Abstract
Background
The red cell distribution width (RDW) reflects the degree of heterogeneity of red blood cells. Elevated RDW is associated both with frailty and with increased mortality in hospital-admitted patients. In this study we evaluate whether high RDW values are associated with mortality in older emergency department (ED) patients with frailty, and if the association is independent of the degree of frailty.
Methods
We included ED patients with the following criteria: ≥ 75 years of age, Clinical Frailty Scale (CFS) score of 4 to 8, and RDW % measured within 48 h of ED admission. Patients were allocated to six classes by their RDW value: ≤ 13%, 14%, 15%, 16%, 17%, and ≥ 18%. The outcome was death within 30 days of ED admission. Crude and adjusted odds ratios (OR) with 95% confidence intervals (CI) for a one-class increase in RDW for 30-day mortality were calculated via binary logistic regression analysis. Age, gender and CFS score were considered as potential confounders.
Results
A total of 1407 patients (61.2% female), were included. The median age was 85 with an inter-quartile range (IQR) of 80–89, median CFS score 6 (IQR: 5–7), and median RDW 14 (IQR: 13–16). Of the included patients, 71.9% were admitted to hospital wards. A total of 85 patients (6.0%) died during the 30-day follow-up. Mortality rate was associated with RDW increase (p for trend < .001). Crude OR for a one-class increase in RDW for 30-day mortality was 1.32 (95% CI: 1.17–1.50, p < .001). When adjusted for age, gender and CFS-score, OR of mortality for one-class RDW increase was still 1.32 (95% CI: 1.16–1.50, p < .001).
Conclusion
Higher RDW values had a significant association with increased 30-day mortality risk in frail older adults in the ED, and this risk was independent of degree of frailty. RDW is a readily available biomarker for most ED patients. It might be beneficial to include it in risk stratification of older frail ED patients to identify those who could benefit from further diagnostic assessment, targeted interventions, and care planning.
Similar content being viewed by others
Background
Red cell distribution width (RDW) is a measure reflecting the degree of heterogeneity of red blood cell size. RDW is calculated by dividing the standard deviation of red blood cell volumes by the mean corpuscular volume (MCV), usually expressed as a percentage value. RDW has traditionally been used for differential diagnosis of anaemia, but, subsequently, RDW elevation has been found to associate with higher short- and long-term increased mortality, both in the general population and in patients with many specific conditions [1,2,3,4,5], such as infections and sepsis [6,7,8], liver cirrhosis [9, 10], diabetic ketoacidosis [11], trauma [12, 13], acute pancreatitis [14], cardiac diseases [8, 15,16,17,18,19], pulmonary embolism [20], COVID-19, and acute respiratory failure [21,22,23]. In hospitals, high RDW has been shown to predict poor prognosis among general patients [24], among surgical patients [25], and among patients with critical illness [26, 27]. Older patients in emergency departments (EDs) and hospital wards have an increased risk of mortality if their RDW is elevated [8, 12, 28,29,30]. Although several mechanisms for this association have been presented [1, 2, 31], defined mechanisms for the association of elevated RDW and increased mortality have not yet been established.
Frailty syndrome, an ageing-related state of vulnerability due to decline in physiological reserves and functions [32], is usually defined either as a clinical phenotype or by calculating accumulated deficits such as diseases, physical and cognitive impairments, psychosocial risks, and geriatric syndromes [33,34,35]. Frailty is an independent predictor of mortality in patients admitted to emergency departments and hospital wards [36,37,38]. Elevated RDW has been shown to associate with frailty besides increased mortality of older ED patients [39,40,41].
Because frailty is related with both increased mortality of older ED patients and elevated RDW, frailty may be a confounder explaining increased mortality of older patients with elevated RDW. We studied whether elevated RDW is a risk predictor in older patients with frailty in the ED, and how frailty stage affects the association between elevated RDW and mortality.
Methods
This study is a secondary analysis of an observational cohort study in frail older ED patients that was performed in an ED of a teaching hospital in Finland. In the primary study we included patients who were ≥ 75 years of age, had a score between 4 to 9 on the 9-point Clinical Frailty Scale (CFS) [34], and were registered residents of the hospital’s service area. ED visit data were collected between December 11th, 2018 and June 7th, 2019. The included patients were followed up from electronic health records. Methods for the primary study have been described in detail in our previous article [42].
