Abstract
Objective
We aimed to evaluate door-to-puncture time (DPT) and door-to-recanalization time (DRT) without directing healthcare by neuro-interventionalist support in the emergency department (ED) by workflow optimization and improving patients’ outcomes.
Methods
Records of 98 consecutive ischemic stroke patients who had undergone endovascular therapy (EVT) between 2018 to 2021 were retrospectively reviewed in a single-center study. Patients were divided into three groups: pre-intervention (2018–2019), interim-intervention (2020), and post-intervention (January 1st 2021 to August 16th, 2021). We compared door-to-puncture time, door-to-recanalization time (DRT), puncture-to-recanalization time (PRT), last known normal time to-puncture time (LKNPT), and patient outcomes (measured by 3 months modified Rankin Scale) between three groups using descriptive statistics.
Results
Our findings indicate that process optimization measures could shorten DPT, DRT, PRT, and LKNPT. Median LKNPT was shortened by 70 min from 325 to 255 min(P < 0.05), and DPT was shortened by 119 min from 237 to 118 min. DRT shortened by 132 min from 338 to 206 min, and PRT shortened by 33 min from 92 to 59 min from the pre-intervention to post-intervention groups (all P < 0.05). Only 21.4% of patients had a favorable outcome in the pre-intervention group as compared to 55.6% in the interventional group (P= 0.026).
Conclusion
This study demonstrated that multidisciplinary cooperation was associated with shortened DPT, DRT, PRT, and LKNPT despite challenges posed to the healthcare system such as the COVID-19 pandemic. These practice paradigms may be transported to other stroke centers and healthcare providers to improve endovascular time metrics and patient outcomes.
Similar content being viewed by others
Introduction
Stroke affects one-fifth of the world’s population and is the leading cause of mortality in China [1]. Endovascular thrombectomy has been proven to reduce disability in ischemic stroke patients with large vessel occlusion when performed within 6 h, or in selected patients up to 24 h post-stroke onset [2]. More favorable patient outcomes are observed when shorter delays in pre-hospital care and cumulative time from symptom recognition to treatment [3, 4]. Endovascular treatment has also been associated with a lower risk of complications, including symptomatic intracranial hemorrhage (sICH), achieving discharge independent walking, and lower in-hospital death or hospice discharge when patients are treated soon after ictus [3, 4]. Current guidelines strongly recommend providers effectively shorten intraarterial therapy time for patients with ischemic stroke to improve patient outcomes and explore process improvement initiatives to optimize patient throughput [5]. One study has demonstrated that the direct involvement of neuro-interventionalists in the emergency department (ED) could shorten the door-to-puncture time (DPT) from 167.2 ± 54.3 min to 135.2 ± 50.0 min (P = 0.040) [6]. Another study showed that multidisciplinary cooperation with regular training and debriefing might also shorten the door-to-needle time (DNT) even during the COVID-19 pandemic [7]. Our Foshan Sanshui District People’s hospital is the only comprehensive tertiary hospital and national stroke center that serves more than 0.8 million people, providing intravenous (IV) thrombolysis and endovascular therapy for acute ischemic stroke patients. Due to staffing availability, our neuro-interventionalists do not respond to the ED for stroke codes. With that in mind, we aimed to shorten the DPT and door-to-recanalization time (DRT) without the involvement of neuro-interventionalist support in the ED through nursing and provider education, process optimization, and faster facilitation of transfer of patients between departments.
Methods
Design and setting
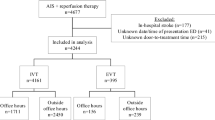
This study included the retrospective analysis of prospectively collected data from 98 consecutive ischemic stroke patients who underwent endovascular therapy from 2018 to 2021 in a single-center study in Foshan Sanshui District People’s Hospital in China. We compared time to interventions across three patient groups according to timing of intervention: pre-intervention (2018–2019; n = 14), interim-intervention (2020; n = 39), and post-intervention (January 1st 2021 to August 16th, 2021; n = 45). Inclusion criteria were as follows: age ≥ 18 years old; admitting diagnosis of acute ischemic stroke due to an acute occlusion of the internal carotid artery, M1 or M2 segments of the middle cerebral artery, or basilar artery; stroke onset or last known well within 24 h of thrombectomy. The hospital institutional review board approved the study protocol Informed consent was waived due to the nature of a retrospective observational study.
