Abstract
Background
Acute coronary syndrome (ACS) is a serious cardiovascular disease that severely affects the quality of life and longevity of patients. MicroRNAs (miRNAs) play a key role in the progression of ACS with significant clinical value. The aim of this study was to examine the clinical value of miR-223-5p in ACS and on the occurrence of major adverse cardiovascular events (MACE) after percutaneous coronary intervention (PCI).
Methods
The plasma expression of miR-223-5p was detected by RT-qPCR. The correlation of miR-223-5p and cTnI or Gensini score was shown by the Pearson method. Risk factors for the development of ACS were analyzed by multivariate logistic regression. The efficacy of miR-223-5p in identifying patients with ACS was shown by ROC curve. The predictive value of miR-223-5p for MACE development in ACS patients within 6 months after PCI was assessed by Kaplan-Meier curve and multivariate Cox regression.
Results
miR-223-5p levels were markedly elevated in ACS patients. miR-223-5p was found to be positively related to cTnI or Gensini score. miR-223-5p was a risk factor for ACS and significantly identified patients with ACS. MACE was more likely to occur after PCI in patients with high miR-223-5p levels, and miR-223-5p was an independent prognostic indicator of MACE.
Conclusions
miR-223-5p had diagnostic value for ACS and predicted MACE after PCI.
Similar content being viewed by others
Background
Acute coronary syndrome (ACS) is an acute ischemic syndrome of the cardiac system due to disruption or erosion of unstable atherosclerotic plaques that are present in the coronary arteries, covering ST-segment elevation myocardial infarction (STEMI), unstable angina pectoris (UA), and non-STEMI (NSTEMI) [1]. Patients with ACS usually present with acute chest pain, but other atypical symptoms may also be present as their first symptoms [2]. In China, the incidence of ACS continues to increase year after year, and most patients with ACS are first seen in the emergency department. The clinical diagnosis of ACS is usually based on clinical symptoms, electrocardiograms, and myocardial enzyme profiles. Despite this, a large number of patients with ACS are misdiagnosed, and even 2–5% have been missed in the emergency department [3,4,5].
ACS is often associated with serious complications, and early intervention can help to minimize serious complications (e.g., sudden death, recurrent myocardial infarction, and cardiac failure) [6]. The application of percutaneous coronary intervention (PCI) has greatly improved the efficiency for ACS patients. However, patients still face the risk of major adverse cardiovascular events (MACE) following surgery, and the prognosis is uncertain. Early prediction of prognosis can provide an important reference for clinical treatment and management [7]. Therefore, the search for biomarkers for the diagnosis and prognosis of ACS is particularly important for timely intervention and effective treatment of ACS patients.
MicroRNAs (miRNAs) can be involved in vascular development, inflammation, thrombosis, etc., and are essential for the normal physiological or pathological mechanisms of the cardiovascular system [8,9,10]. miRNAs have been demonstrated to be key regulators of many cellular events in the pathogenesis of cardiovascular diseases, such as disorders of lipid metabolism, impaired endothelial function, and atherosclerotic plaque formation [11]. miR-663 was reported to influence the development of atherosclerosis by regulating the proliferation of vascular smooth muscle cells, thereby informing atherosclerosis treatment [12]. Recent studies suggested that miRNAs may have a potential biomarker role in the early diagnosis, treatment, and prognosis of ACS [13]. For example, miR-378c was shown to be significantly downregulated in blood and atherosclerotic plaques of ACS patients, which may serve as a biomarker for early atherosclerosis detection and a novel regulator of atherosclerosis [14]. Previously, miR-223-5p showed differential expression in ACS [15], but its value in the diagnosis and prognosis prediction of ACS has not been studied.
In the present study, miR-223-5p was hypothesized as a potential biomarker for ACS diagnosis and prognosis. By fully analyzing the clinical data, this study revealed the significance that miR-223-5p has on the occurrence and prognosis of ACS.