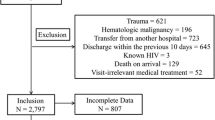
The clinical laboratory service of the ED routinely gives RDW values (% value as integer) for all blood counts tested. Besides the clinical laboratory service, the ED has point-of-care testing equipment available, which does not provide RDW values. Point-of care testing is typically preferred, if more extensive laboratory testing is not anticipated based on patient’s chief complaint or condition. For the secondary analysis conducted here, those patient visits from the primary study who had the CFS score 4–8 and had RDW tested 0–48 h after ED admission were included. If more than one blood count was drawn from a patient within 48 h of ED admission, the result of the first laboratory test was used for the analysis. Patients who had a CFS score of 9 were excluded because such patients are defined as having a short life expectancy < 6 months, but otherwise not living with severe frailty.
Nonparametric baseline data were presented with interquartile ranges (IQR). The outcome measure was 30-day mortality. Patients were allocated to six classes based on their RDW value: ≤ 13%, 14%, 15%, 16%, 17%, and ≥ 18%. We used same cut-off values as a recent study to enable comparison of our results in frail ED patients to general older adult ED patient population [42]. Mortality rate was calculated for each class. The Cochran–Armitage test for trend was used to test the statistical significance of the trend of increasing mortality with higher RDW values.
Crude and adjusted ORs with 95% confidence intervals (CI) of a one-class increase in RDW for 30-day mortality were calculated. Univariate and multivariate models of binary logistic regression analysis were used for crude and adjusted ORs, respectively. Age, sex, and CFS score were considered as potential confounders and were included in the analysis.
As a sensitivity analysis to assess if categorisation of the RDW values has impact on the results, we performed a regression analysis with RDW as continuous variable. We also performed a sensitivity analysis with haemoglobin as a potential confounder, because haemoglobin level is directly related to red blood cells, like RDW is, and may be associated with mortality.
From clinical perspective, we were interested whether RDW is independent of vital parameters. The National Early Warning Score 2 (NEWS2), a widely used prognostic score based on common vital signs, was included in the baseline data for our previous study [42]. We performed an additional testing by adjusting with the NEWS2 besides other potential confounders used in the regression analysis.
A p value of < 0.05 was considered statistically significant. GraphPad Prism software, version 9.4.1 (Graphpad Software LCC) was used for the Cochran–Armitage test. SPSS software, version 28 (IBM) was used for all other statistical analyses.
The primary study which this secondary analysis was based on, was registered at ClinicalTrials.gov on December 20th, 2018, identifier NCT03783234.
Results
A total of 1407 (61.2% female) patient visits were included after excluding 294 visits for patients who either had no blood count drawn within 48 h of ED admission or had only point-of-care blood count testing without RDW-values, as well as four cases for patients with dual peak RDW values (due to previous red cell transfusions), and seven cases for patients with a CFS score of 9. Patient characteristics for the analytical sample are presented in Table 1: median age was 85 (IQR: 80–89), median CFS was 6 (IQR: 5–7), and median RDW % was 14 (IQR: 13–16, range: 12–28). Distribution of the RDW % values in the analytical sample are presented in Fig. 1. Of the included patients, 1011 (71.9%) were admitted to hospital wards.
Follow-up data for 30-day mortality were available for all ED visits. A total of 85 of 1407 (6.0%) of included patients with RDW value available died during the 30-day follow-up. Within 30 days of ED admission, mortality rates were as follows: 9/412 (2.2%) of patients in the RDW ≤ 13% group, 19/358 (5.3%) in the RDW 14% group, 21/262 (8.0%) in the RDW 15% group, 14/149 (9.4%) in the RDW 16% group, 5/80 (6.3%) in the RDW 17% group, and 17/146 (11.6%) in the RDW ≥ 18 group. Mortality rate was significantly higher with an increase in RDW (p for trend < 0.001). Mortality rates are presented in Fig. 2. For comparison, 30-day mortality of excluded patients who had no RDW value available was 8/298 (2.7%).
