Abstract
Background
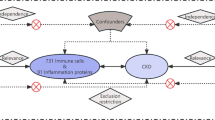
The Caspase activation and recruitment domain 8 (CARD8) protein is a component of innate immunity as a negative regulator of NF- ĸB, and has been associated with regulation of proteins involved in inflammation. Expression of CARD8 mRNA and protein has been identified in human atherosclerotic lesions, and the truncated T30A variant (rs2043211) of CARD8 has been associated with lower C-reactive (CRP) and MCP-1 levels in myocardial infarction patients. The present study examines the role of a genetic variation in the CARD8 gene in relation to a selection of markers of inflammation.
Methods
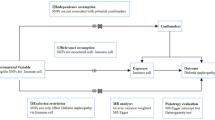
In a cross-sectional study of young healthy individuals (18.0–25.9 yrs, n = 744) the association between the rs2043211 variant in the CARD8 gene and protein markers of inflammation was assessed. Genotyping of the CARD8 C10X (rs2043211) polymorphism was performed with TaqMan real time PCR on DNA from blood samples. Protein levels were studied via Olink inflammation panel (https://olink.com/). Using linear models, we analyzed men and two groups of women with and without estrogen containing contraceptives separately, due to previous findings indicating differences between estrogen users and non-estrogen using women. Genotypes were analyzed by additive, recessive and dominant models.
Results
The minor (A) allele of the rs2043211 polymorphism in the CARD8 gene was associated with lower levels of CCL20 and IL-6 in men (CCL20, Additive model: p = 0.023; Dominant model: p = 0.016. IL-6, Additive model: p = 0.042; Dominant model: p = 0.039). The associations remained significant also after adjustment for age and potential intermediate variables.
Conclusions
Our data indicate that CARD8 may be involved in the regulation of CCL20 and IL-6 in men. No such association was observed in women.
These findings strengthen and support previous in vitro data on IL-6 and CCL20 and highlight the importance of CARD8 as a factor in the regulation of inflammatory proteins. The reason to the difference between sexes is however not clear, and the influence of estrogen as a possible factor important for the inflammatory response needs to be further explored.
Similar content being viewed by others
Background
Cardiovascular diseases (CVDs), such as coronary artery disease (CAD) and cerebrovascular disease are mainly a result of atherosclerosis, which is a slowly progressing chronic inflammatory disease that results in narrowing of large and medium-sized arteries. The atherosclerotic process starts in early life as fatty streaks, which typically evolves asymptomatically over decades, followed by accumulation of inflammatory cells, apoptotic and necrotic cells, debris and cholesterol in the plaque [1]. Among childhood risk factors for CVD are childhood adiposity, hypertension and hyperlipidemia [2]. Inflammation is a central process in the pathogenesis of atherosclerosis and recently we identified CARD8 (also known as TUCAN/CARDINAL/NDDP1) as a factor important for regulation of inflammatory proteins in endothelial cells [3]. The CARD8 protein is a component of the innate immunity and was in an early study identified as a negative regulator of NF-κB via its interaction with the regulatory subunit of the IĸB kinase complex [4]. Furthermore, CARD8 physically binds and regulates procaspase-9 and caspase-1 and regulates generation of IL-1β via caspase-1 [5, 6]. Due to this, CARD8 has been suggested as a part of the NLRP3 inflammasome, although its role in IL-1β release has been debated. The CARD8 protein has also been shown to negatively regulate NLRP3 and IL-1β secretion in vitro in HEK293 cells [7] but in vascular smooth muscle cells, CARD8 did not have any impact on the IL-1β release, possibly indicating on a cell-specific regulation [8].
