Abstract
Background
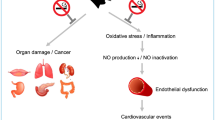
Tobacco use is recognized as a major cause of cardiovascular disease, which is associated with endothelial dysfunction. Endothelial function is evaluated using flow-mediated dilation (FMD), which is a noninvasive method. This meta-analysis aimed to investigate the association between smoking exposure and endothelial function evaluated using FMD values.
Methods
We searched the PubMed, Embase, Web of Science, and Cochrane Library databases for cohort studies of smokers or passive smokers that used FMD to assess endothelial function. The primary outcome of the study was the change in the rate of FMD. The risk of bias was evaluated using the Cochrane Collaboration tool and Newcastle–Ottawa Scale. Further, the weighted mean difference was used to analyze the continuous data.
Results
Overall, 14 of 1426 articles were included in this study. The results of these articles indicated that smoking is a major cause of endothelial dysfunction and altered FMD; a pooled effect size of − 3.15 was obtained with a 95% confidence interval of (− 3.84, − 2.46). Notably, pregnancy status, Asian ethnicity, or health status did not affect heterogeneity.
Conclusions
We found that smoking has a significant negative impact on FMD, and measures such as medication or education for smoking cessation may improve endothelial function and reduce the risk of cardiovascular disease.
Trial Registration
The meta-analysis was registered with PROSPERO on April 5th, 2023 (CRD42023414654).
Similar content being viewed by others
Background
Tobacco-related diseases are recognized as a significant global burden, causing an estimated 7 million deaths per year. Moreover, tobacco and tobacco smoke contain over 7,000 chemicals, most of which are toxic [1]. Compared to nonsmokers, smokers have a 20-year shorter life expectancy, resulting in significant economic implications [2, 3]. Moreover, smoking is well-known as a risk factor for the development and progression of cardiovascular diseases (CVDs), such as ischemic stroke, coronary artery disease, and peripheral artery disease. Approximately 10% of all cardiovascular deaths in adults are caused by cigarette smoking [4].
Cardiovascular morbidity and mortality due to smoking can be attributed to various pathophysiological mechanisms [5]. Notably, endothelial dysfunction is considered a crucial early sign of the development of coronary atherosclerosis, and its early detection may help prevent CVDs. Several studies, both clinical and animal-based, have reported that exposure to cigarette smoke and its components can induce vascular endothelial pathology by reducing the availability of nitric oxide (NO). The reaction of NO with free radicals present in smoke together with direct physical damage to endothelial cells results in altered biosynthesis and reduced activity of NO [6, 7]. Consequently, the ability of the endothelium to maintain its vasodilatory and anti-inflammatory, -thrombotic, and -oxidant effects is impaired.
Assessment of endothelial function is usually performed using flow-mediated dilation (FMD), which is a noninvasive approach for measuring changes in the diameter of the brachial artery in response to shear stress induced by reactive hyperemia [8]. In the present study, we performed a systematic review and meta-analysis to evaluate the association between smoking exposure and endothelial function evaluated using FMD values. Furthermore, the effects of factors such as pregnancy status, Asian ethnicity, and health status of smokers on endothelial function were examined.
Methods
We followed the statements on the Preferred Reporting Items for Systematic Reviews and Meta-analyses and the Meta-analysis of Observational Studies in Epidemiology. The meta-analysis was registered with PROSPERO on April 5th, 2023 (CRD42023414654).
Search strategy
A comprehensive literature search in the PubMed, EMBASE, Cochrane Library, and Web of Science databases was conducted. The date limit was invalid until February 21, 2023. Searches included index and text terms and were limited to titles or abstracts (see the Additional file 1 for the complete search strategy). No language or study design restrictions were employed. The search strategy was adapted to the syntax applicable to each database.
