Abstract
Background
Neutrophil to lymphocyte ratio (NLR), as a recent inflammatory index, has been reported to be a prognostic tool in different diseases. However, implication of this ratio in heart failure (HF) is less investigated. In this systematic review and meta-analysis, we aimed to assess the potential impact of NLR on HF clinical outcomes.
Methods
Relevant English published records in PubMed, Scopus, Embase, and Web of Science were screened up to July 2023. Articles reporting clinical outcomes (follow-up or in-hospital mortality, readmission, HF prediction, extended hospital stay length, pulmonary vascular resistance, atrial fibrillation, renal disease and functional capacity) in HF sufferers were collected for further analysis with addition of NLR difference stratified by death/survived and HF status.
Results
Thirty-six articles (n = 18231) were finally selected which reported NLR in HF sufferers (mean: 4.38, 95% confidence interval (CI): 4.02–4.73). We found 25 articles reported NLR and total mortality (either follow-up death (N = 19): 4.52 (95% CI: 4.03–5.01) or in-hospital death (N = 10): 5.33 (95% CI: 4.08–6.57)) with mean NLR of 4.74 (95% CI: 4.28–5.20). NLR was higher among deceased patients compared to survived ones (standard mean difference: 0.67 (95% CI: 0.48–0.87), P < 0.001)). NLR was found to be related with higher mortality risk (continuous variable: hazard ratio (HR): 1.12, 95% CI: 1.02–1.23, P = 0.013), categorical variable: HR: 1.77, 95% CI: 1.27–2.46, P = 0.001, T2 vs. T1: HR:1.56, 95%CI: 1.21–2.00, P = 0.001, T3 vs. T1: HR:2.49, 95%CI: 1.85–3.35, P < 0.001). Other aforementioned variables were not feasible to analyze due to presence of few studies.
Conclusions
NLR is a simple and acceptable prognostic tool for risk stratification and prioritizing high risk patients in clinical settings, especially in resource limited nations.
Similar content being viewed by others
Introduction
Heart failure (HF) is commonly considered as the end stage of many cardiovascular diseases (CVDs) [1, 2]. This disorder is simply characterized by inability of cardiac tissue pumping the oxygen and blood to meet the metabolic demands of body organs. HF prevalence is still rising rapidly and it has been estimated to increase by 46% in 2030 [3]. Globally, 64.3 million people suffer from this chronic disease leading to a significant economic burden on healthcare system [4]. For instance, approximately $65 billion has been reported as direct HF management cost for each year [5]. Despite substantial improvement in context of implementing new treatment modalities, HF mortality rate is still concerning [6, 7]. Five-year death rate has been indicated to be 42.3% and only 10% of HF sufferers survive after 10 years post HF diagnosis [4, 8]. Therefore, early diagnosis and appropriate delivery of therapeutic interventions are pivotal steps in HF era.
In addition to several previously proved HF risk factors, one of the major pathways in CVDs pathogenesis is related to inflammation and several biomarkers have been introduced in this regard [9,10,11,12,13]. The inflammatory cytokines lead to cardiac cell apoptosis, fibrosis and consequent adverse ventricular remodeling [14]. Neutrophils and lymphocytes are two main arms of inflammation and division of these two blood indices results in introduction of a recent inflammatory index, named neutrophil to lymphocyte ratio (NLR), which has been reported to be a useful prognostic tool in CVDs [15,16,17]. Due to an imbalance between inflammatory and anti-inflammatory pathways in HF, neutrophil apoptosis decreases leading to heightened absolute counts and increased rate of HF occurrence [18,19,20]. On the other hand, decompensated state of HF results in lowering lymphocyte counts and lymphocytopenia has been indicated to be an independent mortality predictor in HF [21]. It seems this simple and inexpensive tool might be prognostic in clinical settings. However, reported data are still controversial. For instance, in Delcea et al. and Davran et al.’s studies, findings were in favor of significant NLR association with HF clinical outcomes [22, 23]. On the other hand, Liu et al. and Pourafkari and colleagues suggested this biomarker might not independently predict HF outcomes. Due to these inconsistencies as well as presence of literature gap, a thorough study is required [24, 25].
In this systematic review and meta-analysis, we aimed to assess the potential effect of NLR on different clinical outcomes among patients with HF.
Materials and methods
Protocol registration
We registered current systematic review and meta-analysis in International Prospective Register of Systematic Reviews (PROSPERO) with identification number of CRD42022350800. There was not any protocol deviation in current study. This study was also implemented based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [26].
Inclusion and exclusion criteria
We conducted a systematic review of the literature and evaluated all English peer-reviewed studies that reported the impact of the NLR on clinical outcomes in HF patients. We structured our research assessment using the population, exposure, comparator, outcomes, and study designs (PECOS) framework. In this context, our study focused on patients suffering from HF as the defined population. Exposure and comparator elements were not applicable, as our primary objective was to examine the potential impact of NLR across all HF patients. Regarding the outcomes, we considered several factors, including mortality, rehospitalization, HF prediction, extended hospital stay, pulmonary vascular resistance, atrial fibrillation (AF), progression to renal disease, and functional capacity. For study designs, the inclusion criteria were studies with cross-sectional, case–control, cohort, and randomized clinical trial (RCT) designs. For the exclusion criteria, we discarded meeting abstracts, editorials, case report, case series and any studies with incomplete desired outcome as well as studies on animal species.
