Abstract
Background
Early diagnosis of atrial fibrillation is important as it is crucial for improving patient outcomes. Fibroblast growth factor-2 (FGF2) may serve as a diagnostic biomarker for heart failure due to its ability to promote cardiac fibrosis and hypertrophy; however, the relationship between FGF2 concentration and heart failure is unclear. Therefore, this study aimed to explore whether FGF2 could aid in distinguishing patients with heart failure from healthy controls and those with dyspnea without heart failure. Additionally, to evaluate the possible correlation between serum FGF2 levels and its diagnostic parameters in patients with heart failure.
Methods
Plasma FGF2 concentration was measured in 114 patients with a complaint of dyspnea (enrolled in the study between January 2022 and August 2022). Based on heart failure diagnosis, the patients were assigned to three groups, as follows: heart failure (n = 80), non-heart-failure dyspnea (n = 34), and healthy controls (n = 36), following physical examination. Possible correlations between serum FGF2 levels and other prognostic parameters in patients with heart failure were analyzed.
Results
Serum FGF2 levels were higher in patients with heart failure (125.60 [88.95, 183.40] pg/mL) than in those with non-heart-failure dyspnea (65.30 [28.85, 78.95] pg/mL) and healthy controls (78.90 [60.80, 87.20] pg/mL) (p < 0.001). Receiver operating characteristic curve analysis identified FGF2 concentration as a significant predictor in heart failure diagnosis, with an area under the curve of 0.8693 (p < 0.0001). Importantly, in the heart failure group, serum FGF2 concentrations correlated with key prognostic parameters for heart failure, such as reduced left ventricular ejection fraction and elevated serum levels of N-terminal pro-B-type natriuretic peptide.
Conclusions
Elevated serum FGF2 level is strongly associated with an increased risk of heart failure and could serve as a useful biomarker to complement vital diagnostic parameters for heart failure.
Similar content being viewed by others
Background
Heart failure (HF) is a complex, life-threatening clinical syndrome with high prevalence, incidence, mortality, and health care costs and a profound socioeconomic burden worldwide [1]. It is currently defined as “a clinical syndrome with symptoms and/or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion” according to the European Society of Cardiology guidelines [2]. HF prevalence in developed countries is 1.5–2.0%, and it accounts for substantial morbidity and mortality worldwide [2, 3]. Therefore, prediction, early diagnosis, and treatment guidance are crucial.
Myocardial fibrosis is closely related to the occurrence and development of HF [2]. Specific biomolecules involved in pathophysiological processes, such as myocardial fibrosis and remodeling, may serve as HF biomarkers [4, 5]. Fibroblast growth factor-2 (FGF2) is a broad-spectrum, potent, mitogenic and angiogenic factor [6] essential in heart development, homeostasis, disease, and repair [7]. FGF2 can promote cardiac hypertrophy and fibrosis [8,9,10,11] through its high-molecular-weight FGF2 isoform (hi-FGF2) [12,13,14]. Our previous study reveals a novel mechanism, by which cells overexpressing hi-FGF2 can induce mitochondria-associated apoptosis which is closely associated with HF [15].The level of hi-FGF2 can serve as a prognostic biomarker to predict the occurrence of HF in patients with atrial fibrillation (AF) [15, 16]. However, the relationship between FGF2 concentration and HF is unclear.
We hypothesized that the FGF2 level may be an HF biomarker due to its ability to promote cardiac fibrosis and hypertrophy. Hence, we aimed to evaluate and compare serum FGF2 levels in patients with HF, those having dyspnea without HF, and healthy controls. To the best of our knowledge, this is the first study to compare FGF2 levels between these groups of individuals. Furthermore, we calculated the area under the receiver operating characteristic curve (AUC) to shed light on the utility of FGF2 levels in HF diagnosis. Similarly, we explored the correlation of FGF2 levels with known prognostic clinical markers of HF, such as N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration and cardiac function parameters (left ventricular ejection fraction [LVEF]), to determine the ability of FGF2 expression level to indicate HF severity.