Data collection
For all patients included in this study, we recorded the following demographics and information: age, sex, past medical history of hypertension, atrial fibrillation (AF), diabetes mellitus (DM), chronic kidney disease (CKD), coronary heart disease (CAD), dyslipidemia, history of stroke, and smoking status. Neurologists measured and recorded the National Institute of Health Stroke Scale (NIHSS), Pre- endovascular therapy (EVT) Alberta Stroke Program Early CT Score (ASPECTS), initial premorbid modified Rankin Scale (mRS), Trial of ORG 10,172 in Acute Stroke Treatment (TOAST) stroke classification, and treatment with IV thrombolysis. DPT, DRT, puncture-to-recanalization time (PRT), and last known normal-to-puncture time (LKNPT) were collected. Three-month mRS scores were evaluated by routine follow-up.
Interventions
Potential improvement points were identified in our hospital by multiple discussions and meetings with medical colleagues, the hospital chief and staff. Table 1 summarizes improvement measures implemented. Each measure was introduced and implemented during the interim-intervention period.
Outcome measurements
The modified Treatment In Cerebral Infarction (mTICI) score was used to assess the recanalization rate [8]. Successful recanalization was defined as TICI 2b to 3. Modified Rankin scale (mRS) scores were determined by phone calls or in-person outpatient appointments and used to assess patient outcomes at 90 days, which was collected by a trained and dedicated stroke nurse navigator following the implementation period, as required for certification of a national stroke center [9]. The favorable outcome was defined as mRS 0–2 at 90 days. Symptomatic intracranial hemorrhage (sICH) was defined by The Heidelberg Bleeding Classification as a new intracranial hemorrhage associated with ≥ 4-point worsening in NIHSS, or ≥ 2-point worsening in a single NIHSS item—neither of which would be attributed to a process other than the hemorrhage [10].
Statistical analyses
The non-parametric Mann–Whitney U test was performed using IBM SPSS version 23 (IBM-Armonk, NY) to analyze non-normally distributed continuous data, reported as medians along with the interquartile range (IQR). Normally distributed data are reported as means with corresponding standard deviations (SD) and compared using the student’s t-test. Results were considered statistically significant if the P-value was less than 0.05. No adjustments were made for multiple hypotheses testing. The results were reported using the STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) guidelines [11].
Results
There were 98 patients evaluated during the study period who were included in the final analysis. There were no statistically significant differences regarding age, sex, cerebrovascular risk factors, mRS pre-treatment, pre-treatment ASPECTS, and IV thrombolysis of study participants between pre-intervention, interim-intervention, and post-intervention groups (Table 2). Admission NIHSS (IQR) of study participants between pre-intervention, interim-intervention and post-intervention groups were 19.0 (11.0, 21.0), 14.0 (11.0, 18.0), and 17.0 (14.0, 21.0) respectively (P = 0.026). There was a significant distribution in stroke mechanisms between the study periods based on TOAST definition (P = 0.028; Table 2).
Post-intervention measures, such as the median LKNPT was shorter post-intervention (255 vs. 325 min, P< 0.05; Table 3, Fig. 1). Similarly, DPT, DRT, and PRT were all significantly (P < 0.05) shorter in the post-intervention period versus pre-intervention period (118 vs. 237 min; 206 vs. 338 min; and PRT 59 vs. 92 min, respectively).
The target goal of DPT ≤ 120 min is illustrated in Table 4 and Fig. 2. The target goal was statistically significant (P = 0.006) and showed consistent improvement.