Inclusion and exclusion criteria
The inclusion criteria were as follows: studies published in English or Chinese, studies on smokers or passive smokers, studies assessing endothelial function using FMD, and observational studies. The exclusion criteria were as follows: abstracts, letters, talks, or reviews; studies in which FMD was not used to assess endothelial function; studies involving animal testing; studies with insufficient data for statistical analysis; studies without nonexposure group; studies whose full text was not available; and duplicate studies with data already included in the meta-analysis.
Data extraction
All included studies were independently examined by three reviewers, and the following data were extracted: author; publication year; study design type; region of data set; cigarette type; N, mean, and standard deviation of the experimental and control groups; inclusion of patient characteristics, including pregnancy status, health status, and sex. Should disagreements occur, a fourth reviewer will be consulted.
Quality assessment
Three reviewers independently scored the quality of the included studies using the Newcastle–Ottawa Scale (NOS) criteria, which can provide a maximum score of 9. This scale evaluates the quality of non-randomized, cohort, and case-control studies in relation to their design, content, and ease of use. When in doubt or should discrepancies occur, a fourth researcher will be consulted.
Statistical analysis methods
Heterogeneity was detected using Q-test and I2-test, and a random-effects model was selected if I2 was greater than 50%. A subgroup analysis was conducted to assess sources of heterogeneity between studies. The following subgroups were considered: tobacco type, pregnancy status, sex, and the region to which the exposed population belonged. Continuous data were combined using weighted mean difference statistics. Statistical significance was set at a p-value less than or equal to 0.05, and STATA 15.1 was used for all statistical analyses.
Results
Basic characteristics
The flowchart for the selection of eligible studies is shown in Fig. 1. The database search identified 1426 publications. The detailed search strategy is presented in Additional file 1. The titles and abstracts of the remaining 861 publications were reviewed after eliminating duplicate records. After a thorough review, 79 of these publications were deemed eligible. After reviewing the full text of 79 articles, 65 of these publications did not satisfy our inclusion criteria. Specifically, one was an animal trial, 28 had insufficient data for statistical analysis, five did not have a nonexposure group, three were not available in full text, seven did not match the study type, and 21 did not match the topic. Finally, 14 articles were determined to be eligible for inclusion in this meta-analysis.
The NOS scores of the included studies are reported in Table 1. Most studies were of good quality, with quality assessment scores of ≥ 7 (out of 9), and only one had a score of 5 (Table 1).
Meta-analysis results
An overview of the characteristics of the included studies is provided in Table 2 [9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Overall, there were 17 observational studies published from 1998 to 2021, and tobacco types were classified into two categories: conventional and hookah. Four studies compared outcomes between pregnant and nonpregnant women, nine studies mentioned sex of the participants, and four studies included unhealthy populations.
The pooled effect size was − 3.15 with a 95% confidence interval of (− 3.84, − 2.46), and the results were statistically significant (P < 0.01). The results showed that smokers had reduced FMD values compared to non-smokers, while reduced FMD values indicated impaired vascular endothelial function. Therefore, smoking may affect the vascular endothelial function of patients and increase the risk of cardiovascular disease (Fig. 2a).
In terms of age, there was no significant difference in the age distribution between the exposed and non-exposed groups (P = 0.9076). As for the gender ratio, it could not be statistically analyzed because there were only three articles in the study that mentioned the gender ratio (Table 3).
Subgroup analysis
Subgroup analysis demonstrated that pregnant and nonpregnant populations were not a source of heterogeneity. Smoking alone reduced FMD regardless of pregnancy (Fig. 2b). Similarly, a good or bad health status was not a cause of heterogeneity (Fig. 2c). Finally, the presence or absence of Asian populations was also not a source of heterogeneity, suggesting the existence of other unknown potential causes of heterogeneity (Fig. 2d).
In terms of tobacco type, the results showed that both conventional tobacco and hookah reduced FMD values, possibly affecting their endothelial function (Cigarettes: P = 0.001, I2 = 85.5%, PI2 = 0.001; Water pipes: P = 0.001, I2 = 85.5%, PI2 = 0.600) (Fig. 3).
