Abstract
Background
Early prognosis evaluation is crucial for decision-making in cardiogenic shock (CS) patients. Dynamic lactate assessment, for example, normalized lactate load, has been a better prognosis predictor than single lactate value in septic shock. Our objective was to investigate the correlation between normalized lactate load and in-hospital mortality in patients with CS.
Methods
Data were extracted from the Medical Information Mart for Intensive Care (MIMIC)-IV database. The calculation of lactate load involved the determination of the cumulative area under the lactate curve, while normalized lactate load was computed by dividing the lactate load by the corresponding period. Receiver Operating Characteristic (ROC) curves were constructed, and the evaluation of areas under the curves (AUC) for various parameters was performed using the DeLong test.
Results
Our study involved a cohort of 1932 CS patients, with 687 individuals (36.1%) experiencing mortality during their hospitalization. The AUC for normalized lactate load demonstrated significant superiority compared to the first lactate (0.675 vs. 0.646, P < 0.001), maximum lactate (0.675 vs. 0.651, P < 0.001), and mean lactate (0.675 vs. 0.669, P = 0.003). Notably, the AUC for normalized lactate load showed comparability to that of the Sequential Organ Failure Assessment (SOFA) score (0.675 vs. 0.695, P = 0.175).
Conclusion
The normalized lactate load was an independently associated with the in-hospital mortality among CS patients.
Similar content being viewed by others
Background
Cardiogenic shock (CS) is a profound cardiac dysfunction leading to diminished cardiac output, critical organ hypoperfusion, and tissue hypoxia. Despite significant advancements in intensive care medicine, high mortality rates in CS, ranging from 40 to 50%, still exist [1]. Arterial lactate, in conditions of oxygen deficiency, is typically elevated in CS. Baseline lactate has been widely used in mortality prediction in CS for decades [2, 3]. Klemm et al. evaluated the efficacy of various lactate measurements within the first 24 h post-ICU admission for predicting 30-day mortality in cardiogenic shock patients, concluding that the 24-hour lactate level is the most effective predictor of mortality in this context [4]. In patients with CS receiving mechanical circulatory support, Scolari et al. found that serum lactate levels and particularly the lactate level after 24 h of treatment were identified as significant independent predictors of 30-day mortality [5]. However, the prognostic accuracy of critically ill patients’ prognosis, as indicated by lactate levels over time, has been shown to be superior with dynamic lactate compared to a single lactate measurement [6, 7].
There are numerous indicators of dynamic lactate changes. Lactate clearance (LC), characterized by the change in lactate levels over time, has been reported with better predictive performance compared to baseline lactate [8]. Another study demonstrated that calculating the weighted average of lactate levels and their changes during the initial 24 h provided superior performance compared to individual measures such as admission, maximum, and minimum lactate levels [7]. Both the lactate value and timing significantly impact clinical outcomes [9]. The concept of normalized lactate load, originally introduced by Zhang et al., served as a comprehensive index that took into account both the lactate value and time [10]. Previous studies have indicated its association with favorable outcomes in septic shock [11] as well as non- septic patients [12]. Nevertheless, the association between this variable and overall mortality has not been validated in a substantial cohort of patients with CS. Therefore, the primary objective of this study was to explore the relationship between normalized lactate load and in-hospital mortality in individuals diagnosed with CS.
Methods
Study design
A retrospective observational study was conducted, including patients diagnosed with CS using the ICD-9/10 code (details can be found in the Supplementary material) [13]. Patients with missing lactate data or those with malignant tumors were excluded from the study. The analysis was exclusively focused on patients initially admitted to the hospital (Fig. 1).