The target goal of DPT ≤ 120 min improved from 7.1% in 2018–2019 to 33.3% in 2020, and 53.30% in 2021 in the post-intervention period (P = 0.006).
No statistically significant difference was observed concerning the rate of pneumonia, TICI post ≥ 2b, mRS at discharge, inpatient mortality or hospice discharge, and patient mortality at three months. In the post-intervention group, 55.6% had a favorable outcome, and only 21.4% had a favorable outcome measured at 3 months (P = 0.026) in the pre-intervention group (Table 5).
Ninety day outcomes according to the interval of DPT are summarized in Table 6, indicating a non-significant trend toward better outcomes among patients who achieved a DPT of 120 min or less, when compared to patients with a DPT of > 180 min.
Discussion
Our findings in this study indicate that process optimization measures can successfully be implemented to shorten DPT, DRT, PRT, and LKNPT according to available hospital resources. We observed significant improvements in both arrivals to arterial puncture as well as the PRT during the study period. An increase in achieving a 90-day favorable outcome (mRS score of 0 to 2) was also observed, with favorable outcomes non-significantly more common among patients with shorter DPT, as has been shown in prior studies [3, 4].
The most recent American Stroke Association (ASA) guidelines recommends a goal for door-to-endovascular treatment time being restricted to within 120 min of stroke-onset [12]. Following the ASA recommendations, our center successfully improved the deadline of DPT ≤ 120 min from 7.1% in 2018–2019 to 33.3% in 2020 and 53.30% in the 2021 post-intervention period (P = 0.006). With every minute counting to manage such cases, a 90-min DPT for receiving endovascular treatment is considered for optimal management [13]. A recent study also demonstrated there were no significant differences in long-term thrombectomy outcomes among proximal anterior circulation patients who were selected based on non-contrast CT compared as compared to those selected with CTP or MRI in the extended window of 6 to 24 h [14]. Therefore, when possible, the patients may be selected without advanced or additional imaging beyond the CT in order to shorter DPT. Delays in stroke care and reperfusion treatment were a global challenge during the COVID-19 pandemic, corresponding to a global decline in the volume of stroke hospitalizations during the COVID-19 period [15,16,17,18]. The Society of Vascular and Interventional Neurology (SVIN) provided a formal guidance statement for recalibrating stroke workflow to protect frontline healthcare workers, their families and colleagues, with individualization of stroke treatment according to patient needs during the COVID-19 pandemic [19].
Strategies to optimize DPT, DRT, PRT, and LKNPT are critical to improve patient outcomes. Once acute stroke patients arrive at the hospital, resources must be allocated to rapidly identify patients with suspected ischemic stroke and intracranial occlusion, and mobilize personnel in order to treat using endovascular interventions. These processes necessitate the involvement of pre-hospital transfer services alongside ED personnel, neurologists, nurses, radiologists, interventionalists, and the hospital administration department. Only by involving each of these stakeholders can the most effective treatment be provided in the timeliest manner.
Our study has some limitations. The study is a retrospective study in a single hospital with a small data sample size. Prospective multicenter studies and larger sample data sizes are required to analyze shortened DRT and patient outcomes. Despite these limitations, we believe accomplishments at our center can provide a framework for other stroke centers to improve their patient outcomes.
Conclusions
This study demonstrated that multidisciplinary cooperation could shorten the time to endovascular treatment, with the potential to improve long-term patient outcomes. We call on stroke centers and healthcare providers to internally review their local paradigms to evaluate where improvements can be made to safely expedite care.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to the non-disclosure agreement in institutional review board restrictions. Availability of data and materials were available by contacting corresponding authors on reasonable request.