Knowledge about the role of CARD8 in general is still limited, although research on CARD8 is increasing. Recently, CARD8 was identified as an inflammasome sensor and studies have shown that CARD8 binds dipeptidyl peptidases (DPP)8 and 9 [9,10,11]. Furthermore, CARD8 is degraded by the core 20S proteasome and controls the activation of the CARD8 inflammasome [12]. The knowledge on the regulatory function of CARD8 in the etiology of CVDs is also limited. In two of our previous studies, we showed upregulation of CARD8 mRNA and protein expression in human atherosclerotic lesions compared to non-atherosclerotic vessels, indicating a pathophysiological role of CARD8 in CVD [3, 13]. On the other hand, the role of genetic alterations in the CARD8 gene has been extensively investigated for the association to various inflammatory diseases, including CVDs [14]. The T30A polymorphism (rs2043211) in exon 5 encoding a C10X alteration of the CARD8 gene and has been extensively studied and causes a premature stop codon that terminates the protein, although the functional aspect of the polymorphism is not completely clear. In one of our previous studies, we have shown an association between lower mRNA expression of CARD8 in atherosclerotic lesions from homozygous polymorphic individuals for the C10X polymorphism rs2043211, where the minor allele was associated with lower C-reactive protein (CRP) and MCP-1 levels [13]. We have also identified that CARD8 acts as a regulator of inflammatory cytokines and chemokines like CXCL1, CXCL6, PDGF-A, MCP-1 and IL-6 in endothelial cells and atherosclerotic lesions [3], which indicates that CARD8 plays an important role for the regulation of proteins involved in inflammation. Knowledge about the role of the rs2043211 CARD8 variant on the expression of proteins involved in inflammation in young healthy adults is however limited.
The aim of the present study was to examine the role of the rs2043211 C10X polymorphism in the CARD8 gene in relation to a selection of markers of inflammation previously shown altered after CARD8 knock down [3]. We have hypothesized that the minor variant of the C10X polymorphism mimics the lack of CARD8 protein.
Methods
Study population, ethics and exclusion criteria
Blood samples from 834 Swedish young self-reported non-smoking healthy individuals (aged 18.0–25.9 years) were collected in the Lifestyle, Biomarkers and Atherosclerosis (LBA) cohort, Örebro University, Sweden.
The LBA study is an epidemiological cross-sectional study on cardiovascular risk factors, including biomarkers, vascular function variables and physical activity assessment. Details about the LBA cohort and sampling were published in Fernström et al. [15]. Informed consent was obtained from all participants. The study was approved by Regional Ethical Review Board, Uppsala, Sweden (Dnr 2014/224) and performed according to the Helsinki declaration.
Out of 834 individuals, we excluded individuals whose CRP level was ≥ 10mg/mL (n = 36), indicating ongoing inflammation, and had missing data in genotype (n = 15; failed quality control) and/or other variables (n = 15). Further 24 individuals were excluded due to missing data in proteins. In total, we therefore included the remaining n = 744 individuals in the present study, whereof 511 were females and 233 males (Fig. 1).
DNA extraction and genotyping of the CARD8 C10X (rs2043211) polymorphism
Genomic DNA was isolated with Wizard Genomic DNA purification kit (Promega, Madison, WI) according to the supplier’s recommendations. DNA concentration was measured with NanoDrop 200 spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and diluted to 20 ng/µl.
Genotyping of the rs2043211 polymorphism in the CARD8 gene was performed for the LBA cohort. Ten nanograms of DNA was amplified in a 10 µl reaction containing 1 × TaqMan Genotyping Mastermix (Applied Biosystems, Foster City, CA) and 1 × TaqMan SNP Genotyping Assay (Applied Biosystems) with predesigned primers and probes C_ _11708080_1_ (Applied Biosystems) according to a TaqMan standard protocol in a QuantStudio 7 Flex Real-Time PCR system (Applied Biosystems) followed by allelic discrimination analysis. Four reactions in each 96-well plate contained no-template control. A random selection of > 10% of all samples were re-genotyped in a new PCR reaction to verify the accuracy of the genotyping. The genotypes were analyzed by three classifications: additive, recessive and dominant models. Additive model assigned values of 0, 0.5 and 1 for the homozygous wildtype (TT), heterozygous (TA) and polymorphic homozygous (AA) genotype groups, respectively. The dominant model compared TA + AA with TT (reference) and the recessive model compared AA vs. TT + TA (reference).
Olink proteomics
Proteomics analysis was performed with Olink (www.olink.com) proteomics of plasma samples from the LBA cohort as described in Pettersson Pablo et al. [16, 17].
Based on the previous results by Paramel et al. [3], we selected the 16 proteins from the Olink Inflammation and CVDIII panels (http://olink.com) with false discovery rate (FDR) levels \(\le\) 5%: CXCL6, CCL20, IL-6, IL-18R1, CXCL-1, ADA, CD40, 4E-BP1, MCP3, MCP1, IL-8, TWEAK, VEGF-A, OPG, t-PA and AXL. Of these, MCP3 was not included in the present study because more than 50% of the individuals had protein levels under the Limit of Detection (LOD) value, resulting in 15 proteins analyzed. The values of the proteins were log2 transformed to fit normal distribution.