Methods
Study population
We enrolled 36 healthy volunteers and 114 patients admitted to the hospital for dyspnea between January 2022 and August 2022. Among the patients with dyspnea, 80 and 34 were determined to have HF and normal cardiac function, respectively. General demographic data were collected. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Shantou University Medical College, Shantou, China (NO. 2021–13), and conducted in accordance with the principles outlined in the Declaration of Helsinki. Plasma was obtained from patients. Written informed consent was obtained from all patients at the study entry.
Inclusion criteria for the HF group were consistent with those of the Framingham diagnostic criteria. If HF was excluded through clinical diagnosis and dyspnea symptoms were observed, a patient was assigned to the non-HF dyspnea group. Unknown coronary, valvular, or myocardial diseases did not affect the enrolment of healthy controls. Exclusion criteria for all groups included the presence of rheumatic heart disease, severe liver and kidney diseases, immune dysfunction, pregnancy, malignancy, or acute myocardial infarction (MI).
Demographic, clinical, and baseline biochemical data were collected for all participants. In addition, echocardiographic parameters in the HF and dyspneic non-HF groups were collected to identify cardiac hypertrophy.
Measurement of serum FGF2 concentration
Baseline peripheral venous blood was collected from all participants in a vacuum tube containing potassium ethylene diamine tetraacetic acid, naturally solidified at 37°C for 30 min, and centrifuged at 1000 g for 15 min. Separated serum was placed in a centrifuge tube and stored at − 80 °C. FGF2 content was measured using an enzyme-linked immunosorbent assay kit (Proteintech Group, Rosemont, IL, USA; stock number EK0342) and Tecan.
Statistical analysis
All statistical analyses were performed using SPSS 26.0 software (version 22.0; IBM Corporation, Armonk, NY, USA) and GraphPad Prism 8 software (GraphPad Software, Inc., Boston, MA, USA). Continuous variables with normal distribution are expressed as means and standard deviations (x ± s), and comparisons between multiple groups were performed using one-way analysis of variance and pairwise comparisons using the Student–Newman–Keuls method. Non-normally distributed variables are expressed as medians and interquartile ranges. The rank sum test was used for comparisons between multiple groups. Categorical clinical variables are presented as counts (percentages) and were compared using the chi-square test.
The capacity of FGF2 level to discriminate between HF, dyspnea without HF, and healthy controls was characterized using a receiver operating characteristic curve, and the AUC was calculated. Considering the relationship between serum FGF2 levels and other variables, such as LVEF and NT-proBNP concentration, was non-linear, Spearman’s correlation coefficient was used to assess these associations. Statistical significance was determined at p < 0.05.
Results
Characteristics of the study population
This study included 114 patients with dyspnea diagnosed with (n = 80) and without (n = 34) HF, along with 36 healthy controls. The clinical characteristics and laboratory findings of each group are shown in Table 1. No significant differences between groups were observed regarding diastolic blood pressure, hemoglobin level, platelet count, and serum sodium, chloride, and phosphorus concentration (p > 0.05). However, significant between-group differences were observed in smoking habits, comorbidities (including hypertension, diabetes, coronary heart disease, chronic kidney disease, atrial fibrillation, MI, and cerebral infarction), drug regimens, white blood cell and neutrophil counts, and high- and low-density lipoprotein cholesterol levels. LVEF% was significantly reduced in patients with HF than in patients with non-HF dyspnea, LVEDD, LVESD, E/E’ and LVM(g) were similarly raised in the HF group. Meanwhile, biochemical indicators, such as cystatin C, FGF2, NT-proBNP, creatinine, and high-sensitivity C-reactive protein, were significantly higher in patients with HF. FGF2 expression levels in each group are shown in Fig. 1. No significant differences were observed in FGF2 expression between patients with dyspnea without HF (65.30 [28.85, 78.95] pg/mL) and healthy controls (78.90 [60.80, 87.20] pg/mL) (p > 0.05). FGF2 concentration was elevated in the HF group (125.60 [88.95, 183.40] pg/mL) compared with that in the non-HF dyspnea and healthy control groups (p < 0.001).