Crude OR of a one-class increase in RDW for 30-day mortality was 1.32 (95% CI: 1.17–1.50, p < 0.001). When adjusted for age, sex and CFS score, OR of a one-class increase was still 1.32 (95% CI: 1.16–1.50, p < 0.001). Crude and adjusted odds ratios are presented in Table 2.
In the sensitivity analysis with RDW as a continuous variable the significance of the results were not changed. Crude and adjusted ORs of 1%-unit increase of RDW for 30-day mortality were: 1.15 (95% CI: 1.06–1.24, p < 0.001), and 1.15 (95% CI: 1.07–1.25, p < 0.001), respectively. The absolute OR values were expectedly lower as scale increased from 6 categorical steps to 16 steps in %-units (range of RDW, 12–28). When haemoglobin level was added as a potential confounder, the adjusted OR of one-class increase in RDW was 1.34 (95% CI: 1.17–1.54, p < 0.001), without significant change in results.
When NEWS2 was added as a confounder, the adjusted OR of one-class increase of RDW for 30-day mortality was slightly lower than without it, but still significant: 1.27 (95%: 1.11–1.47, p < 0.001).
Discussion
In this study, increasing RDW was associated with higher 30-day mortality in frail older ED patients. The association remained significant when adjusted for age, gender and CFS score.
This study shows that the association of higher RDW value and increased mortality applies to the frail older population in an acute care setting. The association is independent of CFS score. The mortality rate increase is similar to those rates shown in a recent large cohort study of general hospital-admitted older patients [29], supporting the hypothesis that RDW is independent of frailty as a risk predictor. A small notch in mortality rate was noted in the group of patients with RDW of 17%. However, since the total trend was statistically significant, we interpret this dip to be variation due to limited sample size.
Many mechanisms, both short- and long-term, have been suggested for the association of elevated RDW and increased mortality. Impaired erythropoiesis and shortened red cell survival due to organ dysfunction, metabolic imbalances, and inflammatory reactions may cause alterations in red cell volumes. Oxidative stress and suppression of the erythrocyte lineage due to alterations in neutrophil and thrombocyte production during inflammation in acute conditions are potential contributors. Other possible causes of higher RDW include poor nutrition and erythrocyte fragmentation [1, 2, 31]. In addition, direct causality of high RDW and impaired intravascular haemodynamics, especially with vascular pathologies, has been presented [31]. Telomere shortening may be a link for poor outcomes in older vulnerable patients, as this is associated with both MCV variation and ageing-related all-cause mortality [43, 44]. Association of RDW increase with mortality was slightly lower, when NEWS2 was added as a potential confounder, which supports that elevated RDW is reflecting both short-, and long-term clinical deterioration.
RDW is a readily available biomarker for most ED patients. In clinical practice, RDW may be overlooked as a marker when clinical state and risks are assessed. Including RDW in patient assessment could lead to better high-risk feature identification, better targeting of further diagnostic work-up, effective interventions, and individualized advanced care planning.
Current risk-assessment methods, including ED triage systems, have limited performance, especially in older adults [45,46,47]. Machine-learning systems are promising tools for objective and more accurate risk assessment in emergency care, and may help in identifying patients who would benefit from targeted interventions [48,49,50]. In this study we have considered RDW as a general predictor. As higher RDW predicts poor outcomes in numerous different conditions, RDW may be associated with other predictive biomarkers in different specific conditions. Independence of RDW in multivariable predictive models could be studied preferably with machine learning methods with large data sets, as many predictive variables, including haemoglobin, white blood cell, and platelet counts, may have nonlinear associations.
Kim et al. stated in their article that RDW value should be included in risk stratification strategies for hospitalized older patients [29]. Based on earlier studies and our results, we agree with those authors and suggest that RDW should also be included in risk stratification of frail patients in emergency departments and hospital wards. Older patients often have non-specific presentations in the ED, and vital signs are less reliable for detecting early clinical deterioration in older patients [51,52,53]. Therefore, older patients, with or without frailty, could be one patient group that would benefit in particular from more comprehensive deep-learning risk-assessment methods. It may be favourable to include RDW among other variables when such artificial intelligence models are studied.