Five individuals had missing values in these protein data, and we replaced these missing data with the mean of observed values. A protein value equal to or greater (or smaller) than 4 standard deviations from the mean were replaced to missing values. The number of such observations differed by protein, but it ranged between 0 (7 proteins) and 5 (CCL20).
Other variables
The variables age, Body Mass Index (BMI; kg/m2), Low density lipoprotein (LDL; mmol/L), triglycerides (mmol/L), insulin (mU/L), and systolic blood pressure (mm Hg) have previously been suggested to be involved in inflammatory processes [18, 19]. BMI, LDL, triglycerides, insulin, and systolic blood pressure were measured according to standard procedures as previously described in Fernström et al. [15].
Further, we examined whether men or women, with the latter being further separated into estrogen-containing contraceptive usage (yes/no) modifies the association between genotyping of the rs2043211 polymorphism and proteins. Among women, estrogen-containing contraceptive usage was self-reported in a questionnaire. Hereafter, women using estrogen containing contraceptive are called “estrogen users (EU)”, and women not using them as “non-estrogen users (NEU)”. Details of the reported estrogen data are described in Pettersson-Pablo et al. [20].
We assumed that the rs2043211 could also be involved in the regulation of inflammatory proteins in a similar pattern as previously described [18, 19]. Therefore, we adjusted for these variables to obtain the extent to which the rs2043211 polymorphism is associated with protein expression independently.
Statistics
The characteristics of the participants were summarized by frequencies, proportions, means and standard deviations (or median and 25 and 75 percentiles for skewed data). Box plots were used to summarize the distribution of protein values by TT, TA and AA separately for men and NEU and EU women. The associations between additive, dominant or recessive models with the fifteen proteins were assessed using linear models for microarray data (using the R package ‘limma’, [21, 22]). It fits a linear model to each protein while analyzing the entire experiment as an integrated whole to increase effectiveness in estimation. For each of additive, dominant, and recessive models, we fitted two models: age-adjusted (Model 1) and a model adjusting for age, BMI, LDL, triglycerides, insulin, and systolic blood pressure (Model 2). All adjustment variables were modeled as piecewise linear functions using linear splines, which model the associations between these variables and proteins using three equally distanced segments. Two segments were used if three segments did not fit data. The estimates from Model 2 are interpreted as the association of the rs2043211 polymorphism with a given protein not mediated through variables adjusted for in the model. Two-sided p-values lower than 0.05 were considered statistically significant. Analytical data were prepared by Stata MP/17 and analyses were conducted using RStudio version 1.2.5033.
Results
Genotype distribution in the CARD8 gene
The allele frequencies of the rs2043211 polymorphism were 69% for the major (T) allele and 31% for the minor (A) allele (Table 1). The rs2043211 polymorphism in the CARD8 gene was found in Hardy Weinberg equilibrium.
Basic characteristics of the LBA cohort based on genotypes in the rs2043211 polymorphism in the CARD8 gene
The distribution of genotype TT, TA or AA for 744 individuals was stratified by men, NEU and EU women (Table 1). The distribution of age and biomarker characteristics are shown in Table 2. The results were presented as mean or median, and standard deviation (SD) or quartiles respectively, depending on the distribution of the variables (insulin and CRP were skewed).
The distribution of covariates such as BMI, blood pressure, LDL, insulin, and CRP were within the age and gender specific expected values, and there appeared to be little differences by genotypes. Distribution of the protein levels by TT, TA or AA for men, NEU and EU women is available in Supplementary material S1.
Association between rs2043211 genotype in the CARD8 gene and inflammatory proteins in the LBA cohort
The CARD8 polymorphism rs2043211 generates a truncated variant in codon 10 of the CARD8 gene (C10X). We evaluated the association between 15 proteins assessed via Olink proteomics and the CARD8 genotype, previously found to be regulated by CARD8 [3]. In the present cohort of healthy, young adults, we assumed a similar effect of the minor (A) allele (encoding the truncated X variant) of the polymorphism rs2043211 as the effect of CARD8 knock-down in HUVECs [3]. The script used is available in Supplementary material S2.