Data extraction
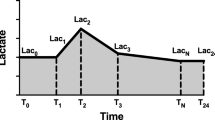
The data used in this study were sourced from the Medical Information Mart for Intensive Care IV (MIMIC-IV, version 2.1) database (Details can be found in Supplementary) [13]. We specifically gathered all lactate measurements and their corresponding measurement timestamps acquired within the initial 24 h following ICU admission to calculate various lactate-related variables, such as normalized lactate load, lactate load, first lactate, maximum lactate, and mean lactate. A Cartesian coordinate graph representing lactate measurements alongside their corresponding timestamps was constructed. The lactate load was computed as the cumulative area under the curve (AUC) of lactate concentration over time. This involved integrating the AUC formed by plotting lactate concentration (mmol/L) against time (hours) from the initial 24 h following ICU admission. The formula for the lactate load was: Lactate Load=(Lactate1 × ΔTime1)+(Lactate2 × ΔTime2)+…+(Lactaten × ΔTimen), where Lactaten was the lactate concentration at the nth measurement, and ΔTime𝑛 was the time interval since the previous measurement. To normalize the lactate load over the total duration of the 24-hour observation period, we divided the total lactate load by 24 h. The formula for normalized lactate load was: Normalized Lactate Load = lactate load/24 h (Fig. 2) [12].
A Cartesian coordinate describing the calculation of Lactate load and normalized Lactate load. “Lactate” represents each Lactate value. “Time” represented their corresponding measurement timestamps. The integral of the curve was computed to quantify the lactate load. The formula for the lactate load was: lactate load=(Lactate1 × ΔTime1)+(Lactate2 × ΔTime2)+…+(Lactaten × ΔTimen), where Lactaten was the lactate concentration at the nth measurement, and ΔTime𝑛 was the time interval since the previous measurement. The normalized lactate load was obtained using the following formula: normalized lactate load = lactate load/24 h
Grouping and outcome
Participants were stratified into four groups according to quartiles of normalized lactate load. The primary outcome was in-hospital mortality.
Statistical analysis
Quantitative data with a normal distribution were presented as means and standard deviations (SD), skewed data as medians and interquartile ranges, and categorical data as numbers and percentages. Patient characteristics were compared between groups utilizing appropriate statistical tests such as analysis of variance, Kruskal-Wallis test, and Chi-square test.
A thorough investigation into the correlation between normalized lactate load levels and in-hospital mortality was conducted. This included binary logistic regression analysis using three distinct models (model 1, 2, and 3), where covariates for model 3 were selected through a stepwise method with a removal criterion of P > 0.05. The results were presented as odds ratios (OR) with 95% confidence intervals (CI). Furthermore, a restricted cubic spline curve (RCS) was constructed based on model 3, incorporating three knots for analysis.
Receiver operating characteristic (ROC) curves were generated to assess the predictive performance of various lactate parameters, and the area under the curve (AUC) values were compared using the DeLong test.
Subgroup analyses were performed to investigate the associations between normalized lactate load levels and in-hospital mortality across different patient subpopulations.
All analyses, including two-tailed statistical tests with a significance level of P < 0.05, were performed using the R software.
Results
Patient characteristics
The study included a total of 1,932 CS patients. Participants were stratified into four groups according to quartiles of normalized lactate load: Quartile 1 with normalized lactate load < 1.53 mmol/L (n = 483), Quartile 2 with 1.53 mmol/L ≤ normalized lactate load < 2.20 mmol/L (n = 483), Quartile 3 with 2.20 mmol/L ≤ normalized lactate load < 3.67 mmol/L (n = 483), and Quartile 4 with normalized lactate load ≥ 3.67 mmol/L (n = 483). Patients with higher normalized lactate load levels exhibited distinct characteristics (Table 1): they were less likely to be of white ethnicity, and had higher heart rates, while their lower systolic blood pressure was lower. Furthermore, patients in the higher quartiles had elevated levels of white blood cells, neutrophil percentage, hemoglobin, glucose, and sodium, while demonstrating lower platelet levels. They had a higher risk of hypertension and respiratory failure, but a lower risk of congestive heart failure and cardiomyopathy. Additionally, these patients received higher proportions of epinephrine, dialysis, mechanical ventilation, and extracorporeal membrane oxygenation (ECMO) treatment. Notably, these patients also had higher levels of normalized lactate load, lactate load, first lactate, maximum lactate, mean lactate, and Sequential Organ Failure Assessment (SOFA) score.