The strengths of this study include the prospectively collected patient data from a clinical setting, that is representative for the frail older ED patient population. Frailty status was assessed systematically with the CFS during ED admission. Baseline and outcome data were available for all patients included.
The study has some limitations. The analysis of this study was based on data collected in a previous prospective study. In this study, 82% of patients who met the eligibility criteria, had an RDW value available. The included patients had higher mortality than patients who had no laboratory testing, or only point-of-care testing available. Chief complaints or acute disease severity were not included in the analyses, but we assume that those patients without blood tests taken were more likely visiting the ED for simple, low-acuity complaints, and therefore the results may not be representative for low-acuity patients.
Conclusion
Higher RDW values were significantly associated with increased 30-day mortality in frail older adults in the ED. In this study, RDW was independent of frailty state as a risk predictor. RDW is a readily available parameter for most ED patients who have laboratory tests. It might be beneficial to include RDW in risk stratification of older frail ED patients in order to identify patients at high risk of adverse outcomes who could benefit from further diagnostic assessment, targeted interventions, and care planning.
Availability of data and materials
The data are not publicly available or available for sharing due to national juridical restrictions. However, further description or analysis of data are available from the authors upon reasonable request. For inquiries, please contact the corresponding author.
Abbreviations
- RDW:
-
Red cell distribution width
- MCV:
-
Mean corpuscular volume (of red blood cells)
- ED:
-
Emergency department
- CFS:
-
Clinical Frailty Scale
- IQR:
-
Interquartile range
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- NEWS2:
-
National Early Warning Score 2
References
Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86–105. https://doi.org/10.3109/10408363.2014.992064.
Pan J, Borné Y, Engström G. The relationship between red cell distribution width and all-cause and cause-specific mortality in a general population. Sci Rep. 2019;9(1):16208. https://doi.org/10.1038/s41598-019-52708-2.
Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169(5):515–23. https://doi.org/10.1001/archinternmed.2009.11.
Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;169(6):588–94. https://doi.org/10.1001/archinternmed.2009.55.
Pilling LC, Atkins JL, Kuchel GA, Ferrucci L, Melzer D. Red cell distribution width and common disease onsets in 240,477 healthy volunteers followed for up to 9 years. PLoS One. 2018;13(9):e0203504. https://doi.org/10.1371/journal.pone.0203504.
Kim SY, Woo SH, Lee WJ, Kim DH, Seol SH, Lee JY, et al. The qSOFA score combined with the initial red cell distribution width as a useful predictor of 30 day mortality among older adults with infection in an emergency department. Aging Clin Exp Res. 2021;33(6):1619–25. https://doi.org/10.1007/s40520-020-01738-2.
Lee JH, Chung HJ, Kim K, Jo YH, Rhee JE, Kim YJ, et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am J Emerg Med. 2013;31(1):72–9. https://doi.org/10.1016/j.ajem.2012.06.004.
Nakashima K, Ohgami E, Kato K, Yoshitomi S, Maruyama T, Harada M. Prognostic significance of red cell distribution width in hospitalized older patients with heart failure or infection. Geriatr Gerontol Int. 2019;19(10):988–92. https://doi.org/10.1111/ggi.13755.
Abdel-Samiee M, Ahmed SamyKohla M, FathyRagab M, Ghanem HS, KamelAbouGabal A, Abdelsameea E. Red cell distribution width as a predictor of outcome in hospitalized cirrhotic patients. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1):e978–85. https://doi.org/10.1097/MEG.0000000000002337.
Turcato G, Campagnaro T, Bonora A, Vignola N, Salvagno GL, Cervellin G, et al. Red blood cell distribution width independently predicts 1-month mortality in acute decompensation of cirrhotic patients admitted to emergency department. Eur J Gastroenterol Hepatol. 2018;30(1):33–8. https://doi.org/10.1097/MEG.0000000000000993.
Dai H, Su X, Li H, Zhu L. Association between red blood cell distribution width and mortality in diabetic ketoacidosis. J Int Med Res. 2020;48(3):300060520911494. https://doi.org/10.1177/0300060520911494.