Additive model
In men, the minor (A) allele of the rs2043211 polymorphism in the CARD8 gene was significantly associated with lower levels of CCL20 (p = 0.023, Model 1; Table 3) and IL-6 (p = 0.042, Model 1; Table 3) in the age adjusted estimate. The association remained after adjustment for BMI, LDL and other potential intermediating variables (CCL20: p = 0.030; IL-6: p = 0.039, Model 2; Table 3). There were no such relations found in NEU or EU women.
A few of the statistically significant observations in this study were not consistent with previous findings: In men and EU women respectively, the minor allele was significantly associated with a higher value in OPG and CXCL1 (Table 3), but the opposite direction as previously reported [3].
Dominant model
In the dominant model in men, where TA + AA were grouped together and compared to TT (reference), the minor allele of CARD8 was significantly associated with lower levels of CCL20 (p = 0.016, Model 1; p = 0.030, Model 2; Table 4) and IL-6 (p = 0.039, Model 1; p = 0.045, Model 2; Table 4). The OPG level showed positive association with the minor allele in Model 1, but the association was slightly weakened after the adjustment (Table 4). The direction of associations in other proteins were inconsistent between men and NEU or EU women.
Recessive model
In the recessive model, MCP-1 was positively associated with the minor allele of CARD8 in the Models 1 and 2 in men, and the IL-18R1 was positively associated with the minor allele of CARD8 in the Model 1 but not Model 2 in NEU women (Table 5). Both observations were in the opposite direction as compared to our previous study (Table 5; [3]). Further, AXL was positively associated with the minor allele of CARD8 in men in Model 1, but not in Model 2, which was in the same direction as in Paramel et al. [3].
Discussion
In the present investigation, we have studied the association between the rs2043211 polymorphism in the CARD8 gene and protein expression levels in a selection of 15 proteins involved in inflammation, evaluated by Olink proteomics in young healthy individuals. We showed that the polymorphism rs2043211 in the CARD8 gene was significantly associated with lower levels of CCL20 and IL-6 in men. Both IL-6 and CCL20 have been associated with the atherosclerotic process. The chemokine CCL20 has been associated with overexpression in atherosclerotic lesions and is involved in the attraction of immune cells [23]. It signals via the Ccr6 receptor and the importance of the CCL20/Ccr6 axis for development of atherosclerosis was demonstrated in ApoE−/− mice lacking Ccr6, which had reduced size of the atherosclerotic lesions [24]. The cytokine IL-6 has both pro- and anti-inflammatory properties [25]. It contributes to atherosclerosis via promotion of smooth muscle cell proliferation, migration and endothelial dysfunction [26]. In addition, IL-6 is involved in the production of acute phase proteins, has chemotactic abilities and can activate the endothelium, thereby facilitating the infiltration of immune cells into the atherosclerotic lesion [23]. On the other hand, IL-6 can also contribute to anti-inflammatory properties, such as inhibition of tumor necrosis factor (TNF)-α and IL-1 [25].
In the present study, the association between the polymorphism rs2043211 in the CARD8 gene and reduced levels of CCL20 and IL-6 in men was significant both in the additive and dominant models, but not in the recessive model. Although the nature of the truncated variant of the CARD8 gene is not clear, our data suggests that being heterozygous for the rs2043211 polymorphism in the CARD8 gene is enough to alter the levels of specific proteins involved in inflammation. For CCL20 and IL-6, these results are consistent with our previous data [3], where we observed that knock down of CARD8 in HUVECs generated a reduction of both CCL20 and IL-6 [3].
The remaining proteins showed inconsistent associations and/or no association in all three models, and this was inconsistent with our previous study [3]. However, in the additive model, we also observed that eight of the 15 proteins were altered in the same direction as in our previous study [3], although with non-significant associations.
We found an allele frequency of 69% for the major (T) allele and 31% for the minor (A) allele. This is in similar frequencies as described in the SNP database at National Center for Biotechnology Information (NCBI; https://www.ncbi.nlm.nih.gov/snp/rs2043211), where 68% of the European population carry the T allele and 32% the A allele. However, when stratifying the women by estrogen use, we observed a minor difference in the distribution of the alleles. Although smaller groups, the examination of women by estrogen use is a strength of our study, as it has been shown to be influential in other studies [18].