Publication bias
Finally, Egger’s test was used to evaluate for publication bias, and the results revealed the absence of publication bias in our study (P = 0.429) (Additional file 2).
Influence analysis
The findings of the study revealed that out of the 17 studies included, none of the remaining 16 studies exhibited statistically significant pooled results when any one study was excluded. This consistency with the original pooled effect size of -3.15 (95% CI: -3.84, -2.46) suggests that the results of the meta-analysis were robust and stable (Additional file 3).
Discussion
Tobacco use is an important risk factor for CVDs, including hypertension, atherosclerosis, and stroke. One of the mechanisms by which smoking contributes to CVD is by altering endothelial function in blood vessels, which is assessed by FMD. An analysis of the 14 articles included in our meta-analysis suggested that smoking can reduce FMD and impair vascular endothelial function; notably, most of the studies were of good quality, with a quality assessment score of ≥ 7. Additionally, no heterogeneous changes in cigar type, pregnancy status, geography, or health status were identified in the subgroup analysis, suggesting the presence of other potential unknown causes.
Impaired FMD is well-known as a predictor of CVD, and smoking is known to decrease FMD in both smokers and nonsmokers. In the study by Celermajer et al. [23], Among smokers, the FMD values are notably reduced by 24% in comparison to non-smoking counterparts, with this detrimental effect persisting beyond four weeks post-smoking cessation. This observation implies a potential deleterious impact of smoking on vascular endothelial function, culminating in compromised vasodilatory capacity. The observed decrement in vasodilation is plausibly linked to the oxidative stress, inflammatory responses, and endothelial cell injury instigated by smoking, all of which are well-established contributors to the pathogenesis of atherosclerosis. The manifestation of FMD alterations is subject to variability influenced by lifestyle choices. In particular, the presence of diabetes mellitus may accentuate the adverse effects on FMD [24], whereas in other scenarios, the impact of smoking parallels that of diabetes [25,26,27]. Many studies have reported that smoking decreases FMD and impairs endothelial function, regardless of pregnancy status. For example, one study reported that smoking acutely decreases FMD in both pregnant and nonpregnant women, indicating that the deleterious effects of smoking on endothelial function are not limited to pregnant women [28]. Subgroup analysis of the study revealed that pregnant and nonpregnant populations did not represent one source of heterogeneity. Further, the deleterious effects of cigarette smoking on endothelial function and FMD are not limited to adults. Several studies have reported that exposure to passive smoke significantly reduces FMD, which further induces endothelial dysfunction, in healthy young adults [29, 30]. Exposure to parental smoking was found to be associated with reduced vascular function in both young children and adolescents, which was particularly evident in those whose parents had smoked during pregnancy [31]. These findings underscore the importance of protecting children and adolescents from exposure to tobacco smoke.
The association between smoking exposure and endothelial function evaluated using FMD values has been investigated in several studies. In a study conducted in Asia, the effect of cigarette smoking on FMD was studied in 71 Japanese men [32]. They found that smokers had a significantly lower FMD than nonsmokers. These studies suggest that smoking is associated with impaired endothelial function through FMD measurement conducted worldwide. Moreover, our results indicate that the presence or absence of Asian populations was not a source of heterogeneity. These investigations collectively illuminate a pervasive correlation between tobacco smoking and compromised endothelial function, as evidenced by assessments of Flow-Mediated Dilation (FMD). Nevertheless, the exactitude of FMD as a biomarker for endothelial dysfunction merits further elucidation. Despite its status as a prevalent non-invasive diagnostic instrument, the uniformity and dependability of FMD assessments may be contingent upon a spectrum of determinants, ranging from lifestyle factors to genetic underpinnings and environmental exposures.