Marom O, Paz I, Topaz G, Ohana N, Yaacobi E. Red cell distribution width-A mortality predictor in older adults with proximal femoral fracture. Arch Gerontol Geriatr. 2022;100:104623. https://doi.org/10.1016/j.archger.2022.104623.
Sakai Y, Wakao N, Matsui H, Watanabe T, Iida H, Katsumi A. Elevated red blood cell distribution width is associated with poor outcome in osteoporotic vertebral fracture. J Bone Miner Metab. 2021;39(6):1048–57. https://doi.org/10.1007/s00774-021-01242-1.
Şenol K, Saylam B, Kocaay F, Tez M. Red cell distribution width as a predictor of mortality in acute pancreatitis. Am J Emerg Med. 2013;31(4):687–9. https://doi.org/10.1016/j.ajem.2012.12.015.
Peng Y, Guan X, Wang J, Ma J. Red cell distribution width is correlated with all-cause mortality of patients in the coronary care unit. J Int Med Res. 2020;48(7):300060520941317. https://doi.org/10.1177/0300060520941317.
Huang S, Zhou Q, Guo N, Zhang Z, Luo L, Luo Y, et al. Association between red blood cell distribution width and in-hospital mortality in acute myocardial infarction. Medicine (Baltimore). 2021;100(15):e25404. https://doi.org/10.1097/MD.0000000000025404.
Wang Z, Chen X, He S. Prognostic value of red blood cell distribution width for mortality in patients with hypertrophic cardiomyopathy. Clin Biochem. 2021;96:19–25. https://doi.org/10.1016/j.clinbiochem.2021.07.002.
Zhang X, Wang Y, Chen N, Liu Y, Xiao J, Lin Z, et al. Red cell distribution width is associated with short-term mortality in critically ill patients with heart failure. ESC Heart Fail. 2022. https://doi.org/10.1002/ehf2.14023.
Szekely Y, Finkelstein A, Bazan S, Halkin A, Abbas Younis M, Erez J, et al. Red blood cell distribution width as a prognostic factor in patients undergoing transcatheter aortic valve implantation. J Cardiol. 2019;74(3):212–6. https://doi.org/10.1016/j.jjcc.2019.04.005.
Zhou XY, Chen HL, Ni SS. Red cell distribution width in predicting 30-day mortality in patients with pulmonary embolism. J Crit Care. 2017;37:197–201. https://doi.org/10.1016/j.jcrc.2016.09.024.
Zhang W, Wang Y, Wang J, Wang S. Association between red blood cell distribution width and long-term mortality in acute respiratory failure patients. Sci Rep. 2020;10(1):21185. https://doi.org/10.1038/s41598-020-78321-2.
Banon T, Wortsman J, Ben Moshe S, Gazit S, Peretz A, Ben Tov A, et al. Evaluating red blood cell distribution width from community blood tests as a predictor of hospitalization and mortality in adults with SARS-CoV-2: a cohort study. Ann Med. 2021;53(1):1410–8. https://doi.org/10.1080/07853890.2021.1968484.
Lorente L, Martín MM, Argueso M, Solé-Violán J, Perez A, Marcos YRJA, et al. Association between red blood cell distribution width and mortality of COVID-19 patients. Anaesth Crit Care Pain Med. 2021;40(1):100777. https://doi.org/10.1016/j.accpm.2020.10.013.
Hunziker S, Stevens J, Howell MD. Red cell distribution width and mortality in newly hospitalized patients. Am J Med. 2012;125(3):283–91. https://doi.org/10.1016/j.amjmed.2011.08.021.doi:10.1016/j.amjmed.2011.08.021.
Abdullah HR, Sim YE, Sim YT, Ang AL, Chan YH, Richards T, et al. Preoperative Red Cell Distribution Width and 30-day mortality in older patients undergoing non-cardiac surgery: a retrospective cohort observational study. Sci Rep. 2018;8(1):6226. https://doi.org/10.1038/s41598-018-24556-z.
Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39(8):1913–21. https://doi.org/10.1097/CCM.0b013e31821b85c6.