Strategy of literature search
Four well-known electronic medical databases including PubMed, Scopus, Embase and Web of Science were screened up to July 2023. In Scopus database, titles, abstracts and keywords were searched. In PubMed, Embase and Web of Science, titles and abstracts were investigated. We used the following search strategy (using medical subject headings (MeSH) and non-MeSH terms) in all aforementioned databases to collect all relevant records: (“neutrophil* to lymphocyte* ratio” OR “neutrophil *-lymphocyte*” OR “neutrophil*-lymphocyte* ratio” OR “neutrophil* to lymphocyte*” OR “neutrophil*-to-lymphocyte* ratio” OR “neutrophil*-to lymphocyte* ratio” OR “neutrophil* to-lymphocyte* ratio” OR “neutrophil */lymphocyte* ratio” OR “neutrophil*/lymphocyte*” OR “nlr”) AND (“heart failure” OR “cardiac failure” OR “heart insufficiency” OR “cardiac insufficiency” OR “congestive heart failure” OR “congestive cardiac failure” OR “decompensated heart failure” OR “decompensated cardiac failure” OR “decompensated heart insufficiency” OR “decompensated cardiac insufficiency” OR “acute decompensated heart failure” OR “acute decompensated cardiac failure” OR “acute decompensated heart insufficiency” OR “acute decompensated cardiac insufficiency” OR “hf”).
Selection process
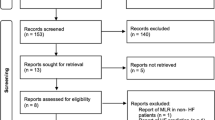
Three authors (M. V., N. B. and SA. E.) carefully screened titles and abstracts and gathered the full-texts of all relevant articles independently within four aforementioned databases. Only one record was considered in case of duplicated articles. Any disagreement was resolved by consensus. We also provided flow-diagram of current study in Fig. 1.
Data gathering process
The following items were screened in each recruited record: first author’s name plus publication year, study design, sample size, male frequency, age (mean ± standard deviation (SD) or median (interquartile range (IQR)), as reported), follow-up period (if applicable), NLR (mean ± SD, median (IQR), as reported), NLR tertiles, quartiles and cut-off points (as reported) as well as HF outcomes (mortality (follow-up or in-hospital death), rehospitalization, HF prediction, extended length of hospital stay, pulmonary vascular resistance, AF, progression to renal disease and functional capacity, as reported).
Risk of bias assessment
In order to evaluate quality and risk of bias in each enrolled article, the following assessment tools were used according to study designs: cross-sectional studies (a critical appraisal tool (AXIS)), case–control studies (national institute of health (NIH) quality assessment tool), cohort studies (Joanna Briggs Institute (JBI) critical appraisal checklist for cohort studies) and RCT (JBI critical appraisal checklists for RCT) [27,28,29,30]. We also assessed the certainty of the pre-defined outcomes using Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework.
Statistical analysis
Pooled effect sizes were provided as mean and hazard ratio (HR) with 95% confidence interval (CI), as appropriate. We used Wan et al.’s and Hozo et al.’s methods to convert median (IQR) and median (range) to mean ± SD for continuous variables, respectively [31, 32]. Cochran’s Q statistic, I2 and tau squared (τ2) were used to assess heterogeneity. We used random effects model to implement downstream analyses. Forest plots were depicted to show NLR mean and HR according to studies reported this index in HF subjects. We also provided NLR forest plots according to all-cause mortality and death/survival as well as HF status. In addition to funnel plots, the Egger’s and Begg’s tests as well as Duval and Tweedie’s trim-and-fill method were used to assess heterogeneity and publication bias. Excel datasheet was utilized for data entrance and all analyses were done using comprehensive meta-analysis (CMA) software (version 2.0).
Results
Study selection and characteristics
After reviewing 1672 articles and elimination of duplicated items as well as other non-relevant articles, we identified 39 articles (n = 27256, age: 70.69 ± 13.53 years, 60.41% male) that reported NLR in HF individuals (Fig. 1) [22,23,24,25, 33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68]. Two articles on same number of participants with similar outcomes which have been performed by the same authors were considered to be a single record [45, 46]. We provided summary of all recruited studies in Table 1. Three articles were not added to assess mean NLR due to report of this biomarker other than mean ± SD or median (IQR) [34, 40, 68]. Total mean NLR in the remaining 36 studies (n = 18231) were found to be 4.38 (95% CI: 4.02–4.73) (Fig. 2).
We found 25 studies reported total mortality (either follow-up or in-hospital death) on 16086 HF sufferers [22,23,24,25, 33, 36, 39, 41, 43, 45,46,47,48,49,50, 52, 53, 57,58,59,60,61,62,63, 67]. Total mean age was 71.62 ± 13.51 years (males: 62%) and 3895 (24.21%) patients died either during admission or follow-up. Figure 3 shows forest plot for mean NLR (4.74, 95% CI: 4.28–5.20). In 19 records (n = 12,427, age: 71.75 ± 13.80, males: 62.36%), follow-up mortality had been reported [22, 24, 25, 33, 36, 37, 39, 41, 43, 45,46,47, 49, 52, 53, 59,60,61, 63, 67]. Mean NLR in HF subjects was determined to be 4.52 (95% CI: 4.03–5.01) (Fig. 4). On the other hand, we found 10 records (n = 5331) reported mortality during hospitalization (age: 71.52 ± 12.94, males: 56.98%), with total NLR mean of 5.33 (95% CI: 4.08–6.57) (Fig. 5) [22,23,24, 37, 48, 50, 57,58,59, 62].