Materials and methods
Study subjects
In this study, 116 patients with ACS who attended the emergency department of General Hospital of Central Theater Command between May 2022 and May 2023 were included in the study, and all of them met the diagnostic criteria of the Guidelines for Rapid Diagnosis and Treatment of Acute Coronary Syndrome in Emergency Department (2019). A total of 80 healthy people who underwent medical checkups during the period were selected as the control group. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of General Hospital of Central Theater Command and conducted with informed consent signed by the patients or guardians.
Inclusion criteria: (1) diagnosis of ACS confirmed by history, physical signs, laboratory tests, electrocardiogram, cardiac ultrasound, coronary CT angiography (CTA), coronary angiography, etc.; (2) age greater than 40 years; (3) no psychiatric disease; (4) all underwent PCI; (5) the patient himself/herself or his/her legal guardian agreed to take part in the study and signed the form, and was able to cooperate with the follow-up visits. Exclusion criteria: (1) unanticipated death or survival less than six months; (2) severe cardiac insufficiency (greater than class III), severe hepatic or renal insufficiency; (3) malignant cardiac arrhythmia, infective endocarditis, aortic coarctation, and cardiac valvular disease, Pulmonary embolism; (4) severe infectious or contagious diseases; (5) concomitant hematologic or autoimmune diseases; (6) breastfeeding or pregnancy; (7) malignant tumors, cerebral infarction, cerebral hemorrhage.
General data such as gender, age, BMI, and history of diabetes or hypertension were collected and recorded for all subjects. Biochemical parameters of ACS and control groups were obtained using cobas c 702 automated biochemistry analyzer (Roche, Germany): triacylglycerol (TG), total cholesterol (TC), low- or high-density lipoprotein cholesterol (LDL-C, HDL-C), white blood cells (WBC), etc. Coronary angiograms were collected from the enrolled ACS patients and quantitatively scored using the Gensini Scoring System for each diseased coronary vessel according to the Coronary Artery Vessel Segmentation Scoring Criteria (CAVSSC) developed by the American Heart Association (AHA) [16], as described in the Table S1.
Blood sample collection
5 mL of venous blood was collected from ACS patients (within 24 h before PCI) using EDTA anticoagulation tubes after admission. Fasting venous blood was collected from the control population. The supernatant plasma was separated at 3000 g for 15 min at 4 °C, placed in EP tubes (RNase-free), and stored at -80 °C.
RT-qPCR
According to the manual, the miR-223-5p in plasma was extracted by miRNeasy Serum/Plasma Kit (Qiagen, Germany) and reverse transcribed to cDNA by SuperRT III miRNA Reverse Transcription Kit (Biosharp, Anhui, China). The cDNA was used as the template for RT⁃qPCR with miRNA QPCR Master Mix (Agilent, USA) on an ABI 7500 system (Applied Biosystems, USA). The RT-qPCR program was as follows: 95°C for 10 min; 95°C for 10 s, 60°C for 15 s, 72°C for 20 s, 40 cycles. The levels of miR⁃223⁃5p were calculated by the 2−ΔΔCt method (internal reference: U6). The forward and reverse primer sequences for miR-223-5p were 5′-CGTGTATTTGACAAGCTG-3’ and 5′-GAACATGTCTGCGTATCTC-3’. The forward and reverse primer sequences for U6 were 5′-GCTTCGGCAGCACATATACT-3’ and 5′-GTGCAGGGTCCGAGGTATTC-3’.
Follow-up and prognostic evaluation
ACS patients were followed up for 6 months after discharge from the hospital in outpatient clinics or by telephone. Follow-up consisted of the occurrence of MACE, which was defined as unintended coronary revascularization, nonfatal AMI, and all-cause death. Patients with ACS were categorized into MACE and non-MACE groups according to the occurrence of MACE.