Diabetes and obesity are known to independently contribute to a decline in flow-mediated dilation (FMD) through the augmentation of oxidative stress and the increased release of inflammatory cytokines from adipose tissue, such as tumor necrosis factor-alpha (TNF-α) and C-reactive protein (CRP) [33]. The chronic low-grade inflammatory state and insulin resistance associated with metabolic syndrome, including hypertension, further exacerbate the reduction in FMD. Studies have demonstrated that, compared to a control group, patients with hypertension exhibit significantly lower FMD values, and the migratory and proliferative functions of endothelial progenitor cells (EPCs) are markedly diminished (P < 0.05). Additionally, a strong positive correlation exists between the migratory and proliferative capacities of circulating EPCs and FMD (migration: r = 0.56, proliferation: r = 0.41, P < 0.05) [34]. In individuals with type 2 diabetes, chronic smokers have been found to have impaired microvascular reactivity, with a significant reduction in flow-mediated microvascular dilation response compared to non-smokers [35]. The deleterious effects of smoking on cardiovascular health that lead to endothelial dysfunction and reduced FMD are well-established, regardless of the presence of an underlying disease. In the current study, the patient characteristics were confined to either a healthy cohort or healthy pregnant women, thereby eliminating confounding factors that could distort the imaging outcomes. As a result, the findings are expected to be robust. Various studies on smoking cessation interventions have reported significant improvements in vascular endothelial function in successful quitters, regardless of the intervention, medication [36,37,38,39] or education for smoking cessation [39,40,41]. Furthermore, Schroeter et al. revealed that smoking-induced endothelial dysfunction is dose-dependent and reversible upon cessation of smoking [42]. Given that pregnancy status, Asian ethnicity, or health status did not affect heterogeneity, the existence of other unknown potential causes was considered, such as genotype or other environmental pollutant exposures. A previous study investigated the effect of a genetic variation in the promoter region of interleukin-6 (IL-6) gene on endothelial function in healthy volunteers. The findings of that study revealed that individuals carrying the C allele of the IL-6–174G > C polymorphism had impaired endothelial function compared to those carrying the GG genotype [43]. This finding highlights the potential role of the IL-6 gene in the CVD development via its endothelial function effects.
Conclusions
The results of this study revealed that smoking alone is a major contributor to endothelial dysfunction and FMD impairment, regardless of pregnancy status, Asian ethnicity, and health status. Other possible causes of endothelial dysfunction and FMD impairment may include genotype variations.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- FMD:
-
Flow-mediated dilation
- CVDs:
-
Cardiovascular diseases
- NO:
-
Nitric oxide
- NOS:
-
Newcastle–Ottawa Scale
- IL-6:
-
Interleukin-6
References
Cui M, Cui R, Liu K, Dong JY, Imano H, Hayama-Terada M, et al. Associations of Tobacco Smoking with impaired endothelial function: the circulatory risk in communities Study (CIRCS). J Atheroscler Thromb. 2018;25:836–45.
Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer. 2009;9:655–64.
Max W, Sung HY, Shi Y. Deaths from secondhand smoke exposure in the United States: economic implications. Am J Public Health. 2012;102:2173–80.
Ezzati M, Henley SJ, Thun MJ, Lopez AD. Role of smoking in global and regional cardiovascular mortality. Circulation. 2005;112:489–97.
Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34:509–15.
Papathanasiou G, Mamali A, Papafloratos S, Zerva E. Effects of smoking on cardiovascular function: the role of nicotine and carbon monoxide. Health Sci J. 2014;8:274.
Hahad O, Arnold N, Prochaska JH, Panova-Noeva M, Schulz A, Lackner KJ, et al. Cigarette smoking is related to endothelial dysfunction of resistance, but not conduit arteries in the General Population-results from the Gutenberg Health Study. Front Cardiovasc Med. 2021;8:674622.
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial artery reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65.
Nicolau LG, Martins WP, Gallarreta FM, Lima JC, Filho FM. Influence of pregnancy and smoking on brachial artery flow-mediated dilation values and time until maximum response. Arch Gynecol Obstet. 2011;284:313–7.