Thirteen records were selected reporting NLR in dead as well as survived HF subjects [22, 24, 33, 39, 41, 47, 50, 57, 58, 60, 62, 63, 67]. Of 7365 patients, 2299 (31.21%) died. Deceased patients had significantly higher NLR values (7.61, 95% CI: 6.38–8.85) than survivors (4.82, 95% CI: 3.79–5.84) (Fig. 6). Forest plot (Fig. 7) also showed a statistically significant difference in NLR between dead and survived individuals (standardized mean difference: 0.67, 95% CI: 0.48–0.87, P < 0.001). Fourteen records (NLR as continuous variable in seven studies, NLR as dichotomous variable in the remaining ones) reported all-cause mortality through multi-variated adjusted HR models based on NLR [23,24,25, 33, 34, 36, 37, 43, 47, 52, 60, 61, 63, 68]. Due to inconsistent HR and 95% CI in one record in each group, six studies reported NLR as a continuous variable and six studies reported as a dichotomous variable were finally selected. Increasing NLR was associated with higher hazard of death (continuous variable: HR: 1.12, 95% CI: 1.02–1.23, P = 0.013 (Fig. 8); dichotomous variable: HR: 1.77, 95% CI: 1.27–2.46, P = 0.001) (Fig. 9). We also analyzed mortality based on NLR tertiles (5 out of 6 studies due to inconsistent CIs). Patients in higher NLR tertiles had higher mortality risk than those in the lowest tertile (T2 vs. T1: HR: 1.56, 95% CI: 1.21–2.00, P = 0.001; T3 vs. T1: HR: 2.49, 95% CI: 1.85–3.35, P < 0.001) (Fig. 10).
In order to perform subgroup analysis to investigate the probable NLR difference between HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF), 14 articles were reported this biomarker among HF sufferers with either preserved (N = 4) or reduced (N = 10) ejection fraction [22, 33, 35, 36, 38, 43, 44, 49, 51, 54, 57, 58, 61, 64]. Figure 11 shows the forest plot for mean NLR in HFpEF and HFrEF groups. The results failed to prove any significant difference (NLR: 4.67, 95% CI: 3.58–5.76 vs. NLR: 4.17, 95% CI: 3.55–4.80, respectively).
Six articles reported readmission and/or death in HF individuals based on NLR [25, 34, 37, 45, 65, 66]. In Maeda et al.’s study on 669 HF subjects, 255 patients experienced death or HF readmission during the median follow-up of 476 days [65]. In another study, patients with NLR > 2.53 had higher risk of HF rehospitalization (HR: 1.75, 95% CI: 1.26–2.42, P = 0.001) but not cardiac death (HR: 1.20, 95% CI: 0.56–2.61, P = 0.640) [25]. Li et al. found that HF patients with higher NLR values had higher odds of the primary endpoint (death, HF readmission, or non-fatal myocardial infarction) than those with lower NLR values (odds ratio (OR): 1.631, 95% CI: 1.182–2.248, P = 0.019) [66]. Curran and colleagues followed 1622 HF patients for a median of 18 months. During this time, 406 (25.03%) hospitalizations and 447 (27.55%) deaths were reported. A multivariable-adjusted HR model revealed that each SD increase in NLR was associated with a 1.18-fold increase in the risk of mortality and/or rehospitalization (HR: 1.18, 95% CI: 1.11–1.26, P < 0.001). Further analysis based on NLR tertiles showed similar results (T2 vs. T1: HR: 1.33, 95% CI: 1.06–1.67, P = 0.014; T3 vs. T1: HR: 1.72, 95% CI: 1.37–2.15, P < 0.001) [34]. Yan et al.’s findings on 1355 old HF individuals revealed 3rd NLR tertile was associated with higher risk of readmission rather than the 1st tertile (HR: 1.461, 95% CI: 1.108–1.927, P = 0.007) [46]. Another study on 321 HF patients indicated HF readmission rate was 19.3% after median (IQR) follow-up period of 23 [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] months and patients with higher NLR had 2.70 (95% CI: 1.58–4.61, P < 0.001) times increased likelihood of readmission [37].
Four cross-sectional articles reported utility of NLR as a tool to predict HF [35, 42, 44, 64]. Wang and colleagues selected 141 HFpEF patients with New York heart association (NYHA) II- IV and 48 ones with NYHA I as controls, and found NLR was an independent HF presence predictor (OR: 1.388, 95% CI: 1.031–1.870, P = 0.031) [64]. In another study on 172 HFpEF patients and 173 controls, multi-variable adjusted regression model revealed NLR was independently associated with HFpEF (OR: 2.351, 95% CI: 1.464–3.776, P < 0.001) [35]. In contrast, another study on 40 HF with left ventricular ejection fraction (LVEF) < 40% and 30 healthy controls indicated this biomarker was insignificantly associated with odds of HF detection (OR: 0.644, 95% CI: 0.317–1.309, P = 0.224) [44]. Kone et al. enrolled 81 and 24 patients with moderate (NYHA I, II) and severe (NYHA III, IV) HF, respectively, and found patients with NLR of more than 3.0 had 6.78 (95% CI: 1.40–32.80, P = 0.017) times higher chances of severe HF rather than the lower group [42].
In terms of hospital stay, a study of 1299 HF patients found that 22.1% had a longer hospital stay (defined as more than seven days admission), and NLR > 3.68 was associated with 1.48-fold higher odds of a longer stay (95% CI: 1.05–2.08, P = 0.025) [23].