Statistical analysis
SPSS 23.0 was employed for statistical analysis, and GraphPad 9.0 was used to visualize the results. Mean ± SD was used for the data conforming to the normal distribution, and the number of cases was presented for count data. Comparisons between the two groups were made by chi-square test or t-test. The risk factors for the occurrence of ACS were evaluated by multivariate logistic regression. The diagnostic efficacy of plasma miR-223-5p for ACS was analyzed using the ROC curve, and the correlation of plasma miR-223-5p and cTnI or Gensini score was analyzed by the Pearson method. Different levels of miR-223-5p were analyzed with the possibility of MACE after PCI through Kaplan-Meier curve. Multivariate Cox regression was carried out to analyze the independent predictors of MACE in patients with ACS. P < 0.05 indicated statistically significant differences.
Results
Upregulation of miR-223-5p correlated with the development of ACS
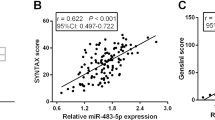
The baseline data of ACS patients and the control group were first compared, and no significant differences were shown in terms of sex, age, BMI, LDL-C, HDL-C, and history of hypertension or diabetes mellitus (P > 0.05). Nevertheless, the levels of TC, TG, and WBC were markedly higher in ACS patients than that of the control group (P < 0.05, Table 1). Plasma miR-223-5p levels were dramatically elevated in ACS patients according to the results of RT-qPCR (P < 0.0001, Fig. 1A). Furthermore, miR-223-5p was positively correlated with cTnI (r = 0.684, 95% CI = 0.573–0.770, Fig. 1B), a biomarker of myocardial injury, and Gensini score (r = 0.715, 95% CI = 0.612–0.794, Fig. 1C), a quantitative assessment of coronary artery stenosis severity.
MiR-223-5p possessed potential diagnostic value in ACS
Multivariate logistic regression results indicated that miR-223-5p (OR = 8.404, 95% CI = 4.044–17.465) was a risk factor in the development of ACS (P < 0.001, Fig. 2A). Consistently, the ROC curve confirmed the significant value of miR-223-5p in identifying ACS patients, showing the AUC value of which was 0.829 (95% CI = 0.773–0.886, Fig. 2B) with the sensitivity and specificity of 0.664 and 0.888, respectively.
MiR-223-5p predicted MACE after PCI in patients with ACS
Here, MACE occurred in 27 patients within 6 months after PCI in the ACS patients. The levels of NT-proBNP, CK-MB, cTnI, and Gensini score in the MACE group were markedly elevated by comparison with the non-MACE group (P < 0.001, Table 2), while other features such as sex and age were not remarkably different (P > 0.05). The levels of miR-223-5p were markedly elevated in patients who underwent MACE after PCI versus the non-MACE group (P < 0.0001, Fig. 3A). Subsequently, the predictive value of miR-223-5p for the occurrence of MACE after PCI was examined. All ACS patients were grouped according to mean miR-223-5p levels, and the Kaplan-Meier curve indicated that ACS patients with high miR-223-5p expression were at higher risk of MACE after PCI (log-rank, P = 0.030, Fig. 3B). In addition, multivariate Cox regression demonstrated that plasma miR-223-5p (HR = 4.202, 95% CI = 1.286–13.725), cTnI (HR = 2.731, 95% CI = 1.046–7.128), and Gensini score (HR = 3.194, 95% CI = 1.101–9.264) were independent predictors of MACE in patients after PCI (P < 0.05, Fig. 3C).