Association between normalized lactate load and in-hospital mortality
The overall in-hospital mortality rate was 36.1%. A significant trend was observed, indicating a higher in-hospital mortality with increasing normalized lactate load (quartile 4 vs. quartile 1: 62.7% vs. 24.2%, P < 0.001) as shown in Table 1. Binary logistic regression analysis was performed to confirm the direct impact of normalized lactate load on in-hospital mortality. In Model 1, a significant association between elevated levels of normalized lactate load and increased risk of in-hospital mortality was observed (quartile 4 vs. quartile 1: OR, 95% CI: 5.27, 4.00–6.97, P < 0.001, P for trend < 0.001). Model 2 revealed a positive correlation between normalized lactate load and mortality (quartile 4 vs. quartile 1: OR, 95% CI: 5.39, 4.07–7.16, P < 0.001, P for trend < 0.001). Even after adjusting for additional confounding variables in Model 3, a significant association persisted between higher quartiles of normalized lactate load and an increased risk of in-hospital mortality (quartile 4 vs. quartile 1: OR, 95% CI: 3.56, 2.52–5.06, P < 0.001, P for trend < 0.001). Furthermore, when considering normalized lactate load as a continuous variable, each unit increase was associated with approximately 0.72-fold, 0.73-fold, and 0.47-fold increases in the risk of in-hospital mortality in Model 1, Model 2, and Model 3, respectively (Table 2).
In Fig. 3, the ROC curves visually depicted the predictive capability of normalized lactate load for in-hospital mortality, yielding an AUC of 0.675 [95% CI: 0.654–0.696]. This performance surpassed that of the first lactate (0.675 vs. 0.646, De-long test P < 0.001), maximum lactate (0.675 vs. 0.651, De-long test P < 0.001), and mean lactate (0.675 vs. 0.669, De-long test P = 0.003). Notably, it demonstrated comparability to the predictive performance of SOFA score (0.675 vs. 0.695, De-long test P = 0.175).
In Fig. 4, the association between in-hospital mortality and normalized lactate load was visualized using RCS with model 3 applied. Upon adjusting for potential confounding factors, an evident linear relationship between normalized lactate load and in-hospital mortality was identified, with a non-linear p-value of 0.081.
Subgroup analysis
In all subgroups, except for those categorized by myocarditis and ethnicity, elevated levels of normalized lactate load were significantly associated with an increased risk of in-hospital mortality. Significant interactions were notably detected among subgroups including septic shock, heart rate, platelets, cerebrovascular disease, atrial fibrillation, diabetes, and respiratory failure (Table can be found in the Supplementary material).
Discussion
Our study indicated a significant correlation between normalized lactate load and in-hospital mortality in patients with CS. Increased normalized lactate load was associated with an elevated risk of in-hospital death, even after adjusting for potential confounders. Notably, normalized lactate load exhibited superior predictive ability for in-hospital mortality compared to the first lactate value, maximum lactate value, and mean lactate value, and was comparable to the SOFA score.
In patients with CS, reduced cardiac output leads to inadequate tissue perfusion and decreased oxygen consumption, further resulting in increased lactate levels [14]. Elevated lactate levels in CS are associated with adverse outcomes due to a complex interplay of factors. The primary driver is tissue hypoperfusion from reduced cardiac output, which shifts metabolism towards anaerobic glycolysis, escalating lactate production. Impaired lactate clearance, often due to hepatic dysfunction, further exacerbates this accumulation. Additionally, adrenergic stimulation during shock increases glycolysis, potentially overwhelming the tricarboxylic acid cycle’s capacity to metabolize pyruvate, thereby augmenting lactate concentrations [15, 16]. Numerous studies have shown the importance of lactate in predicting the outcome of critically ill patients [17, 18]. In a recent study, baseline lactate value was incorporated in the early risk stratification model: IABP-SHOCK II ((Intra-aortic Balloon Pump in Cardiogenic Shock II) score in patients with CS [19]. However, the severity of organ damage is determined by the combination of lactate concentration and duration, and a single lactate value might not be comprehensive. Lactate load, defined as the AUC of lactate concentration over time, representes a composite measure of both lactate concentration and duration. Moreover, normalized lactate load, calculated by dividing AUC by time interval, provides an estimate of average intensity of hyperlactatemia. Chen et al. documented that normalized lactate load emerged as an independent risk factor for 28-day mortality in patients with septic shock, demonstrating a superior prognostic impact compared to baseline and maximum lactate levels [11]. In non-septic shock patients, the predictive ability of normalized lactate load was also superior to a single lactate value, although the accuracy was lower compared to sepsis patients [12]. In critically ill pediatric patients, normalized lactate load was identified as an independent predictor of adverse outcomes [20]. In a prior investigation, a significant association was established between normalized lactate levels and the incidence of acute kidney injury in patients undergoing cardiopulmonary bypass surgery [10]. Our study is the first to find the correlation between normalized lactate load and in-hospital mortality in individuals with CS.