With regard to the right heart characteristics, an observational study reported pulmonary vascular resistance (PVR) (median (IQR)) and right ventricular systolic pressure (RVSP) (median (IQR)) were significantly different between patients with higher NLR values compared to the other group (PVR: NLR > 6: 407 (186–690) dyn*s*cm−5 vs. NLR ≤ 6: 142 (99.5–244.3) dyn*s*cm−5, P = 0.0386), RVSP: NLR > 6: 60 (40-65) mmHg vs. NLR ≤ 6: 40 (32–49), P = 0.0438). They concluded this biomarker could be a useful tool to assess HF progression [38]. One record on 1355 HF individuals (mean NLR: 3.2 ± 3.1) suggested this biomarker could be an independent risk factor for AF (OR: 1.079, 95% CI: 1.027–1.134, P = 0.003) [45]. In two studies, NLR association with renal disease was investigated. In the first one, NLR was found to be an independent predictor of kidney disease progression (HR: 1.361, 95% CI: 1.102–1.680, P = 0.003) among HFrEF patients with AF [51]. Likewise in another study, NLR was determined to be independently associated with chronic kidney disease (OR: 1.170, 95% CI: 1.054–1.298, P = 0.003) [46]. Finally, functional capacity was assessed with NLR status in one study, indicating this biomarker as an independent predictor of poor functional class in HF (OR: 3.085, 95% CI: 1.520–6.260, P = 0.002) [54].
Six articles reported specific NLR tertiles and their associations with clinical HF outcomes [23, 46, 49, 56, 62, 68]. Liu and colleagues assessed the association of NLR and in-hospital mortality on 1169 acute HF subjects, and reported 32 (17.49%), 58 (31.69%), and 93 (50.82%) deaths during hospital admission in each NLR tertile. Patients within the highest NLR tertile had significantly increased chance of in-hospital mortality in comparison to the 1st tertile (OR: 1.06, 95% CI: 1.00–1.11, P = 0.035) [62]. In Davison et al.’s study on 1823 acute HF patients, NLR was suggested as an independent predictor of one- and six-month all-cause mortality (HR: 1.66, 95% CI: 1.22–2.25, P = 0.001 and HR: 1.27, 95% CI: 1.08–1.50, P = 0.003, respectively) [68]. Delcea and colleagues found patients within the 3rd tertile died more frequently during admission (T1: 2 (0.5%), T2: 7 (1.6%) and T3: 28 (6.4%), P < 0.001) [23]. Yan et al. stated that HF patients in the highest NLR tertile had a higher risk of major cardiac events (MCE) (composite of cardiac death and HF rehospitalization) during the median follow-up of 18 months (HR: 1.425, 95% CI: 1.109–1.832, P = 0.006) [46]. Wasilewski et al. indicated 2nd and 3rd NLR tertiles had been associated with increased hazard of long-term mortality (T2 vs. T1: HR: 1.37, 95% CI: 1.06–1.77, P = 0.014, T3 vs. T1: HR: 2.31, 95% CI: 1.82–2.92, P < 0.0001, respectively) after follow-up for a median (IQR) of 660 (331–1074) days [49]. Similarly, another study proved patients within the 2nd and 3rd NLR tertiles had 1.61 (95% CI: 1.01–2.37, P = 0.02) and 1.55 (95% CI: 1.02–2.36, P = 0.04) times increased risk of experiencing primary outcome, defined as death and/or heart transplantation [56].
Two records reported different NLR quartiles and their associations with mortality [40, 60]. Wu et al. found that patients in the highest NLR quartile had a higher risk of mortality than those in the lowest quartile during a median follow-up of 66 months (HR: 1.59, 95% CI: 1.18–2.15, P = 0.002) [60]. Another study of 5580 acute HF patients found that those in the highest NLR quartile had significantly higher odds of in-hospital death (OR 2.23, 95% CI: 1.44–3.44, P < 0.001) and mortality after three-year follow-up (Q3 vs. Q1: OR: 1.35, 95% CI: 1.16–1.55, P < 0.001; Q4 vs. Q1: OR: 1.44, 95% CI: 1.24–1.67, P < 0.001) [40].
Nineteen articles reported specific NLR cut-off points and evaluated their relations with different clinical outcomes including the followings: in-hospital or long-term mortality, extended length of hospital stay, HF prediction, rehospitalization, renal disease progression, acute HF differentiation from respiratory infections, poor functional capacity, cardiovascular events plus its composite with all-cause mortality, and prediction of cardiovascular outcomes, defined as cardiac death, non-fatal myocardial infarction, and HF rehospitalization [23, 25, 33, 37, 39,40,41, 48, 50, 51, 53, 54, 57, 58, 61, 63, 64, 66, 67]. Detailed information of each cut-off value is represented in Table 2.
Risk of bias assessment
Tables S1 and S2 showed the results of risk of bias assessment. Six manuscripts performed in a cohort format and others had cross-sectional designs. NLR was defined as division of absolute neutrophil counts over absolute lymphocyte counts [22,23,24,25, 33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68]. No study had significant risk of bias and we included all of them for the downstream analysis.
Publication bias and GRADE assessment
Heterogeneity details as well as funnel plot associated with total NLR mean in HF are shown in Table S3 and Figure S1, respectively. Although funnel plot was in favor of asymmetry (Egger’s test (P = 0.00001), Begg’s test (P = 0.014)), Duval and Tweedie’s trim-and-fill method revealed similar point estimate between observed and adjusted values (4.376, 95% CI: 4.019–4.733), suggesting no probable publication bias.