Abbreviations
- AF:
-
Atrial fibrillation
- ASPECTS:
-
Alberta stroke program early CT score
- ASA:
-
American stroke association
- CAD:
-
Coronary heart disease
- CE:
-
Cardioembolism
- CKD:
-
Chronic kidney disease
- DM:
-
Diabetes mellitus
- ED:
-
Emergency department
- EVT:
-
Endovascular therapy
- DNT:
-
Door-to-needle time
- DPT:
-
Door-to-puncture
- DRT:
-
Door-to-recanalization time
- ICA:
-
Internal carotid artery
- IQR:
-
Interquartile range
- IV:
-
Intravenous
- LAA:
-
Large-artery atherosclerosis
- LKNPT:
-
Last known normal time to-puncture time
- mRS:
-
Modified Rankin Scale
- mTICI:
-
Modified treatment in cerebral infarction
- NIHSS:
-
National Institute of Health Stroke Scale
- PRT:
-
Puncture-to-recanalization time
- SD:
-
Standard deviations
- sICH:
-
Symptomatic intracranial hemorrhage
- SOE:
-
Stroke of undetermined etiology
- STROBE:
-
STrengthening the Reporting of OBservational Studies in Epidemiology
- SUE:
-
Stroke of undetermined etiology
- SVIN:
-
Society of Vascular and Interventional Neurology
- SVO:
-
Small vessel occlusion
- TOAST:
-
Trial of ORG 10,172 in acute stroke treatment
References
Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, Sandercock P, Wang Y, Huang Y, Cui L, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405.
Campbell BCV, Khatri P. Stroke. Lancet. 2020;396(10244):129–42.
Jahan R, Saver JL, Schwamm LH, Fonarow GC, Liang L, Matsouaka RA, Xian Y, Holmes DN, Peterson ED, Yavagal D, et al. Association Between Time to Treatment With Endovascular Reperfusion Therapy and Outcomes in Patients With Acute Ischemic Stroke Treated in Clinical Practice. JAMA. 2019;322(3):252–63.
Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, Campbell BC, Nogueira RG, Demchuk AM, Tomasello A, et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA. 2016;316(12):1279–88.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–418.
Kim SH, Nam TM, Jang JH, Kim YZ, Kim KH, Kim DH, Lee H, Jin SC, Lee CH. Improving Door-To-Puncture Time in Mechanical Thrombectomy with Direct Care from a Neurointerventionalist in the Emergency Department. World Neurosurg. 2021;152:e455–61.
Chen Y, Nguyen TN, Wellington J, Mofatteh M, Yao W, Hu Z, Kuang Q, Wu W, Wang X, Sun Y, et al. Shortening Door-to-Needle Time by Multidisciplinary Collaboration and Workflow Optimization During the COVID-19 Pandemic. J Stroke Cerebrovasc Dis. 2022;31(1):106179–106179.
Dargazanli C, Fahed R, Blanc R, Gory B, Labreuche J, Duhamel A, et al. Modified Thrombolysis in Cerebral Infarction 2C/Thrombolysis in Cerebral Infarction 3 Reperfusion Should Be the Aim of Mechanical Thrombectomy. Stroke. 2018;49(5):1189–96.
Banks JL, Marotta CA. Outcomes Validity and Reliability of the Modified Rankin Scale: Implications for Stroke Clinical Trials. Stroke. 2007;38(3):1091–6.
von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, Treurniet KM, Majoie CB, Marquering HA, Mazya MV, et al. The Heidelberg Bleeding Classification: Classification of Bleeding Events After Ischemic Stroke and Reperfusion Therapy. Stroke. 2015;46(10):2981–6.
Elm EV, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8.
Leifer D, Bravata DM, Connors JJ, Hinchey JA, Jauch EC, Johnston SC, Latchaw R, Likosky W, Ogilvy C, Qureshi AI, et al. Metrics for Measuring Quality of Care in Comprehensive Stroke Centers: Detailed Follow-Up to Brain Attack Coalition Comprehensive Stroke Center Recommendations. Stroke. 2011;42(3):849–77.
Ota T, Sato M, Amano T, Matsumaru Y. Door-to-Needle Time Under 60 Minutes and Picture-to-Puncture Under 90 Minutes: Initiatives and Outcomes in Reducing Time to Recanalization for Cerebral Major Artery Occlusion. Neurol Med Chir (Tokyo). 2016;56(12):725–30.
