–99.
Ramamoorthy A, Pacanowski MA, Bull J, Zhang L. Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clin Pharmacol Ther. 2015;97(3):263–73.
Sharma A, Palaniappan L. Improving diversity in medical research. Nat Rev Dis Primers. 2021;7(1):74.
Agoritsas T, Deom M, Perneger TV. Study design attributes influenced patients’ willingness to participate in clinical research: a randomized vignette-based study. J Clin Epidemiol. 2011;64(1):107–15.
Brown DR, Topcu M. Willingness to participate in clinical treatment research among older African Americans and Whites. Gerontologist. 2003;43(1):62–72.
Ding EL, Powe NR, Manson JE, Sherber NS, Braunstein JB. Sex differences in perceived risks, distrust, and willingness to participate in clinical trials: a randomized study of cardiovascular prevention trials. Arch Intern Med. 2007;167(9):905–12.
Moorcraft SY, Marriott C, Peckitt C, Cunningham D, Chau I, Starling N, Watkins D, Rao S. Patients’ willingness to participate in clinical trials and their views on aspects of cancer research: results of a prospective patient survey. Trials. 2016;17:17.
Noirmain C, Gil-Wey B, Pichon I, Brindel P, Haller G. Factors associated with patient willingness to participate in anaesthesia clinical trials: a vignette-based cross-sectional study. BMC Med Res Methodol. 2020;20(1):67.
Kong Q, Mei H, Lai Y, Shi S, Li Y, He L, Qin HY. Barriers and facilitators to participation in clinical trial among lymphoma patients from Sun Yat-sen University Cancer Center in China: An observation study. Medicine (Baltimore). 2017;96(37):e8062.
Sedrak MS, Freedman RA, Cohen HJ, Muss HB, Jatoi A, Klepin HD, Wildes TM, Le-Rademacher JG, Kimmick GG, Tew WP, et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin. 2021;71(1):78–92.
Trauth JM, Musa D, Siminoff L, Jewell IK, Ricci E. Public attitudes regarding willingness to participate in medical research studies. J Health Soc Policy. 2000;12(2):23–43.
Wood D, Kosa K, Brown D, Ehrlich OG, Higgins PDR, Heller C. Preferences of Adult Patients With Inflammatory Bowel Disease for Attributes of Clinical Trials: Evidence From a Choice-Based Conjoint Analysis. Crohn’s Colitis 360. 2019;2(1):otz048.
Cheung YK, Wood D, Zhang K, Ridenour TA, Derby L, St Onge T, Duan N, Duer-Hefele J, Davidson KW, Kronish I, et al. Personal preferences for Personalised Trials among patients with chronic diseases: an empirical Bayesian analysis of a conjoint survey. BMJ Open. 2020;10(6):e036056.
Crocker JC, Ricci-Cabello I, Parker A, Hirst JA, Chant A, Petit-Zeman S, Evans D, Rees S. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. BMJ. 2018;363:k4738.
Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, Johnson FR, Mauskopf J. Conjoint analysis applications in health–a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–13.
Eysenbach G. Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res. 2004;6(3):e34.
Syriopoulou E, Bower H, Andersson TM, Lambert PC, Rutherford MJ. Estimating the impact of a cancer diagnosis on life expectancy by socio-economic group for a range of cancer types in England. Br J Cancer. 2017;117(9):1419–26.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
WorldData.info: Average income around the world. https://www.worlddata.info/average-income.php (2022). Accessed 9 May 2022.
Heidenreich S, Phillips-Beyer A, Flamion B, Ross M, Seo J, Marsh K. Benefit-Risk or Risk-Benefit Trade-Offs? Another Look at Attribute Ordering Effects in a Pilot Choice Experiment. Patient. 2021;14(1):65–74.
RAND Corporation: 36-Item Short Form Survey (SF-36). https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html (2022). Accessed 17 Mar 2022.
Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79.
EuroQol: EQ-5D. https://euroqol.org/ (2022). Accessed 09 May 2022.