Heterogeneity information as well as funnel plot among recruited studies reported total mortality (either follow-up or in-hospital death) are shown in Table S3 and Figure S2, respectively (Egger’s test (P = 0.00001), and Begg’s test (P = 0.026)). However, Duval and Tweedie’s trim-and-fill method showed similar point estimates and intervals between observed and adjusted values (4.739, 95% CI: 4.283–5.195). Further information on publication bias and heterogeneity indices of enrolled records reporting follow-up death are provided in Figure S3 and Table S3, respectively. The results of Egger’s (P = 0.00005) and Begg’s (P = 0.008) tests were in favor of presence of funnel plot asymmetry. However, Duval and Tweedie’s trim-and-fill method showed no probable publication bias (similar observed and adjusted point estimate: 4.523, 95% CI: 4.034–5.012). Heterogeneity indices showed considerable heterogeneity in enrolled records indicating in-hospital mortality (Table S3). We also provided funnel plot in Figure S4, indicating asymmetry (Egger’s test (P = 0.030), Begg’s test (P = 0.500)), but no probable publication bias (Duval and Tweedie’s trim-and-fill method observed and adjusted point estimate: 5.327, 95% CI: 4.084–6.570).
Heterogeneity indices for studies reported NLR among dead or survived HF subjects are shown in Table S3, with further provision of the funnel plot in Figure S5. Our findings suggested presence of possible publication bias (observed point estimate: 5.933, 95% CI: 5.265–6.601, adjusted point estimate: 5.115, 95% CI: 4.485–5.744). Certainty of this outcome is shown in Table S4. Funnel plot, Egger’s (P = 0.220), and Begg’s (P = 0.129) tests indicated symmetry (Figure S6) and no publication bias (similar observed and adjusted point estimate: 1.120, 95% CI: 1.023–1.226) among studies reported this biomarker’s impact, as a continuous variable, on mortality HR. In terms of NLR based on HF status, the heterogeneity indices are provided in Table S3. Finally, we provided the certainty of all aforementioned outcomes in Table S5.
Discussion
We found that mean NLR in HF patients was 4.38 (95% CI: 4.02–4.73). Each unit increase in this biomarker has been associated with 1.12 (95% CI: 1.02–1.23, P = 0.013) times increased mortality risk and this risk was higher among patients with higher NLR values than proposed cut-offs (HR: 1.77, 95% CI: 1.27–2.46, P = 0.001). Also, being in a higher NLR tertile had been associated with increased death likelihood (T2 vs. T1: HR: 1.56, 95% CI: 1.21–2.00, P = 0.001, T3 vs. T1: HR: 2.49, 95% CI: 1.85–3.35, P < 0.001). Furthermore, NLR values were significantly higher in deceased HF subjects compared to survived ones (standard mean difference: 0.67 95% CI: 0.48–0.87, P < 0.001). Since this ratio can be easily calculated during admission, it seems NLR could be a useful tool in health care settings for appropriate patients’ risk stratification. Summary figure of NLR association with different clinical outcomes in HF sufferers is provided in Fig. 12.
To date, only one systematic review and meta-analysis was done to assess prognostic utility of NLR in HF. Although the reported mortality HR was significant (HR: 1.28, 95% CI: 1.14–1.43), some points should be noted. They only searched two databases and the literature screening was up to September 2017 and they finally enrolled nine eligible studies to assess all-cause mortality [69].
Although the exact pathophysiological relation between higher NLR and worsening of cardiovascular outcomes has to be elucidated, inflammation is recognized as a main player. It has been previously reported long-term mortality in HF subjects increased as white blood cells increase. Secretion of different inflammatory cytokines including C-reactive protein (CRP), tumor necrosis factor-α (TNF-α) and interleukin (IL)-1 results in reduction in cardiac activity [70,71,72]. Moreover, neutrophils release multiple proteolytic enzymes like elastase, acid phosphatase and myeloperoxidase leading to destructive effects on cardiac tissue [73, 74]. The secretion of these inflammatory signals, coupled with increased release of granulocyte-monocyte colony-stimulating factor, lipopolysaccharides, hypoxia signals, and free radicals during the inflammatory process, ultimately prolongs the lifespan of neutrophils and induces detrimental effects on the heart [75,76,77].
On the other hand, lymphocytes play an immunomodulatory action by inducing the expression of tissue inhibitor of metalloproteinase-1 [78]. Activation of hypothalamic–pituitary–adrenal axis in context of HF, as a stressful condition, causes increased cortisol secretion from adrenal glands. This hormone induces lymphocyte apoptosis and consequent lymphocytopenia [19, 78, 79]. Also, TNF-α has been suggested as a culprit in diminishing lymphocyte counts in this regard [80]. In addition to apoptosis, other potential mechanisms proposed to induce lymphocytopenia include neurohormonal activation and downregulation of lymphocyte proliferation and differentiation [81, 82].
NLR has also been implicated in other non-CVDs including irritable bowel syndrome, multiple sclerosis, spontaneous intracerebral hemorrhage, as well as malignancies [83,84,85,86,87]. The strength of NLR as a potential prognostic tool might be attributed to two different immunologic pathways. The first one is associated with neutrophils with a rapid response. On the contrary, lymphocytes modulate a more adaptive and chronic immune system response [15]. Another possible mechanism could be related to NLR association with autonomic nervous system in a way that this ratio could imply sympathetic over parasympathetic autonomic nervous system tone. In case of sympathetic stimulation, granulocyte numbers increase. Conversely, parasympathetic down-stimulation results in decreasing lymphocyte counts leading to higher NLR values [88]. Interestingly, NLR has been suggested to be a better tool rather than its independent components (neutrophils and lymphocytes) for mortality prediction among HF sufferers [56, 59]. Given that complete blood count is routinely conducted for HF patients upon admission and typically provides information about leukocyte subsets, the measurement of NLR for effective risk stratification and the prioritization of high-risk HF patients without the need for additional costly tests presents an interesting prospect for healthcare facilities.