Discussion
As a disease of coronary atherosclerotic plaque tissue after invasion and abnormal rupture, ACS can lead to incomplete and complete occlusion thrombosis, resulting in cardiac physiological function damage and necrosis of cardiomyocytes, which has a serious negative impact on the life safety of the patients [17]. Therefore, it is of great value to explore new indicators related to ACS. In recent years, the role of miRNAs in cardiovascular diseases has gradually attracted attention. The study of the expression and regulatory mechanisms associated with miRNAs in different disease states has crucial significance for the diagnosis and therapy of cardiovascular disease as well as for the assessment of the disease progression [18, 19]. Some studies confirmed that miRNAs are related to the onset and development of ACS. miR-9 has been shown to inhibit atherosclerosis associated with ACS by negatively regulating SDC2 in a mouse model of atherosclerosis and is considered a potential therapeutic target for ACS patients with atherosclerosis [20]. Currently, miRNAs are increasingly recognized as important evaluators of ACS severity and coronary artery disease. For example, miR-143 and miR-145 were markedly decreased in ACS patients and were considered promising biomarkers for assessing the severity of ACS [21]. Here, miR-223-5p expression was increased in ACS patients, which was consistent with the findings reported by Elbaz et al. [15]. Furthermore, miR-223-5p was positively related to cTnI and Gensini score, which were the clinical indicators in evaluating the occurrence and severity of ACS. These findings indicated that miR-223-5p correlated with myocardial injury and the severity of coronary artery lesions.
With the gradual deepening of research on miRNAs, their diagnostic value in cardiovascular and cerebrovascular diseases is gradually being recognized. miR-137 and miR-106b were identified as biomarkers of myocardial ischemia that could identify patients with coronary artery disease in the early stages [22]. Elevated serum miR-377 levels were found to possess high diagnostic value in patients with ACS and to modulate myocardial injury (e.g., inflammatory response and endothelial damage) [23]. In previous studies, miR-223-5p was reported to serve as a biomarker of Parkinson’s disease, prostate cancer, and non-small cell lung cancer [24,25,26]. Herein, miR-223-5p was appraised as a risk factor for ACS and showed favorable efficacy in the diagnosis of ACS, identifying ACS patients with high sensitivity and specificity from healthy populations. Previously, miR-223-5p was also detected to be upregulated in the heart of myocardial ischemia/reperfusion (I/R) mice and regulate necrotic apoptosis of the heart, which may provide new therapeutic opportunities for I/R [27]. Therefore, miR-223-5p may be a critical factor in the development of ACS.
PCI is the major treatment for patients with ACS, which can quickly open the occluded blood vessels in a short period, promptly restore blood reperfusion to the myocardium in the ischemic region, and save the lives of patients [28]. Although the use of PCI can improve the clinical symptoms of ACS, and enhance the survival rate and life quality of patients, the occurrence of in-stent thrombosis and post-procedure MACE has negative impacts on the prognosis of patients [29]. It has been reported that some miRNAs were predictors of MACE after PCI, such as miR-483-5p, miR-186-5p, and miR-497-5p [30,31,32]. Another study also noted that miR-223-5p could be an important prognostic factor for vulvar cancer [33]. Thus, the prognostic value of miR-223-5p in ACS was explored by analyzing clinical data. In the present study, miR-223-5p was upregulated in ACS patients who develop MACE, and higher expression of miR-223-5p represented a greater possibility of MACE development. In addition, miR-223-5p, along with cTnI and Gensini score, was ascertained as an independent predictor of MACE in ACS patients within 6 months following PCI. The above illustrated that miR-223-5p is a promising biomarker for ACS to predict MACE after PCI treatment, which may be helpful in developing a treatment plan for patients.
Although the clinical significance of miR-223-5p in ACS was unveiled in this paper, the research is still limited by the sample size, sample source, and follow-up time. Moreover, future studies should further explore the specific biological functions and mechanisms of miR-223-5p in ACS.
In conclusion, upregulated miR-223-5p was strongly associated with the severity of ACS and showed high value in the diagnosis and prognosis prediction of ACS, indicating that miR-223-5p is promising as a new biomarker for ACS.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Atwood J. Management of Acute Coronary Syndrome. Emerg Med Clin North Am. 2022;40(4):693–706.
McGarry M, Shenvi CL. Identification of Acute Coronary Syndrome in the Elderly. Emerg Med Clin North Am. 2021;39(2):339–46.
Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of Acute Coronary syndromes: a review. JAMA. 2022;327(7):662–75.
Barstow C. Acute coronary syndrome: presentation and diagnostic evaluation. FP Essent. 2020;490:11–9.