Strengths
The data in the present study represent a unique contribution to the knowledge on CARD8, since very few studies have so far been published on the association between CARD8 genotype and biomarkers of inflammation. The present study fills a gap in the research field via its preclinical approach by studying a young non-smoking healthy cohort. Although the focus of the LBA study is to discover biomarkers for early atherosclerosis, the results of the present study does not limit to CVDs, since it mirrors the general cytokine profile in young healthy individuals. Identification of markers of inflammation in healthy individuals may in the future contribute to decisions on early prevention management and thereby public policies related to health.
Limitations
In the present study, we have utilized a cohort of young Swedish healthy non-smoking individuals to study the association between CARD8 genotype and expression of chemokines and cytokines. However, the design of the present study does not permit conclusions on biomedical mechanistical causality between CARD8 genotype and the expression of inflammatory proteins per se. Another potential limitation of the present study was that we could not statistically control for the physiological estrogen levels in the women participating in the study. Estrogen use increases the levels of CRP which has been observed both by others and by us [19, 20, 27]. In the present study we assumed that proteins involved in inflammation are also affected by estrogen use, like CRP. In general, most of the significant associations in the present study were observed in men. This may be a result of NEU and EU women being differentially influenced by either physiological cyclic variations and/or supplementary estrogen compared to men. However, we did not have data to adjust for cyclic variations, and the investigation of reasons of the differences in the associations by men and NEU and EU women is beyond the scope of the present study.
Another potential limitation of the present study is the generally low variance of inflammatory markers in the dataset. This is however expected in healthy young individuals.
In our previous publication [3], we showed that knock-down via siRNA of CARD8 in human umbilical vein endothelial cells (HUVECs) resulted in lower expression of markers of inflammation. The comparability between our previous in vitro study made on HUVECs [3] and the present study in healthy young individuals is limited. Adjustments for risk factors on proteomics data based on in vitro cell culture were however not relevant in our previous investigation due to the nature of the study material and thereby not completely possible to compare. In the present study, we have adjusted for age (Model 1) and established risk factors for CVD (Model 2). However, with a few exceptions, adjustment according to Model 1 and Model 2 in the present study show similar results, implicating that the traditional risk factors play a limited role for the regulation of the levels of proteins involved in inflammation in relation to CARD8 genotype.
Future directions
Additional in vitro studies on the mechanistic effect of the truncated variant of CARD8 on inflammatory markers are warranted, especially under the influence of estrogen as a possible factor important for the inflammatory response. Another logical step will be to replicate the present study in an older and/or diseased study cohort.
Conclusions
Collectively, in the present study using the LBA cohort which consists of healthy young adults, we have shown an association between the polymorphism rs2043211 encoding a truncated variant of the CARD8 gene and lower levels of CCL20 and IL-6 levels in men. Our data indicate that CARD8 may be involved in the regulation of these proteins. No such associations were, however, evident for women, and the reason to this is still unclear.
Availability of data and materials
Data sets generated and analyzed in the current study are not public available due to research subject confidentiality but are aware in a de-identified form from the corresponding author and PI upon reasonable request. The samples are being stored in Örebro Biobank no 454.
Abbreviations
- 4E-BP1:
-
Eukaryotic translation initiation factor 4E-binding protein 1
- ADA:
-
Adenosine deaminase
- Apo:
-
Apolipoprotein
- AXL:
-
Tyrosine-protein kinase receptor UFO
- BMI:
-
Body mass index
- CAD:
-
Coronary artery disease
- CARD8:
-
Caspase activation and recruitment domain 8
- CCL20:
-
C–C motif ligand 20
- Ccr6:
-
C–C motif chemokine receptor 6
- CD:
-
Cluster of differentiation
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- CXCL:
-
(C-X-C motif) ligand
- DNA:
-
Deoxyribonucleic acid
- EU:
-
Estrogen users
- FDR:
-
False discovery rate
- HEK:
-
Human embryonic kidney
- HUVEC:
-
Human umbilical vein endothelial cells
- IL:
-
Interleukin
- LBA:
-
Lifestyle, biomarkers and atherosclerosis
- LDL:
-
Low density lipoproteins
- LOD:
-
Limit of detection
- MCP:
-
Monocyte Chemoattractant Protein
- mRNA:
-
Messenger Ribonucleic acid
- NEU:
-
Non-estrogen users
- NF:
-
Nuclear factor
- NLRP3:
-
NACHT, LRR and PYD domains-containing protein 3
- OPG:
-
Osteoprotegerin
- PCR:
-
Polymerase chain reaction
- PDGF:
-
Platelet-derived growth factor
- SD:
-
Standard deviation
- SNP:
-
Single nucleotide polymorphism
- TNF:
-
Tumor necrosis factor
- t-PA:
-
Tissue-type plasminogen activator
- TWEAK:
-
TNF-related weak inducer of apoptosis
- VEGF:
-
Vascular endothelial growth factor
References
Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–12.