Nguyen TN, Abdalkader M, Nagel S, Qureshi MM, Ribo M, Caparros F, Haussen DC, Mohammaden MH, Sheth SA, Ortega-Gutierrez S, et al. Noncontrast Computed Tomography vs Computed Tomography Perfusion or Magnetic Resonance Imaging Selection in Late Presentation of Stroke With Large-Vessel Occlusion. JAMA Neurol. 2022;79(1):22–31.
Siegler JE, Zha AM, Czap AL, Ortega-Gutierrez S, Farooqui M, Liebeskind DS, Desai SM, Hassan AE, Starosciak AK, Linfante I, et al. Influence of the COVID-19 Pandemic on Treatment Times for Acute Ischemic Stroke: The Society of Vascular and Interventional Neurology Multicenter Collaboration. Stroke. 2021;52(1):40–7.
Wang J, Chaudhry SA, Tahsili-Fahadan P, Altaweel LR, Bashir S, Bahiru Z, Fang Y, Qureshi AI. The impact of COVID-19 on acute ischemic stroke admissions: Analysis from a community-based tertiary care center. J Stroke Cerebrovasc Dis. 2020;29(12):105344.
Nogueira RG, Abdalkader M, Qureshi MM, Frankel MR, Mansour OY, Yamagami H, Qiu Z, Farhoudi M, Siegler JE, Yaghi S, et al. Global impact of COVID-19 on stroke care. Int J Stroke. 2021;16(5):573–84.
Nogueira RG, Qureshi MM, Abdalkader M, Martins SO, Yamagami H, Qiu Z, Mansour OY, Sathya A, Czlonkowska A, Tsivgoulis G, et al. Global Impact of COVID-19 on Stroke Care and IV Thrombolysis. Neurology. 2021;96(23):e2824.
Nguyen TN, Abdalkader M, Jovin TG, Nogueira RG, Jadhav AP, Haussen DC, Hassan AE, Novakovic R, Sheth SA, Ortega-Gutierrez S, et al. Mechanical Thrombectomy in the Era of the COVID-19 Pandemic: Emergency Preparedness for Neuroscience Teams. Stroke. 2020;51(6):1896–901.
Acknowledgements
The authors thank all colleague for data collection and efforts to shorten door to puncture time.
Funding
The projected is supported by Foshan Medical Technology Innovation Platform Construction Foundation (FS0AA-KJ218-1301–0012), Foshan the 14th Five-Year Plan Key Discipline Foundation-Department of Neurology and Foshan Competitive Talent Support Project Fund (Brain-Heart Talent Project-Build the Brain-Heart Comorbidity Muti-disciplinary Medical Center ) .
Author information
Authors and Affiliations
Contributions
Yimin Chen and Shuiquan Yang drafted the manuscript. Weiping Yao, Shuiquan Yang, Zhaohui Hu, Zhou Huang designed the study. James E. Siegler, Mohammad Mofatteh, and Jack Wellington analyzed the data and revised the paper. Wenjun Liang and Gan Chen did the EVT and analyzed the cases. Jiale Wu, Rongshen Yang, Juanmei Chen and Yajie Yang collected the data, followed up patients and analyzed the cases. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by Foshan Sanshui District People’s Hospital Institute Review board. The informed consent was waived due to the retrospective observational nature of the study by Foshan Sanshui District People’s Hospital Institute Review Board. All the studies were carried out in the accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
James E. Siegler reports consulting fees for Ceribell and speakers bureau for AstraZeneca, both unrelated to the present work. Other authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, S., Yao, W., Siegler, J.E. et al. Shortening door-to-puncture time and improving patient outcome with workflow optimization in patients with acute ischemic stroke associated with large vessel occlusion. BMC Emerg Med 22, 136 (2022). https://doi.org/10.1186/s12873-022-00692-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12873-022-00692-8