Jia L, Cui S, Yang J, Jia Q, Hao L, Jia R, et al. Red blood cell distribution width predicts long-term mortality in critically ill patients with acute kidney injury: a retrospective database study. Sci Rep. 2020;10(1):4563. https://doi.org/10.1038/s41598-020-61516-y.
Kim SH, Yeon JH, Park KN, Oh SH, Choi SP, Kim YM, et al. The association of Red cell distribution width and in-hospital mortality in older adults admitted to the emergency department. Scand J Trauma Resusc Emerg Med. 2016;24:81. https://doi.org/10.1186/s13049-016-0274-8.
Kim KM, Nerlekar R, Tranah GJ, Browner WS, Cummings SR. Higher red cell distribution width and poorer hospitalization-related outcomes in elderly patients. J Am Geriatr Soc. 2022;70(8):2354–62. https://doi.org/10.1111/jgs.17819.
Wang AY, Ma HP, Kao WF, Tsai SH, Chang CK. Red blood cell distribution width is associated with mortality in elderly patients with sepsis. Am J Emerg Med. 2018;36(6):949–53. https://doi.org/10.1016/j.ajem.2017.10.056.
Ananthaseshan S, Bojakowski K, Sacharczuk M, Poznanski P, Skiba DS, PrahlWittberg L, et al. Red blood cell distribution width is associated with increased interactions of blood cells with vascular wall. Sci Rep. 2022;12(1):13676. https://doi.org/10.1038/s41598-022-17847-z.
Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–75. https://doi.org/10.1016/S0140-6736(19)31786-6.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. https://doi.org/10.1093/gerona/56.3.m146.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. https://doi.org/10.1503/cmaj.050051.
Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. https://doi.org/10.1016/j.cger.2010.08.009.
Romero-Ortuno R, Wallis S, Biram R, Keevil V. Clinical frailty adds to acute illness severity in predicting mortality in hospitalized older adults: An observational study. Eur J Intern Med. 2016;35:24–34. https://doi.org/10.1016/j.ejim.2016.08.033.
Rueegg M, Nissen SK, Brabrand M, Kaeppeli T, Dreher T, Carpenter CR, et al. The clinical frailty scale predicts 1-year mortality in emergency department patients aged 65 years and older. Acad Emerg Med. 2022;29(5):572–80. https://doi.org/10.1111/acem.14460.
Lee JH, Park YS, Kim MJ, Shin HJ, Roh YH, Kim JH, et al. Clinical Frailty Scale as a predictor of short-term mortality: a systematic review and meta-analysis of studies on diagnostic test accuracy. Acad Emerg Med. 2022. https://doi.org/10.1111/acem.14493.
Li CM, Chao CT, Chen SI, Han DS, Huang KC. Elevated red cell distribution width is independently associated with a higher frailty risk among 2,932 community-dwelling older adults. Front Med (Lausanne). 2020;7:470. https://doi.org/10.3389/fmed.2020.00470.
Kim KM, Lui LY, Browner WS, Cauley JA, Ensrud KE, Kado DM, et al. Association Between Variation in Red Cell Size and Multiple Aging-Related Outcomes. J Gerontol A Biol Sci Med Sci. 2021;76(7):1288–94. https://doi.org/10.1093/gerona/glaa217.
Li Q, Chen X, Han B. Red blood cell distribution width is associated with frailty in older inpatients in China: Sex differences in a cross-sectional study. Exp Gerontol. 2021;150:111392. https://doi.org/10.1016/j.exger.2021.111392.
Kemp K, Alakare J, Harjola VP, Strandberg T, Tolonen J, Lehtonen L, et al. National Early Warning Score 2 (NEWS2) and 3-level triage scale as risk predictors in frail older adults in the emergency department. BMC Emerg Med. 2020;20(1):83. https://doi.org/10.1186/s12873-020-00379-y.
Kozlitina J, Garcia CK. Red blood cell size is inversely associated with leukocyte telomere length in a large multi-ethnic population. PLoS One. 2012;7(12):e51046. https://doi.org/10.1371/journal.pone.0051046.
Wang Q, Zhan Y, Pedersen NL, Fang F, Hägg S. Telomere length and all-cause mortality: a meta-analysis. Ageing Res Rev. 2018;48:11–20. https://doi.org/10.1016/j.arr.2018.09.002.