Although data are still limited in association of NLR with AF and renal disease, some possibilities should be considered. In terms of AF, it has been reported that AF occurrence increases with aging, and inflammation has been attributed to AF initiation; thus, co-existence of HF and AF could be predictable among elderly population [89, 90]. Also, any neuro-hormonal and structural alterations in one condition can negatively affect the other disease [91]. For renal disease, despite the fact that the exact mechanism has not been identified yet, the mutual inflammatory cytokines (CRP, IL-1, IL-6 and TNF-α) can invade renal tissue causing interstitial fibrosis, tubular injury and infiltration of different inflammatory cells [51]. Therefore, co-occurrence of HF and chronic renal disease might be associated with worsen clinical outcomes and NLR could be a useful prognostic tool in this regard.
Several strengths could be considered in current study. We tried our best to include all published articles without any time limitations. We also screened four most well-known electronic databases and used a comprehensive search strategy to recruit all potential records.
Limitations
Current study was not free from limitations. We only enrolled English records and some non-English articles might be missed. There was significant funnel plot asymmetry, probably due to different sample sizes and designs in each study which led to considerable inter-study heterogeneity and possible publication bias. However, other possible sources of this asymmetry, including inadequate analysis, selective analysis or selective outcome reporting should be considered [92]. We were unable to assess NLR difference stratified by gender. Although we implemented HR analysis according to binary variable as well as NLR tertiles, interpretation should be done with cautions due to variable tertile ranges and cut-off points reported in each record. Also, the certainty of evidence ranged from very low to low, most commonly due to considerable heterogeneity among included studies and insufficient number of available studies for most of HF clinical outcomes.
Conclusions
In conclusion, this systematic review and meta-analysis indicated NLR could be used as a practical prognostic tool for risk stratification and prioritizing high risk patients in the first place during admission and might be used as an independent factor for HF evaluation, especially in resource limited countries. Complementary studies are required clarifying the prognostic capability of NLR.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are not publicly available due to confidential issues but are available from the corresponding author on reasonable request.
Abbreviations
- AF:
-
Atrial Fibrillation
- AXIS:
-
A critical appraisal tool
- CI:
-
Confidence Interval
- CMA:
-
Comprehensive Meta-Analysis
- CRP:
-
C-Reactive Protein
- CVDs:
-
CardioVascular Diseases
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- HF:
-
Heart Failure
- HFpEF:
-
Heart Failure with preserved Ejection Fraction
- HFrEF:
-
Heart Failure with reduced Ejection Fraction
- HR:
-
Hazard Ratio
- IL:
-
Interleukin
- IQR:
-
Interquartile Range
- JBI:
-
Joanna Briggs Institute
- LVEF:
-
Left Ventricular Ejection Fraction
- MCE:
-
Major Cardiac Event
- MeSH:
-
Medical Subject Headings
- NIH:
-
National Institute of Health
- NLR:
-
Neutrophil to Lymphocyte Ratio
- NYHA:
-
New York Heart Association
- OR:
-
Odds Ratio
- PECOS:
-
Population, Exposure, Comparator, Outcomes, Study designs
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- PVR:
-
Pulmonary Vascular Resistance
- RCT:
-
Randomized Clinical Trial
- RVSP:
-
Right Ventricular Systolic Pressure
- SD:
-
Standard Deviation
- TNF-α:
-
Tumor Necrosis Factor- α
References
Members ATF, Dickstein K, CohenSolal A, Filippatos G, McMurray JJ, Ponikowski P, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European society of cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). European Heart J. 2008;29(19):2388–442.
Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30–41.
Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–743.
Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22(8):1342–56.
Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171(3):368–76.
Amirpour A, Vakhshoori M, Zavar R, Zarei H, Sadeghi M, Yavari B. The effect of 3-month growth hormone administration and 12-month follow-up duration among heart failure patients four weeks after myocardial infarction: a randomized double-blinded clinical trial. Cardiovascular Ther. 2021;2021:2680107.
Rahimi F, Vakhshoori M, Heidarpour M, Nouri F, Heshmat-Ghahdarijani K, Fakhrolmobasheri M, et al. Metolazone Add-On Therapy in Heart Failure: A Cohort Study from Persian Registry of Cardiovascular Disease/Heart Failure (PROVE/HF). Critical Care Res Pract. 2021;2021:3820292.
Roger VL. Epidemiology of heart failure. Circ Res. 2013;113(6):646–59.
Heidarpour M, Bashiri S, Vakhshoori M, Heshmat-Ghahdarijani K, Khanizadeh F, Ferdowsian S, et al. The association between platelet-to-lymphocyte ratio with mortality among patients suffering from acute decompensated heart failure. BMC Cardiovasc Disord. 2021;21(1):1–10.
Koosha P, Roohafza H, Sarrafzadegan N, Vakhshoori M, Talaei M, Sheikhbahaei E, et al. High sensitivity C-reactive protein predictive value for cardiovascular disease: a nested case control from Isfahan cohort study (ICS). Global Heart. 2020;15(1):3.
Farsani AY, Vakhshoori M, Mansouri A, Heidarpour M, Nikouei F, Garakyaraghi M, et al. Relation between hemoconcentration status and readmission plus mortality rate among Iranian individuals with decompensated heart failure. Int J Prevent Med. 2020;11:163.