Heffernan KS, Karas RH, Patvardhan EA, Kuvin JT. Endothelium-dependent vasodilation is associated with exercise capacity in smokers and non-smokers. Vasc Med. 2010;15:119–25.
Corretti MC, Plotnick GD, Vogel RA. Smoking correlates with flow-mediated brachial artery vasoactivity but not cold pressor vasoactivity in men with coronary artery disease. Int J Card Imaging. 1998;14:11–7.
Hashimoto H, Maruhashi T, Yamaji T, Harada T, Han Y, Takaeko Y, et al. Smoking status and endothelial function in Japanese men. Sci Rep. 2021;11:95.
Miyata S, Noda A, Ito Y, Iizuka R, Shimokata K. Smoking acutely impaired endothelial function in healthy college students. Acta Cardiol. 2015;70:282–5.
Suzuki K, Washio T, Tsukamoto S, Kato K, Iwamoto E, Ogoh S. Habitual cigarette smoking attenuates shear-mediated dilation in the brachial artery but not in the carotid artery in young adults. Physiol Rep. 2020;8:e14369.
Ozaki K, Hori T, Ishibashi T, Nishio M, Aizawa Y. Effects of chronic cigarette smoking on endothelial function in young men. J Cardiol. 2010;56:307–13.
Esen AM, Barutcu I, Acar M, Degirmenci B, Kaya D, Turkmen M, et al. Effect of smoking on endothelial function and wall thickness of brachial artery. Circ J. 2004;68:1123–6.
Quinton AE, Cook CM, Peek MJ. The relationship between cigarette smoking, endothelial function and intrauterine growth restriction in human pregnancy. BJOG. 2008;115:780–4.
Tanriverdi H, Evrengul H, Kuru O, Tanriverdi S, Seleci D, Enli Y, et al. Cigarette smoking induced oxidative stress may impair endothelial function and coronary blood flow in angiographically normal coronary arteries. Circ J. 2006;70:593–9.
Hamesa M, Gaber R. The effects of pipe water smoking on endothelial function in healthy non smoker volunteers. Artery Res. 2016;15:1–5.
Diab OA, Abdelrahim EM, Esmail M. Effect of water pipe tobacco smoking on plasma high sensitivity C reactive protein level and endothelial function compared to cigarette smoking. Egypt Heart J. 2015;67:233–41.
Yao Fengjuan HY, Lin H, Donghong L, Rui F, Yanqiu L, Koon L. Deng Chunhua, Sun Xiangzhou. Study of the effects of smoking on vascular endothelial function in patients with ED. Chin J Male Sci. 2011;17:414–7.
Ludwig A, Jochmann N, Kertesz A, Kuhn C, Mueller S, Gericke C, et al. Smoking decreases the level of circulating CD34 + progenitor cells in young healthy women–a pilot study. BMC Womens Health. 2010;10:20.
Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter D, Miller O, Sullivan I, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5.
Qazi SU, Qamar U, Maqsood MT, Gul R, Ansari SA, Imtiaz Z, et al. Efficacy of Allopurinol in improving endothelial dysfunction: a systematic review and Meta-analysis. High Blood Press Cardiovasc Prev. 2023;30:539–50.
Grech J, Norman IJ, Sammut R. Helping smokers with diabetes quit: a scoping review of the interventions utilised, and the challenges and barriers to smoking cessation. Prim Care Diabetes. 2023;17:119–28.
Peng K, Chen G, Liu C, Mu Y, Ye Z, Shi L, et al. Association between smoking and glycemic control in diabetic patients: R esults from the R isk E valuation of c A ncers in C hinese diabe T ic I ndividuals: a l ON gitudinal (REACTION) study. J Diabetes. 2018;10:408–18.
Durlach V, Verges B, Al-Salameh A, Bahougne T, Benzerouk F, Berlin I, et al. Smoking and diabetes interplay: a comprehensive review and joint statement. Diabetes Metab. 2022;48:101370.