Zachariasse JM, van der Hagen V, Seiger N, Mackway-Jones K, van Veen M, Moll HA. Performance of triage systems in emergency care: a systematic review and meta-analysis. BMJ Open. 2019;9(5):e026471. https://doi.org/10.1136/bmjopen-2018-026471.
Brouns SHA, Mignot-Evers L, Derkx F, Lambooij SL, Dieleman JP, Haak HR. Performance of the Manchester triage system in older emergency department patients: a retrospective cohort study. BMC Emerg Med. 2019;19(1):3. https://doi.org/10.1186/s12873-018-0217-y.
Grossmann FF, Zumbrunn T, Frauchiger A, Delport K, Bingisser R, Nickel CH. At risk of undertriage? Testing the performance and accuracy of the emergency severity index in older emergency department patients. Ann Emerg Med. 2012;60(3):317-25.e3. https://doi.org/10.1016/j.annemergmed.2011.12.013.
Grant K, McParland A, Mehta S, Ackery AD. Artificial Intelligence in Emergency Medicine: Surmountable Barriers With Revolutionary Potential. Ann Emerg Med. 2020;75(6):721–6. https://doi.org/10.1016/j.annemergmed.2019.12.024.
Kirubarajan A, Taher A, Khan S, Masood S. Artificial intelligence in emergency medicine: A scoping review. J Am Coll Emerg Physicians Open. 2020;1(6):1691–702. https://doi.org/10.1002/emp2.12277.
Ehrlich H, McKenney M, Elkbuli A. The niche of artificial intelligence in trauma and emergency medicine. Am J Emerg Med. 2021;45:669–70. https://doi.org/10.1016/j.ajem.2020.10.050.
Nemec M, Koller MT, Nickel CH, Maile S, Winterhalder C, Karrer C, et al. Patients presenting to the emergency department with non-specific complaints: the Basel Non-specific Complaints (BANC) study. Acad Emerg Med. 2010;17(3):284–92. https://doi.org/10.1111/j.1553-2712.2009.00658.x.
Nissen SK, Candel BGJ, Nickel CH, de Jonge E, Ryg J, Bogh SB, et al. The Impact of Age on Predictive Performance of National Early Warning Score at Arrival to Emergency Departments: Development and External Validation. Ann Emerg Med. 2022;79(4):354–63. https://doi.org/10.1016/j.annemergmed.2021.09.434.
Pirneskoski J, Lääperi M, Kuisma M, Olkkola KT, Nurmi J. Ability of prehospital NEWS to predict 1-day and 7-day mortality is reduced in older adult patients. Emerg Med J. 2021;38(12):913–8. https://doi.org/10.1136/emermed-2019-209400.
Acknowledgements
We thank Susanne Lehvonen for helping in data acquisition, Elina Lehtelä for data processing, Robert Davis for English language editing, and the personnel of Jorvi ED for their collaboration.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. This study was supported by state funding for university-level health research, granted by Helsinki University Hospital, and by the Department of Emergency Medicine and Services, Helsinki University Hospital. JA and KK were supported by personal grants for the study from the Finnish Association for Emergency Medicine. JA was also supported by a grant from the Finnish Medical Foundation. The funding bodies played no role in the design of the study, data collection, data analysis, or interpretation of data, or in writing of the manuscript.
Author information
Authors and Affiliations
Contributions
The author contributions for this study are as follows: study design: JA, KK, TS, MC, JT, VH; data acquisition: JA, KK, MC, VH; analysis: JA, KK, TS, MC, JT, VH; writing – original draft: JA; writing – review and editing: JA, KK, TS, MC, JT, VH. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. The study protocol and data acquisition were approved by the Ethics Committee II of Helsinki University Hospital (reference number HUS/1171/2018). The Ethics Committee II of Helsinki University Hospital waived the need of consent from patients for the observational data in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alakare, J., Kemp, K., Strandberg, T. et al. Red cell distribution width and mortality in older patients with frailty in the emergency department. BMC Emerg Med 23, 24 (2023). https://doi.org/10.1186/s12873-023-00801-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12873-023-00801-1