Bondariyan N, Vakhshoori M, Sadeghpour N, Shafie D. Prognostic value of shock index, modified shock index, and age-adjusted derivatives in prediction of in-hospital mortality in patients with acute decompensated heart failure: persian registry of cardiovascular disease/heart failure study. Anatolian J Cardiology/Anadolu Kardiyoloji Dergisi. 2022;26(3):210–7.
Heidarpour M, Sourani Z, Vakhshoori M, Bondariyan N, Emami SA, Fakhrolmobasheri M, et al. Prognostic utility of shock index and modified shock index on long-term mortality in acute decompensated heart failure; Persian Registry of cardioVascular diseasE/Heart Failure (PROVE/HF) study. Acta Cardiologica. 2022;78:1–10.
Van Linthout S, Tschöpe C. Inflammation–cause or consequence of heart failure or both? Curr Heart Fail Rep. 2017;14(4):251–65.
Bhat T, Teli S, Rijal J, Bhat H, Raza M, Khoueiry G, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11(1):55–9.
Silberman S, Abu-Yunis U, Tauber R, Shavit L, Grenader T, Fink D, et al. Neutrophil-lymphocyte ratio: prognostic impact in heart surgery. Early outcomes and late survival. Ann Thorac Surg. 2018;105(2):581–6.
Kim S, Eliot M, Koestler DC, Wu W-C, Kelsey KT. Association of neutrophil-to-lymphocyte ratio with mortality and cardiovascular disease in the Jackson heart study and modification by the duffy antigen variant. JAMA cardiology. 2018;3(6):455–62.
Tracchi I, Ghigliotti G, Mura M, Garibaldi S, Spallarossa P, Barisione C, et al. Increased neutrophil lifespan in patients with congestive heart failure. Eur J Heart Fail. 2009;11(4):378–85.
Maisel A, Knowlton K, Fowler P, Rearden A, Ziegler M, Motulsky H, et al. Adrenergic control of circulating lymphocyte subpopulations. Effects of congestive heart failure, dynamic exercise, and terbutaline treatment. J Clin Invest. 1990;85(2):462–7.
Vaduganathan M, Greene SJ, Butler J, Sabbah HN, Shantsila E, Lip GY, et al. The immunological axis in heart failure: importance of the leukocyte differential. Heart Fail Rev. 2013;18(6):835–45.
Rudiger A, Burckhardt OA, Harpes P, Müller SA, Follath F. The relative lymphocyte count on hospital admission is a risk factor for long-term mortality in patients with acute heart failure. Am J Emerg Med. 2006;24(4):451–4.
Davran GB, Davran AÇ, Karabag T. The relationship of prognostic nutritional index with prognosis and inflammation in patient with heart failure and reduced ejection fraction. Nutr Health. 2022;22:02601060221103017.
Delcea C, Buzea CA, Vijan A, Draghici A, Stoichitoiu LE, Dan G-A. Comparative role of hematological indices for the assessment of in-hospital outcome of heart failure patients. Scandinavian Cardiovascular J. 2021;55(4):227–36.
Pourafkari L, Wang CK, Tajlil A, Afshar AH, Schwartz M, Nader ND. platelet–lymphocyte ratio in prediction of outcome of acute heart failure. Biomark Med. 2018;12(1):63–70.
Liu L, Chen Y, Xie J. Association of GNRI, NLR, and FT3 with the clinical prognosis of older patients with heart failure. Int Heart J. 2022;63(6):1048–54.
Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313(16):1657–65.
Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open. 2016;6(12):e011458.
Study Quality Assessment Tools. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews Checklist for Cohort Studies Available from: https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Cohort_Studies2017_0.pdf.
The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews Checklist for Randomized Controlled Trials. Available from: https://jbi.global/sites/default/files/2019-05/JBI_RCTs_Appraisal_tool2017_0.pdf.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):1–13.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):1–10.
Sadeghi MT, Esgandarian I, Nouri-Vaskeh M, Golmohammadi A, Rahvar N, Teimourizad A. Role of circulatory leukocyte based indices in short-term mortality of patients with heart failure with reduced ejection fraction. Med Pharmacy Rep. 2020;93(4):351.
Curran FM, Bhalraam U, Mohan M, Singh JS, Anker SD, Dickstein K, et al. Neutrophil-to-lymphocyte ratio and outcomes in patients with new-onset or worsening heart failure with reduced and preserved ejection fraction. ESC heart failure. 2021;8(4):3168–79.
Bai B, Cheng M, Jiang L, Xu J, Chen H, Xu Y. High neutrophil to lymphocyte ratio and its gene signatures correlate with diastolic dysfunction in heart failure with preserved ejection fraction. Front Cardiovas Med. 2021;8:578.
Arfsten H, Cho A, Prausmüller S, Spinka G, Novak J, Goliasch G, et al. Inflammation-based scores as a common tool for prognostic assessment in heart failure or cancer. Front Cardiovascular Med. 2021;8:725903.
Angkananard T, Inthanoo T, Sricholwattana S, Rattanajaruskul N, Wongsoasu A, Roongsangmanoon W. The predictive role of neutrophil-to-lymphocyte ratio (NLR) and mean platelet volume-to-lymphocyte ratio (MPVLR) for cardiovascular events in adult patients with acute heart failure. Mediators Inflamm. 2021;2021:6889733.
Urbanowicz TK, Olasińska-Wiśniewska A, Michalak M, Straburzyńska-Migaj E, Jemielity M. Neutrophil to lymphocyte ratio as noninvasive predictor of pulmonary vascular resistance increase in congestive heart failure patients: single-center preliminary report. Adv Clin Experimental Med. 2020;29(11):1313–7.