FGF2 levels in patients with HF, those having dyspnea without HF, and healthy controls. Data represent mean ± SD (n = 3). Statistical significance was calculated via one-way analysis of variance, * p < 0.05; ** p < 0.01; *** p < 0.001. Abbreviations: FGF2, fibroblast growth factor 2; HF, heart failure; SD, standard deviation
Diagnostic accuracy of FGF2 concentration
The AUC value of FGF2 concentration for distinguishing patients with HF and healthy controls was 0.8693 (95% confidence interval [CI]: 0.8064–0.9322), whereas the value distinguishing patients with HF, non-HF dyspnea, and healthy controls was 0.8954 (95% CI: 0.8486–0.9420) (Fig. 2). Similarly, the AUC value distinguishing HF from non-HF dyspnea was high, suggesting the robustness of the diagnostic power of FGF2 concentration (AUC, 0.9232; 95% CI: 0.8749–0.9715).
Correlation of FGF2 with other HF parameters
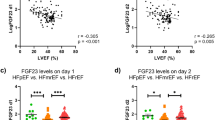
To investigate whether FGF2 levels are associated with disease severity, HF type, or HF etiology, we classified patients with HF according to LVEF, New York Heart Association (NYHA) grade, HF type, and underlying etiology. The results are illustrated in Fig. 3. No differences were observed in FGF2 levels between the LVEF > 45% and LVEF < 45% groups (p > 0.05) nor between the different NYHA groups. Although the level of circulating FGF2 increased as the NYHA grades increased (Ι–III), no significant differences were observed between the groups (p > 0.05). FGF2 levels are not significantly elevated in the HF(+)AF(+) cohort compared to the HF(+)AF (−)group(p > 0.05) (Fig. S1). Additionally, patients with different HF types (reduced ejection fraction, mildly reduced ejection fraction, and preserved ejection fraction) and etiologies did not differ significantly (p > 0.05). These results suggest that FGF2 concentration is limited in distinguishing HF with different severities, types, and causes.
Correlation of FGF2 with other HF parameters. (a) Circulating levels of FGF2 in healthy controls (n = 36) and HF cases with ejection fraction (EF) < 45% (n = 16) and EF > 45% (n = 64). (b) Expression levels of FGF2 according to New York Heart Association cardiac function classification. (c) Expression levels of FGF2 in different HF types. (d) Expression levels of FGF2 in groups with different HF etiologies. ** p < 0.01; *** p < 0.001. Abbreviations: FGF2, fibroblast growth factor 2; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction
To further explore whether serum FGF2 concentration is associated with functional changes in the heart and HF severity, the correlations of FGF2 concentration with LVEF and NT-proBNP concentration were analyzed. Serum FGF2 concentration had a weak negative correlation with LVEF (rs = − 0.4304, p < 0.0001) and a moderate positive correlation with NT-proBNP concentration (rs = 0.5678, p < 0.0001) (Fig. 4).
Discussion
This study demonstrated that elevated FGF2 concentrations were observed in patients with HF relative to patients with non-HF dyspnea and healthy controls. FGF2 level correlated with crucial prognostic parameters for HF, such as reduced LVEF and elevated levels of NT-proBNP, it could serve as a vital biomarker for HF.
Mechanisms of HF are complex and related to the structure and systolic remodeling of the atrial and ventricular myocardium. Increased oxygen demand because of various causes drives the heart to initiate an adaptive remodeling process. Cardiac remodeling includes pathological cardiomyocyte hypertrophy and fibrosis. However, persistent fibrosis progression and extracellular matrix formation can increase myocardial stiffness, causing ventricular systolic and diastolic dysfunction and HF [4]. Certain biomolecules mediating inflammatory and pathophysiological processes, such as myocardial fibrosis and remodeling, can be HF biomarkers [5, 6]. Fibroblasts, myofibroblasts, and extracellular matrix components actively shape and respond to atrial fibrosis. FGF2 is important in atrial fibrosis and is located in the cytoplasm, nucleus, and extracellular matrix in developmental and adult atria [17]. FGF2 is crucial for in vitro myocardial remodeling [18], and the FGF2-FGF receptor-1 axis is a potential therapeutic target for treating cardiac hypertrophy [19].