Early assessment of prognosis and initiation of mechanical circulatory was crucial for patients with CS. However, there was a lack of markers to guide early treatment decisions. In patients with CS, lactate levels are elevated due to tissue and organ hypoperfusion. Target organs failure including kidney and liver failure caused by venous congestion, might further contribute to decreased lactate clearance [21, 22]. The prognostic value of a single value of lactate or LC was evaluated in previous study. In a sub-analysis of the IABP-SHOCK II trial and corresponding registry, LC was defined as the exact time difference between admission and 8 h after admission. A lactate level 8 h after admission was superior in mortality prediction compared to admission value or LC [23]. However, in a post hoc analysis of the DOREMI (Dobutamine Compared to Milrinone in the Treatment of Cardiogenic Shock) trial, complete LC was a strong predictor of in-hospital mortality in different time [24]. In our study, lactate load was calculated as the AUC of the lactate, which was more intuitive and could reflect the concentration and duration of lactate. Meanwhile, patients in IABP-SHOCK II and DOREMI trials were highly selected as they were in randomized control trials. Our study conducted subgroup analyses on heterogeneous patients, which may provide more representative results. These findings suggested that normalized lactate load was a robust predictor of mortality, consistent across diverse physiological profiles.
Lactate area, as a similar indicator with lactate load, was also evaluated in previous studies. One observational study calculated lactate area as the sum of the AUC of serial lactate levels measured every 6 h during the 24 h after admission [25]. The lactate area showed more substantial predictive power (AUC = 0.83) than the initial lactate level (AUC = 0.70) and 24 h lactate clearance (AUC = 0.72). In another study, a higher lactate area in patients with septic shock also indicated higher 28-day mortality [26]. However, the lactate area was not comparable when the length of lactate testing differed in different patients. The normalized lactate load, which represented the average lactate load during the same period, was standardized by time to enhance comparability. Our study focused on normalized lactate load, revealing a significant association between an elevated normalized lactate load and increased in-hospital mortality. Furthermore, the predictive efficacy of normalized lactate load proved to be more robust than that of initial lactate, maximum lactate, and mean lactate. This was consistent with previous findings in patients with septic shock [11]. Notably, the widely used SOFA score evaluates the prognosis of critically ill patients, including those with CS, through a complex assessment involving multiple indicators [27,28,29,30,31,32]. Encouragingly, our study revealed that normalized lactate load exhibited a predictive capability for in-hospital mortality comparable to the traditional prognostic indicator, SOFA score. Furthermore, the calculation of normalized lactate load was simpler compared to SOFA as it relied on a single lactate dimension. These findings imply that in resource-limited primary hospitals, for patients with CS, when obtaining the necessary indicators for calculating SOFA is challenging, normalized lactate load derived from routine laboratory tests can serve as an alternative predictive marker. Its utilization enables the prompt identification of high-risk and high-mortality patients, facilitating timely clinical interventions to enhance patient outcomes.
Limitation
However, our study faced specific limitations. As a retrospective analysis, determining the specific etiologies of CS for each case proved challenging, with diagnoses based solely on ICD codes rather than formal clinical criteria. This reliance on administrative rather than clinical coding may impact the accuracy and specificity of identifying genuine CS cases. Additionally, excessive missing data for inflammatory markers such as CRP (C-reactive protein) and PCT (procalcitonin) precluded their inclusion in the baseline assessment. The use of the U.S.-specific MIMIC-IV database potentially limits the generalizability of our findings. Moreover, the treatment of nearly 20% of the patients with dialysis could have influenced lactate values, impacting the assessment of normalized lactate load, which although superior to single lactate measurements in predicting mortality, presents negligible absolute differences in AUC, limiting its practical clinical application. The study was also constrained to evaluating only in-hospital mortality. Long-term outcomes and their relationship with normalized lactate load require further investigation. Lastly, while normalized lactate load offers higher predictive value for in-hospital mortality than initial or maximum lactate values, its complex calculation could hinder rapid clinical deployment. Balancing predictive accuracy with operational simplicity is essential, suggesting future research should aim to simplify methodologies to enhance clinical utility.