Pool LR, Aguayo L, Brzezinski M, Perak AM, Davis MM, Greenland P, et al. Childhood risk factors and adulthood cardiovascular disease: A systematic review. J Pediatr. 2021;232:118-26.e23.
Paramel GV, Karadimou G, Göthlin Eremo A, Ljungberg LU, Hedin U, Olofsson PS, et al. Expression of CARD8 in human atherosclerosis and its regulation of inflammatory proteins in human endothelial cells. Sci Rep. 2020;10:9108.
Bouchier-Hayes L, Conroy H, Egan H, Adrain C, Creagh EM, MacFarlane M, et al. CARDINAL, a novel caspase recruitment domain protein, is an inhibitor of multiple NF-kappa B activation pathways. J Biol Chem. 2001;276:44069–77.
Pathan N, Marusawa H, Krajewska M, Matsuzawa S, Kim H, Okada K, et al. TUCAN, an antiapoptotic caspase-associated recruitment domain family protein overexpressed in cancer. J Biol Chem. 2001;276:32220–9.
Razmara M, Srinivasula SM, Wang L, Poyet JL, Geddes BJ, DiStefano PS, et al. CARD-8 protein, a new CARD family member that regulates caspase-1 activation and apoptosis. J Biol Chem. 2002;277:13952–8.
Ito S, Hara Y, Kubota T. CARD8 is a negative regulator for NLRP3 inflammasome, but mutant NLRP3 in cryopyrin-associated periodic syndromes escapes the restriction. Arthritis Res Ther. 2014;16:R52.
Tangi TN, Elmabsout AA, Bengtsson T, Sirsjö A, Fransén K. Role of NLRP3 and CARD8 in the regulation of TNF-α induced IL-1β release in vascular smooth muscle cells. Int J Mol Med. 2012;30:697–702.
Linder A, Bauernfeind S, Cheng Y, Albanese M, Jung C, Keppler OT, et al. CARD8 inflammasome activation triggers pyroptosis in human T cells. EMBO J. 2020;39: e105071.
Griswold AR, Ball DP, Bhattacharjee A, Chui AJ, Rao SD, Taabazuing CY, et al. DPP9’s Enzymatic Activity and Not Its Binding to CARD8 Inhibits Inflammasome Activation. ACS Chem Biol. 2019;14:2424–9.
Sharif H, Hollingsworth LR, Griswold AR, Hsiao JC, Wang Q, Bachovchin DA, et al. Dipeptidyl peptidase 9 sets a threshold for CARD8 inflammasome formation by sequestering its active C-terminal fragment. Immunity. 2021;54(1392–1404): e10.
Hsiao JC, Neugroschi AR, Chui AJ, Taabazuing CY, Griswold AR, Wang Q, et al. A ubiquitin-independet proteasome pathway controls activation of the CARD8 inflammasome. J Biol Chem. 2022;298: 102032.
Paramel GV, Folkersen L, Strawbridge RJ, Ateia Elmabsout A, Särndahl E, Lundman P, et al. CARD8 gene encoding a protein of innate immunity is expressed in human atherosclerosis and associated with markers of inflammation. Clin Sci (Lond). 2013;125:401–7.
Paramel GV, Sirsjö A, Fransén K. Role of genetic alterations in the NLRP3 and CARD8 genes in health and disease. Mediators Infl. 2015;2015:ID 846782.
Fernström M, Fernberg U, Eliason G, Hurtig-Wennlöf A. Aerobic fitness is associated with low cardiovascular disease risk: the impact of lifestyle on early risk factors for atherosclerosis in young healthy Swedish individuals - the Lifestyle, Biomarker, and Atherosclerosis study. Vasc Health Risk Manag. 2017;13:91–9.
Pettersson-Pablo P, Cao Y, Breimer LH, Nilsson TK, Hurtig-Wennlöf A. Pulse wave velocity, augmentation index, and carotid intima-media thickness are each associated with different inflammatory protein signatures in young healthy adults: The lifestyle, biomarkers and atherosclerosis study. Atherosclerosis. 2020;313:150–5.