Similarly, FGF2 expression is reportedly upregulated in patients with pressure or volume overload, causing left or right ventricular hypertrophy [20], and increased FGF2 levels are closely associated with human atrial fibrosis [19]. Although the findings of previous studies [21, 22] suggested that FGF2 plays a pivotal role in atrial fibrosis and remodeling, the role of hi-FGF2 in mediating atrial extracellular matrix regulation, fibrosis, and remodeling is controversial. Li et al. [23] demonstrated that increased levels of hi-FGF2 were closely interrelated with fibrotic human atria and might accelerate atrial fibrosis via the mitogen-activated protein kinase signaling pathway. Conversely, in a mouse model of MI, the lack of low molecular weight FGF2 resulted in greater MI scar formation [19]. As opposed to the low molecular weight FGF2 subtype, hi-FGF2 induces cardiomyocyte hypertrophy in vitro [18]. Ling-Yue et al. [16] confirmed that hi-FGF2 concentration can help predict the occurrence of HF in patients with atrial fibrillation and is an independent risk factor. Here, we demonstrated that the circulating levels of FGF2 are specifically elevated in patients with HF. Although hi-FGF2 accounts for 70% of the total cardiac FGF2 [23], whether hi-FGF2 dominates the circulating level remains unknown. However, building on the results of this study, we speculate that the circulating FGF2 primarily exists as hi-FGF2. Further studies are needed to confirm this hypothesis.
The symptoms and signs of some non-vascular diseases, such as anemia, lung disease, kidney disease, and thyroid disease, may be clinically similar to those of HF. To explore FGF2 specificity, we enrolled healthy participants and patients having dyspnea without HF as controls. The results showed that patients with HF had significantly higher FGF2 levels than those having non-HF dyspnea and healthy controls. The AUC was 0.8963, demonstrating that FGF2 can distinguish HF from other causes of dyspnea and is specific for HF. The results were less affected by cardiac cell injury due to the exclusion of patients with recent acute MI. Therefore, FGF2 may be a specific, novel HF biomarker that facilitates more accurate HF diagnosis, detection, and monitoring of cardiac injury before it develops to an irreversible stage.
Our data revealed a negative correlation between FGF2 concentration and LVEF, indicating a possible relationship between FGF2 level and impaired cardiac function and that FGF2 level may stratify the risk for HF. The risk of HF increases as the NT-proBNP level increases [24], and the NT-proBNP level is closely related to HF severity, NYHA grade, end-diastolic pressure, and the degree of hemodynamic disturbance [25]. NT-proBNP level is a robust biomarker for predicting readmission and mortality in patients with acute decompensated HF [26]. It has great value for diagnosis and short- and long-term prognostic evaluation in patients with concurrent dyspnea and suspected or confirmed acute HF [27, 28]. In this study, we found that FGF2 level was moderately correlated with NT-proBNP level, indicating that FGF2 level may be associated with the prognosis of patients with HF and could potentially be used for their risk stratification. However, follow-up data are needed to support this conjecture.
This study had certain limitations. Although we included patients with non-HF dyspnea, the overall number of cases with missing data was small. Hence, larger studies are needed to increase the validity and reliability of the results. Our results suggest an association between FGF2 level and known, vital, prognostic HF parameters. However, using FGF2 levels for risk stratification in patients with HF remains to be validated with long-term follow-up assessment.
Conclusions
This study found that FGF2 level may be a potential biomarker for distinguishing patients with HF from those with non-HF dyspnea. Additionally, increased FGF2 expression levels were correlated with vital prognostic parameters, such as increased NT-proBNP level and decreased LVEF.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the receiver operating characteristic curve
- CI:
-
Confidence interval
- FGF2:
-
Fibroblast growth factor 2
- HF:
-
Heart failure
- AF:
-
Atrial fibrillation
- hi-FGF2:
-
High-molecular-weight fibroblast growth factor 2 isoform
- LVEF:
-
Left ventricular ejection fraction
- MI:
-
Myocardial infarction
- NT-proBNT:
-
N-terminal pro-B-type natriuretic peptide
- NYHA:
-
New York heart association
References
Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118:3272–87.
Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, heart failure Association of the European Society of cardiology, Japanese heart failure society and writing Committee of the Universal Definition of heart failure: endorsed by the Canadian heart failure society, heart failure Association of India, Cardiac Society of Australia and new Zealand, and Chinese heart failure association. Eur J Heart Fail. 2021;23:352–80.
Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–46.
Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–65.
Emdin M, Aimo A, Vergaro G, Bayes-Genis A, Lupón J, Latini R, et al. sST2 predicts outcome in chronic heart failure beyond NT-proBNP and high-sensitivity troponin T. J Am Coll Cardiol. 2018;72:2309–20.
Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, et al. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation. 2017;135:e1054–91.
Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–66.
Detillieux KA, Sheikh F, Kardami E, Cattini PA. Biological activities of fibroblast growth factor-2 in the adult myocardium. Cardiovasc Res. 2003;57:8–19.
Pellieux C, Foletti A, Peduto G, Aubert JF, Nussberger J, Beermann F, et al. Dilated cardiomyopathy and impaired cardiac hypertrophic response to angiotensin II in mice lacking FGF-2. J Clin Invest. 2001;108:1843–51.
House SL, House BE, Glascock B, Kimball T, Nusayr E, Schultz JEJ, et al. Fibroblast growth factor 2 mediates isoproterenol-induced cardiac hypertrophy through activation of the extracellular regulated kinase. Mol Cell Pharmacol. 2010;2:143–54.
Virag JA, Rolle ML, Reece J, Hardouin S, Feigl EO, Murry CE. Fibroblast growth factor-2 regulates myocardial infarct repair: effects on cell proliferation, scar contraction, and ventricular function. Am J Pathol. 2007;171:1431–40.
Schultz JE, Witt SA, Nieman ML, Reiser PJ, Engle SJ, Zhou M, et al. Fibroblast growth factor-2 mediates pressure-induced hypertrophic response. J Clin Invest. 1999;104:709–19.
Koleini N, Nickel BE, Nagalingam RS, Landry NM, Fandrich RR, Cheung DYC, et al. Elimination of endogenous high molecular weight FGF2 prevents pressure-overload-induced systolic dysfunction, linked to increased FGFR1 activity and NR1D1 expression. Cell Tissue Res. 2021;385:753–68.
Liao S, Bodmer JR, Azhar M, Newman G, Coffin JD, Doetschman T, et al. The influence of FGF2 high molecular weight (HMW) isoforms in the development of cardiac ischemia-reperfusion injury. J Mol Cell Cardiol. 2010;48:1245–54.
Hong X, Yu Z, Chen Z, Jiang H, Niu Y, Huang Z. High molecular weight fibroblast growth factor 2 induces apoptosis by interacting with complement component 1 Q subcomponent-binding protein in vitro. J Cell Biochem. 2018;119(11):8807–17.
Sun LY, Qu X, Chen LZ, Chen XX, Zheng GS, Wang ZT, et al. High molecular weight fibroblast growth factor-2 as a promising prognostic biomarker to predict the occurrence of heart failure in atrial fibrillation patients. Heart Vessel. 2017;32:1506–12.
Nusayr E, Sadideen DT, Doetschman T. FGF2 modulates cardiac remodeling in an isoform- and sex-specific manner. Physiol Rep. 2013;1:e00088.
Matsumoto E, Sasaki S, Kinoshita H, Kito T, Ohta H, Konishi M, et al. Angiotensin II-induced cardiac hypertrophy and fibrosis are promoted in mice lacking Fgf16. Genes Cells. 2013;18:544–53.
Kardami E, Jiang ZS, Jimenez SK, Hirst CJ, Sheikh F, Zahradka P, et al. Fibroblast growth factor 2 isoforms and cardiac hypertrophy. Cardiovasc Res. 2004;63:458–66.
He ZY, Feng B, Yang SL, Luo HL. Intracardiac basic fibroblast growth factor and transforming growth factor-beta 1 mRNA and their proteins expression level in patients with pressure or volume-overload right or left ventricular hypertrophy. Acta Cardiol. 2005;60:21–5.