Kim JW, Park CG, Hong SJ, Park SM, Rha SW, Seo HS, et al. Acute and chronic effects of cigarette smoking on arterial stiffness. Blood Press. 2005;14:80–5.
Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol. 2008;51:1760–71.
Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–10.
Juonala M, Magnussen CG, Raitakari OT. Parental smoking produces long-term damage to vascular function in their children. Curr Opin Cardiol. 2013;28:569–74.
Kawano H, Motoyama T, Hirai N, Kugiyama K, Yasue H, Ogawa H. Smoking cessation reverses endothelial dysfunction in healthy young smokers. J Am Coll Cardiol. 2010;35:905–9.
Liu W, Xiong Y, Li L. Research Progress on the mechanism of obesity-Induced Cardiovascular Disease. Adv Clin Med. 2023;13:12887.
Ji B, Wang Z, Huang Z, Liu W. Change of the functional activity of endothelial progenitor cells and their correlation with FMD in primary hypertension patients. Adv Clin Med. 2021;11:6143.
Low BH, Lin YD, Huang BW, Chia T, Bau JG, Huang HY. Impaired microvascular response to muscle stretching in chronic smokers with type 2 diabetes. Front Bioeng Biotechnol. 2020;8:602.
Lu Yan YX, Jiang Yinong Z. Effects of varenicline tartrate smoking cessation on vascular endothelial function. J Dalian Med Univ. 2010;32:4.
Young JM, Shand BI, McGregor PM, Scott RS, Frampton CM. Comparative effects of enzogenol and vitamin C supplementation versus vitamin C alone on endothelial function and biochemical markers of oxidative stress and inflammation in chronic smokers. Free Radic Res. 2006;40:85–94.
Mah E, Pei R, Guo Y, Ballard KD, Barker T, Rogers VE, et al. Gamma-tocopherol-rich supplementation additively improves vascular endothelial function during smoking cessation. Free Radic Biol Med. 2013;65:1291–9.
Mah E, Pei R, Guo Y, Masterjohn C, Ballard KD, Taylor BA, et al. Greater gamma-tocopherol status during acute smoking abstinence with nicotine replacement therapy improved vascular endothelial function by decreasing 8-iso-15(S)-prostaglandin F2alpha. Exp Biol Med (Maywood). 2015;240:527–33.
Jodoin I, Bussières LM, Tardif J-C, Juneau M. Effect of a short-term primary prevention program on endothelium-dependent vasodilation in adults at risk for atherosclerosis. Can J Cardiol. 1999;15:83–8.
Blane DN, Mackay D, Guthrie B, Mercer SW. Smoking cessation interventions for patients with coronary heart disease and comorbidities: an observational cross-sectional study in primary care. Br J Gen Pract. 2017;67:e118–29.
Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, et al. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103:1024–9.
Brull DJ, Leeson CP, Montgomery HE, Mullen M, deDivitiis M, Humphries SE, et al. The effect of the Interleukin-6-174G > C promoter gene polymorphism on endothelial function in healthy volunteers. Eur J Clin Invest. 2002;32:153–7.
Acknowledgements
Not applicable.
Funding
This study is supported by the National Natural Science Foundation of China (No. 82000252, 82200390); Postdoctoral program of Shaoxing People’s Hospital(2021BSQDJ01); The 551 talent project of Zhejiang Province.
Author information
Authors and Affiliations
Contributions
X.J. contributed to study design. P.Z. and F.P. contributed to drafting and editing of the manuscript. L.M. contributed to data collection. W.T. contributed to statistical analysis. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study is a meta-analysis which contains no participants and ethics approval and consent to participate is not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jia, X., Zhang, P., Meng, L. et al. The association between smoking exposure and endothelial function evaluated using flow-mediated dilation values: a meta-analysis. BMC Cardiovasc Disord 24, 292 (2024). https://doi.org/10.1186/s12872-024-03915-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-03915-x