Conclusion
In patients with CS, the normalized lactate load has been recognized as an independent predictor of in-hospital mortality, exhibiting a predictive capacity that was comparable to the SOFA score. This underscores the potential value of normalized lactate load as a prognostic marker and its utility in guiding treatment decisions for individuals with CS.
Data availability
The data used in this study was from the Medical Information Mart for Intensive Care IV (MIMIC-IV, version 2.1) database (https://physionet.org/content/mimiciv/2.2/). The authors were approved to access to the database through (ID:12353225).
Abbreviations
- BMI:
-
Body Mass Index
- SOFA:
-
Sequential Organ Failure Assessment
- ECMO:
-
Extracorporeal Membrane Oxygenation
- OR:
-
Odds Ratio
- CI:
-
Confidence Interval
- CS:
-
Cardiogenic Shock
- MIMIC:
-
Medical Information Mart for Intensive Care
- ROC:
-
Receiver Operating Characteristic
- AUC:
-
Areas Under The Curves
- LC:
-
Lactate Clearance
- RCS:
-
Cubic Spline Curve
References
Merdji H, Levy B, Jung C, Ince C, Siegemund M, Meziani F. Microcirculatory dysfunction in cardiogenic shock. Ann Intensive Care. 2023;13(1):38.
Huckabee WE. Abnormal resting blood lactate. I. The significance of hyperlactatemia in hospitalized patients. Am J Med. 1961;30:840–8.
Weil MH, Afifi AA. Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock). Circulation. 1970;41(6):989–1001.
Klemm G, Markart S, Hermann A, Staudinger T, Hengstenberg C, Heinz G et al. Lactate as a predictor of 30-Day mortality in cardiogenic shock. J Clin Med. 2024;13(7).
Scolari FL, Schneider D, Fogazzi DV, Gus M, Rover MM, Bonatto MG, et al. Association between serum lactate levels and mortality in patients with cardiogenic shock receiving mechanical circulatory support: a multicenter retrospective cohort study. BMC Cardiovasc Disord. 2020;20(1):496.
Slottosch I, Liakopoulos O, Kuhn E, Scherner M, Deppe AC, Sabashnikov A, et al. Lactate and lactate clearance as valuable tool to evaluate ECMO therapy in cardiogenic shock. J Crit Care. 2017;42:35–41.
Nichol A, Bailey M, Egi M, Pettila V, French C, Stachowski E, et al. Dynamic lactate indices as predictors of outcome in critically ill patients. Crit Care. 2011;15(5):R242.
Attana P, Lazzeri C, Chiostri M, Picariello C, Gensini GF, Valente S. Lactate clearance in cardiogenic shock following ST elevation myocardial infarction: a pilot study. Acute Card Care. 2012;14(1):20–6.
Zhang Z, Chen K, Ni H, Fan H. Predictive value of lactate in unselected critically ill patients: an analysis using fractional polynomials. J Thorac Dis. 2014;6(7):995–1003.
Zhang Z, Ni H. Normalized lactate load is associated with development of acute kidney injury in patients who underwent cardiopulmonary bypass surgery. PLoS ONE. 2015;10(3):e0120466.
Chen H, Gong SR, Yu RG. Association between normalized lactate load and mortality in patients with septic shock: an analysis of the MIMIC-III database. BMC Anesthesiol. 2021;21(1):16.
Chen H, Gong SR, Yu RG. Increased normalized lactate load is associated with higher mortality in both sepsis and non-sepsis patients: an analysis of the MIMIC-IV database. BMC Anesthesiol. 2022;22(1):79.
Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. 2023;10(1):1.
Lazzeri C, Valente S, Chiostri M, Gensini GF. Clinical significance of lactate in acute cardiac patients. World J Cardiol. 2015;7(8):483–9.
Kubiak GM, Tomasik AR, Bartus K, Olszanecki R, Ceranowicz P. Lactate in cardiogenic shock - current understanding and clinical implications. J Physiol Pharmacol. 2018;69(1):15–21.