Zhang H, Wang T, Zhang K, Liu Y, Huang F, Zhu X, et al. Deletion of soluble epoxide hydrolase attenuates cardiac hypertrophy via down-regulation of cardiac fibroblasts-derived fibroblast growth factor-2. Crit Care Med. 2014;42:e345–54.
Li M, Yi X, Ma L, Zhou Y. Hepatocyte growth factor and basic fibroblast growth factor regulate atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease via the mitogen-activated protein kinase signaling pathway. Exp Ther Med. 2013;6:1121–6.
Santiago JJ, McNaughton LJ, Koleini N, Ma X, Bestvater B, Nickel BE, et al. High molecular weight fibroblast growth factor-2 in the human heart is a potential target for prevention of cardiac remodeling. PLoS One. 2014;9:e97281.
Bouwens E, Brankovic M, Mouthaan H, Baart S, Rizopoulos D, van Boven N, et al. Temporal patterns of 14 blood biomarker candidates of cardiac remodeling in relation to prognosis of patients with chronic heart failure-the bio- SH i FT study. J Am Heart Assoc. 2019;8:e009555.
Stienen S, Salah K, Eurlings LW, Bettencourt P, Pimenta JM, Metra M, et al. Challenging the two concepts in determining the appropriate pre-discharge N-terminal pro-brain natriuretic peptide treatment target in acute decompensated heart failure patients: absolute or relative discharge levels. Eur J Heart Fail. 2015;17:936–44.
Masson S, Latini R, Anand IS, Vago T, Angelici L, Barlera S, et al. Direct comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the valsartan heart failure (Val-HeFT) data. Clin Chem. 2006;52:1528–38.
Januzzi JL Jr, Sakhuja R, O’donoghue M, Baggish AL, Anwaruddin S, Chae CU, et al. Utility of amino-terminal pro-brain natriuretic peptide testing for prediction of 1-year mortality in patients with dyspnea treated in the emergency department. Arch Intern Med. 2006;166:315–20.
Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilised heart failure: an international pooled analysis of 1256 patients: the international collaborative of NT-proBNP study. Eur Heart J. 2006;27:330–7.
Acknowledgments
Not applicable.
Funding
This work was supported by the Shantou Medical and Health Science and Technology Program, No. SFK [2020] 66–23.
Author information
Authors and Affiliations
Contributions
YZL: Conceptualization, Methodology, Software. CZH: Data curation, Writing- Original draft preparation. CZH and YZL contributed equally to this study and are considered co-first authors. ZJT: Software, Validation. JHY and WN-K.: Visualization, Investigation. ZYQ: Supervision. HXB and FHB: The chief investigators; Writing- Reviewing and Editing. HXB and FHB contributed equally to this study. All authors contributed to the writing of the final manuscript. All authors declare no relevant financial disclosures.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study followed the principles outlined in the Declaration of Helsinki with approval from the Ethics Committee of the Second Affiliated Hospital of Shantou University Medical College, Shantou, China (NO. 2021–13). Written informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
ROC curve: HF vs non HF. Table S2 Circulating levels of FGF2 in healthy controls (n=36) and HF cases with ejection fraction (EF)<45% (n=16) and EF>45% (n=64). Table S3 Expression levels of FGF2 according to New York Heart Association cardiac function classification. Table S4 Expression levels of FGF2 in different HF types. Table S5 Expression levels of FGF2 in groups with different HF etiologies. Table S6 Spearman correlation of FGF2 level with N-terminal pro-B-type natriuretic peptide level. Table S7 Spearman correlation of FGF2 level with EF. Figure S1 FGF2 levels in patients with HF(-) AF(-),HF(+)AF(-)and HF(+)AF(+).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, Z.L., Cai, Z.H., Zheng, J.T. et al. Serum fibroblast growth factor-2 levels complement vital biomarkers for diagnosing heart failure. BMC Cardiovasc Disord 24, 109 (2024). https://doi.org/10.1186/s12872-024-03768-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-03768-4