Pettersson-Pablo P, Nilsson TK, Breimer LH, Hurtig-Wennlöf A. IGFBP-1 and IGFBP-2 are associated with a decreased pulse-wave velocity in young, healthy adults. BMC Cardiovasc Disord. 2021;21:131.
Pettersson-Pablo P, Nilsson TK, Breimer LH, Hurtig-Wennlöf A. Body fat percentage is more strongly associated with biomarkers of low-grade inflammation than traditional cardiometabolic risk factors in healthy young adults - the Lifestyle, Biomarkers, and Atherosclerosis study. Scand J Clin Lab Invest. 2019;79:182–7.
Fransén K, Pettersson C, Hurtig-Wennlöf A. CRP levels are significantly associated with CRP genotype and estrogen use in The Lifestyle, Biomarker and Atherosclerosis (LBA) study. BMC Cardiovasc Disord. 2022;22:170.
Pettersson-Pablo P, Cao Y, Bäckström T, Nilsson TK, Hurtig-Wennlöf A. Body fat percentage and CRP correlate with composite score of vascular risk markers in healthy, young adults – The Lifestyle, Biomarkers and Atherosclerosis (LBA) study. BMC Cardiovasc Dis. 2020;20:77.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43: e47.
Patel H, Ashton NJ, Dobson RJB, Andersson LM, Yilmaz A, Blennow K, et al. Proteomic blood profiling in mild, severe and critical COVID-19 patients. Sci Rep. 2021;11:6357.
Calvayrac O, Rodríguez-Calvo R, Alonso J, Orbe J, Martín-Ventura JL, Guadall A, et al. CCL20 is increased in hypercholesterolemic subjects and is upregulated by LDL in vascular smooth muscle cells: role of NF-κB. Arterioscler Thromb Vasc Biol. 2011;31:2733–41.
Wan W, Lim JK, Lionakis MS, Rivollier A, McDermott DH, Kelsall BL, et al. Genetic deletion of chemokine receptor Ccr6 decreases atherogenesis in ApoE-deficient mice. Circ Res. 2011;109:374–81.
Reiss AB, Siegart NM, De Leon J. Interleukin-6 in atherosclerosis: atherogenic or atheroprotective? Clin Lipidol. 2017;1:14–23.
Feng Y, Ye D, Wang Z, Pan H, Lu X, Wang M, et al. The role of interleukin-6 family members in cardiovascular diseases. Front Cardiovasc Med. 2022;9: 818890.
Raitakari M, Mansikkaniemi K, Marniemi J, Viikari JS, Raitakari OT. Distribution and determinants of serum high-sensitive C-reactive protein in a population of young adults: The Cardiovascular Risk in Young Finns Study. J Intern Med. 2005;258:428–34.
Acknowledgements
The authors of the study would like to thank the students at Örebro University that have been involved in DNA extraction for excellent technical assistance.
Funding
Open access funding provided by Örebro University. This work was supported by The Knowledge Foundation HÖG2017 #20170191 (PI Karin Fransén); The Knowledge Foundation HÖG2019 #20190088 (PI Karin Fransén); Örebro University, Faculty of Medicine and Health #2019–06-13 (PI Karin Fransén); Asset Management Arm (AFA) life insurance #130275 (PI Anita Hurtig-Wennlöf).
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: KF, AH, AHW. Performed the experiments: KF, GPV. Analyzed the data: KF, AH, AHW. Contributed to reagents/materials/analysis tools: KF, AHW. Wrote the manuscript: KF, AH, AHW. Coordinator of the study: KF.
Corresponding author
Ethics declarations
Ethical approval and consent to participate.
Informed consent was obtained from all participants. The study was approved by Regional Ethical Review Board, Uppsala, Sweden (Dnr 2014/224) and performed according to the Helsinki declaration.
Consent for publication
N/A.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary material S1.
Distribution of proteins by TT, TA, AA, stratified by men, women with and without estrogen contraceptive use. Supplementary material S2. The script used in the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fransén, K., Hiyoshi, A., Paramel, G.V. et al. Association between C10X polymorphism in the CARD8 gene and inflammatory markers in young healthy individuals in the LBA study. BMC Cardiovasc Disord 24, 103 (2024). https://doi.org/10.1186/s12872-024-03765-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-03765-7