Shi Z, Zhao C, Hu J, Dai Q, Guan M, Zhong C, et al. The application of traditional Chinese medicine injection on patients with Acute Coronary Syndrome during the Perioperative period of percutaneous coronary intervention: a systematic review and Meta-analysis of Randomized controlled trials. Evid Based Complement Alternat Med. 2020;2020:3834128.
Jones DE, Braun M, Kassop D. Acute Coronary Syndrome: common complications and conditions that mimic ACS. FP Essent. 2020;490:29–34.
Zhang P, Wu L, Zou TT, Zou Z, Tu J, Gong R, et al. Machine learning for early prediction of major adverse Cardiovascular events after first percutaneous coronary intervention in patients with Acute myocardial infarction: Retrospective Cohort Study. JMIR Form Res. 2024;8:e48487.
Lin X, Zhan JK, Wang YJ, Tan P, Chen YY, Deng HQ, et al. Function, role, and clinical application of MicroRNAs in Vascular Aging. Biomed Res Int. 2016;2016:6021394.
Juni RP, Kocken JMM, Abreu RC, Ottaviani L, Davalan T, Duygu B, et al. MicroRNA-216a is essential for cardiac angiogenesis. Mol Ther. 2023;31(6):1807–28.
Du H, Zhao Y, Yin Z, Wang DW, Chen C. The role of miR-320 in glucose and lipid metabolism disorder-associated diseases. Int J Biol Sci. 2021;17(2):402–16.
Kramna D, Riedlova P, Jirik V. MicroRNAs as a potential biomarker in the diagnosis of Cardiovascular diseases. Med (Kaunas). 2023;59(7):1329.
Deng Z, Li L. Effect of miR-663 on atherosclerosis by regulating the proliferation of vascular smooth muscle cells in lipid plaques. Vascular. 2023;31(6):1240–52.
Churov A, Summerhill V, Grechko A, Orekhova V, Orekhov A. MicroRNAs as potential biomarkers in atherosclerosis. Int J Mol Sci. 2019;20(22):5547.
Tian S, Cao Y, Wang J, Bi Y, Zhong J, Meng X, et al. The miR-378c-Samd1 circuit promotes phenotypic modulation of vascular smooth muscle cells and foam cells formation in atherosclerosis lesions. Sci Rep. 2021;11(1):10548.
Elbaz M, Faccini J, Laperche C, Grousset E, Roncalli J, Ruidavets JB et al. Identification of a miRNA based-signature Associated with Acute Coronary Syndrome: evidence from the FLORINF Study. J Clin Med. 2020;9(6):1674.
Rampidis GP, Benetos G, Benz DC, Giannopoulos AA, Buechel RR. A guide for Gensini score calculation. Atherosclerosis. 2019;287:181–3.
Li L, Sun G, Yu J, Shan G, Su L, Dong G. Identification of predictors for the comprehensive clinical risk and severity of coronary lesions of acute coronary syndrome. Front Cardiovasc Med. 2023;10:1046895.
Ma XN, Lu L, Huang YT, Cen CQ, Su FY, Shi Y, et al. Research Progress of Exosomal microRNA in Cardiovascular Disease and its forensic application prospects. Fa Yi Xue Za Zhi. 2022;38(2):258–62.
Smirnova AV, Sukhorukov VN, Karagodin VP, Orekhov AN. [Epigenetic factors in atherogenesis: microRNA]. Biomed Khim. 2016;62(2):134–40.
Zhang R, Song B, Hong X, Shen Z, Sui L, Wang S. microRNA-9 inhibits vulnerable plaque formation and vascular remodeling via suppression of the SDC2-Dependent FAK/ERK signaling pathway in mice with atherosclerosis. Front Physiol. 2020;11:804.
Meng L, Yu X, Han H, Jia X, Hu B, Zhang L, et al. Circulating miR-143 and miR-145 as promising biomarkers for evaluating severity of coronary artery stenosis in patients with acute coronary syndrome. Clin Biochem. 2023;111:32–40.