Rubright JD, Cary MS, Karlawish JH, Kim SY. Measuring how people view biomedical research: Reliability and validity analysis of the Research Attitudes Questionnaire. J Empir Res Hum Res Ethics. 2011;6(1):63–8.
Jonker MF, Donkers B, de Bekker-Grob E, Stolk EA. Attribute level overlap (and color coding) can reduce task complexity, improve choice consistency, and decrease the dropout rate in discrete choice experiments. Health Econ. 2019;28(3):350–63.
Goossens LMA, Jonker MF, Rutten-van Mölken M, Boland MRS, Slok AHM, Salomé PL, van Schayck OCP, In ’t Veen J, Stolk EA, Donkers B. The Fold-in, Fold-out Design for DCE Choice Tasks: Application to Burden of Disease. Med Decis Making. 2019;39(4):450–60.
ChoiceMetrics Pty Ltd: Ngene 1.3 User Manual and Reference Guide. http://www.choice-metrics.com/NgeneManual130.pdf (2021). Accessed 06 Sep 2022.
Profillidis VA, Botzoris GN. Chapter 5 - Statistical Methods for Transport Demand Modeling. In: Profillidis VA, Botzoris GN, editors. Modeling of Transport Demand. Elsevier; 2019. p. 163–224.
Masyn KE. Latent class analysis and finite mixture modeling. In: editors. The Oxford handbook of quantitative methods: Statistical analysis, Vol 2. New York, NY, US: Oxford University Press; 2013. p. 551–611.
Gayet-Ageron A, Rudaz S, Perneger T. Study design factors influencing patients’ willingness to participate in clinical research: a randomised vignette-based study. BMC Med Res Methodol. 2020;20(1):93.
Banks MA. Core Concept: In the wake of COVID-19, decentralized clinical trials move to center stage. Proc Natl Acad Sci U S A. 2021;118(47):e2119097118.
Goodson N, Wicks P, Morgan J, Hashem L, Callinan S, Reites J. Opportunities and counterintuitive challenges for decentralized clinical trials to broaden participant inclusion. NPJ Digit Med. 2022;5(1):58.
Quaife M, Terris-Prestholt F, Di Tanna GL, Vickerman P. How well do discrete choice experiments predict health choices? A systematic review and meta-analysis of external validity. Eur J Health Econ. 2018;19(8):1053–66.
de Bekker-Grob EW, Swait JD, Kassahun HT, Bliemer MCJ, Jonker MF, Veldwijk J, Cong K, Rose JM, Donkers B. Are Healthcare Choices Predictable? The Impact of Discrete Choice Experiment Designs and Models. Value Health. 2019;22(9):1050–62.
Acknowledgements
We thank Esraa Aldalooj, PhD (Evidera, London, UK) for assistance with the preliminary statistical analysis. Medical writing was provided by Jonathan M. Pitt, PhD (Evidera, Paris, France) and funded by Evidera.
Funding
This study was funded by Evidera.
Author information
Authors and Affiliations
Contributions
CT, SM, and KM contributed to conception and planning of the work. CT and SM contributed to acquisition of the data. NK contributed to analysis of the data. CT and SM contributed to writing the first draft of the manuscript. All authors contributed to interpretation of the data and critical revision of the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by Ethical & Independent (E&I) Review Services, a fully accredited Institutional Review Board, for central ethical approval (E&I study number: 21038–01; approved March 23, 2021). The informed consent form was reviewed by the Institutional Review Board, who provided a waiver in order to conduct the study without the need to collect signed informed consent. Nonetheless, all participants reviewed the informed consent form online and indicated their consent to participate by selecting the relevant option at the end of the online form. The study was performed in accordance with the Declaration of Helsinki and other relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
CT, SM, NK, and KM are employees of Evidera.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Thomas, C., Mulnick, S., Krucien, N. et al. How do study design features and participant characteristics influence willingness to participate in clinical trials? Results from a choice experiment. BMC Med Res Methodol 22, 323 (2022). https://doi.org/10.1186/s12874-022-01803-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12874-022-01803-6