Attana P, Lazzeri C, Picariello C, Dini CS, Gensini GF, Valente S. Lactate and lactate clearance in acute cardiac care patients. Eur Heart J Acute Cardiovasc Care. 2012;1(2):115–21.
Bruno RR, Wernly B, Flaatten H, Fjolner J, Artigas A, Bollen Pinto B, et al. Lactate is associated with mortality in very old intensive care patients suffering from COVID-19: results from an international observational study of 2860 patients. Ann Intensive Care. 2021;11(1):128.
Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3(1):12.
Poss J, Koster J, Fuernau G, Eitel I, de Waha S, Ouarrak T, et al. Risk stratification for patients in cardiogenic shock after Acute myocardial infarction. J Am Coll Cardiol. 2017;69(15):1913–20.
Patki VK, Antin JV, Khare SH. Persistent hyperlactatemia as the predictor of poor outcome in critically Ill children: a Single-Center, prospective, observational cohort study. J Pediatr Intensive Care. 2017;6(3):152–8.
Shirakabe A, Matsushita M, Shibata Y, Shighihara S, Nishigoori S, Sawatani T, et al. Organ dysfunction, injury, and failure in cardiogenic shock. J Intensive Care. 2023;11(1):26.
Bellomo R. Bench-to-bedside review: lactate and the kidney. Crit Care. 2002;6(4):322–6.
Fuernau G, Desch S, de Waha-Thiele S, Eitel I, Neumann FJ, Hennersdorf M, et al. Arterial lactate in cardiogenic shock: Prognostic Value of Clearance Versus single values. JACC Cardiovasc Interv. 2020;13(19):2208–16.
Marbach JA, Di Santo P, Kapur NK, Thayer KL, Simard T, Jung RG, et al. Lactate Clearance as a surrogate for mortality in cardiogenic shock: insights from the DOREMI Trial. J Am Heart Assoc. 2022;11(6):e023322.
Kim YA, Ha EJ, Jhang WK, Park SJ. Early blood lactate area as a prognostic marker in pediatric septic shock. Intensive Care Med. 2013;39(10):1818–23.
Khodashahi R, Sarjamee S. Early lactate area scores and serial blood lactate levels as prognostic markers for patients with septic shock: a systematic review. Infect Dis (Lond). 2020;52(7):451–63.
Antonelli M, Moreno R, Vincent JL, Sprung CL, Mendoca A, Passariello M, et al. Application of SOFA score to trauma patients. Sequential Organ Fail Assess Intensive Care Med. 1999;25(4):389–94.
Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on sepsis-related problems of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–800.
Laimoud M, Alanazi M. The validity of SOFA score to Predict Mortality in Adult patients with cardiogenic shock on venoarterial extracorporeal membrane oxygenation. Crit Care Res Pract. 2020;2020:3129864.
Akin S, Caliskan K, Soliman O, Muslem R, Guven G, van Thiel RJ, et al. A novel mortality risk score predicting intensive care mortality in cardiogenic shock patients treated with veno-arterial extracorporeal membrane oxygenation. J Crit Care. 2020;55:35–41.
Miller RJH, Southern D, Wilton SB, James MT, Har B, Schnell G, et al. Comparative prognostic accuracy of risk prediction models for cardiogenic shock. J Intensive Care Med. 2020;35(12):1513–9.
Lambden S, Laterre PF, Levy MM, Francois B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019;23(1):374.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
XW and LY designed the study and drafted the manuscript. JX, JQ and KZ applied for the database, made statistical analysis and drafted the tables and figures. JX and LY revised the manuscript. All authors approved the final manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Institutional Review Committee of Beijing Anzhen Hospital. Human participants data used in this study were derived from the MIMIC-IV.As stated on the official website: “The collection of patient information and creation of the research resource was reviewed by the Institutional Review Board at the Beth Israel Deaconess Medical Center, who granted a waiver of informed consent and approved the data sharing initiative.” (https://physionet.org/content/mimiciv/2.2/).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, X., Yuan, L., Xu, J. et al. Normalized lactate load as an independent prognostic indicator in patients with cardiogenic shock. BMC Cardiovasc Disord 24, 348 (2024). https://doi.org/10.1186/s12872-024-04013-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-04013-8