Elgebaly SA, Christenson RH, Kandil H, Ibrahim M, Rizk H, El-Khazragy N et al. Nourin-dependent miR-137 and miR-106b: novel biomarkers for early diagnosis of myocardial ischemia in coronary artery Disease patients. Diagnostics (Basel). 2021;11(4):703.
Zhang Q, Yang L, Wan G, Zhang X, Wang Y, Zhao G. Serum miR-377 can be used as a diagnostic marker for Acute Coronary Syndrome and can regulate proinflammatory factors and endothelial Injury markers. Acta Med Okayama. 2022;76(6):723–30.
Citterio LA, Mancuso R, Agostini S, Meloni M, Clerici M. Serum and Exosomal miR-7-1-5p and miR-223-3p as Possible Biomarkers for Parkinson’s Disease. Biomolecules. 2023;13(5):865.
Wei Y, Peng J, He S, Huang H, Lin L, Zhu Q, et al. Mir-223-5p targeting ERG inhibits prostate cancer cell proliferation and migration. J Cancer. 2020;11(15):4453–63.
Dou L, Han K, Xiao M, Lv F. Mir-223-5p suppresses Tumor Growth and Metastasis in Non-small Cell Lung Cancer by Targeting E2F8. Oncol Res. 2019;27(2):261–8.
Qin D, Wang X, Li Y, Yang L, Wang R, Peng J, et al. MicroRNA-223-5p and – 3p cooperatively suppress necroptosis in Ischemic/Reperfused hearts. J Biol Chem. 2016;291(38):20247–59.
Fanaroff AC, Nathan AS. Percutaneous coronary intervention in Acute Coronary Syndrome and cardiogenic shock: ensuring Access while maintaining quality. JACC Cardiovasc Interv. 2022;15(8):887–9.
Li M, Hou J, Gu X, Weng R, Zhong Z, Liu S. Incidence and risk factors of in-stent restenosis after percutaneous coronary intervention in patients from southern China. Eur J Med Res. 2022;27(1):12.
Zhao Y, Song X, Ma Y, Liu X, Peng Y. Circulating mir-483-5p as a novel diagnostic biomarker for acute coronary syndrome and its predictive value for the clinical outcome after PCI. BMC Cardiovasc Disord. 2023;23(1):360.
Li Z, Wu J, Wei W, Cai X, Yan J, Song J, et al. Association of serum mir-186-5p with the prognosis of Acute Coronary Syndrome patients after percutaneous coronary intervention. Front Physiol. 2019;10:686.
Chen T, Zhang X, Qian W, Zhou R, Su M, Ma Y, Serum. Mir-497-5p serves as a diagnostic biomarker for acute coronary syndrome and predicts the occurrence of major adverse cardiovascular events after percutaneous coronary intervention. Bioengineered. 2022;13(4):8266–76.
de Melo Maia B, Rodrigues IS, Akagi EM, Soares do Amaral N, Ling H, Monroig P, et al. MiR-223-5p works as an oncomiR in vulvar carcinoma by TP63 suppression. Oncotarget. 2016;7(31):49217–31.
Acknowledgements
Not applicable.
Funding
This study was funded by “Effect of interaction between autophagy and apoptosis on acute remodeling of myocardial Cx43 protein in ischemia-reperfusion-injured rat myocardium and intervention of metoprolol”, Grant numbers [WJ2018H0068].
Author information
Authors and Affiliations
Contributions
SHZ and GFY made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, and draft of the manuscript. YJC provided assistance for data acquisition, data analysis and statistical analysis. WZL revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of General Hospital of Central Theater Command and conducted with informed consent signed by the patients or guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, S., Yang, G., Chen, Y. et al. miR-223-5p serves as a diagnostic biomarker for acute coronary syndrome and its predictive value for the clinical outcome after PCI. BMC Cardiovasc Disord 24, 423 (2024). https://doi.org/10.1186/s12872-024-04088-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-04088-3