Köse A, Çelik A, Altınsoy E, Bozkurt Babus S, Erdoğan S. The effect of emergency department visits and inflammatory markers on one-year mortality in patients with heart failure. Cardiovasc Toxicol. 2021;21(1):49–58.
Cho JH, Cho H-J, Lee H-Y, Ki Y-J, Jeon E-S, Hwang K-K, et al. Neutrophil-lymphocyte ratio in patients with acute heart failure predicts in-hospital and long-term mortality. J Clin Med. 2020;9(2):557.
Turcato G, Sanchis-Gomar F, Cervellin G, Zorzi E, Sivero V, Salvagno GL, et al. Evaluation of neutrophil-lymphocyte and platelet-lymphocyte ratios as predictors of 30-day mortality in patients hospitalized for an episode of acute decompensated heart failure. J Med Biochem. 2019;38(4):452.
Kone A, Cherif MS, Prabin D, Dumre SP, Doumbouya AI, Kapche DF, et al. Modifiable predictors of severe heart failure in Morocco: a descriptive study using routinely collected health data. Pan African Med J. 2019;34:6.
Boralkar KA, Kobayashi Y, Amsallem M, Ataam JA, Moneghetti KJ, Cauwenberghs N, et al. Value of neutrophil to lymphocyte ratio and its trajectory in patients hospitalized with acute heart failure and preserved ejection fraction. Am J Cardiol. 2020;125(2):229–35.
Yurtdaş M, Özdemir M, Aladağ N. Investigation of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and mean platelet volume in patients with compensated heart failure. JAREM J Acad Res Med. 2018;8(2):67.
Yan W, Li R-J, Jia Q, Mu Y, Liu C-L, He K-L. Neutrophil-to-lymphocyte ratio compared to N-terminal pro-brain natriuretic peptide as a prognostic marker of adverse events in elderly patients with chronic heart failure. J Geriatric Cardiol. 2017;14(2):127.
Yan W, Liu C, Li R, Mu Y, Jia Q, He K. Usefulness of the neutrophil-to-lymphocyte ratio in predicting adverse events in elderly patients with chronic heart failure. International heart journal. 2016:16–049.
Huang W-M, Cheng H-M, Huang C-J, Guo C-Y, Lu D-Y, Lee C-W, et al. Hemographic indices are associated with mortality in acute heart failure. Sci Rep. 2017;7(1):1–9.
Siniorakis EE, Tsarouhas K, Tsitsimpikou C, Batila TC, Panta S, Zampelis KG, et al. Neutrophil to lymphocyte (N/L) and platelet to lymphocyte (P/L) ratios in differentiating acute heart failure from respiratory infection. Curr Vasc Pharmacol. 2017;15(5):477–81.
Wasilewski J, Pyka Ł, Hawranek M, Osadnik T, Kurek A, Skrzypek M, et al. Prognostic value of neutrophil-to-lymphocyte ratio in predicting long-term mortality in patients with ischemic and nonischemic heart failure. Pol Arch Med Wewn. 2016;126(3):166–73.
Liu S, Wang P, Shen P-P, Zhou J-H. Predictive values of red blood cell distribution width in assessing severity of chronic heart failure. Med Sci Monitor. 2016;22:2119.
Argan O, Ural D, Kozdag G, Sahin T, Bozyel S, Aktas M, et al. Associations between neutrophil gelatinase associated lipocalin, neutrophil-to-lymphocyte ratio, atrial fibrillation and renal dysfunction in chronic heart failure. Med Sci Monitor. 2016;22:4765.
Fu S, Xie L, Li D, Ye P, Luo L. The predictive capacity and additional prognostic power of N-terminal pro-B-type natriuretic peptide in Chinese elderly with chronic heart failure. Clin Interv Aging. 2015;10:359.
Durmus E, Kivrak T, Gerin F, Sunbul M, Sari I, Erdogan O. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are predictors of heart failure. Arq Bras Cardiol. 2015;105:606–13.
Çakıcı M, Çetin M, Doğan A, Oylumlu M, Aktürk E, Polat M, et al. Neutrophil to lymphocyte ratio predicts poor functional capacity in patients with heart failure. Turk Kardiyol Dern Ars. 2014;42(7):612–20.
Budak YU, Huysal K, Demirci H. Correlation between mean platelet volume and B-type natriuretic peptide concentration in emergency patients with heart failure. Biochemia Medica. 2015;25(1):97–102.
Benites-Zapata VA, Hernandez AV, Nagarajan V, Cauthen CA, Starling RC, Tang WW. Usefulness of neutrophil-to-lymphocyte ratio in risk stratification of patients with advanced heart failure. Am J Cardiol. 2015;115(1):57–61.
Turfan M, ErdoĞan E, Tasal A, Vatankulu MA, Jafarov P, Sönmez O, et al. Neutrophil-to-lymphocyte ratio and in-hospital mortality in patients with acute heart failure. Clinics. 2014;69:190–3.
Tasal A, Erturk M, Uyarel H, Karakurt H, Bacaksiz A, Vatankulu MA, et al. Utility of the neutrophil to lymphocyte ratio for predicting in-hospital mortality after levosimendan infusion in patients with acute decompensated heart failure. J Cardiol. 2014;63(6):418–23.
Uthamalingam S, Patvardhan EA, Subramanian S, Ahmed W, Martin W, Daley M, et al. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol. 2011;107(3):433–8.
Wu C-C, Wu C-H, Lee C-H, Cheng C-I. Association between neutrophil percentage-to-albumin ratio (NPAR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and long-term mortality in community-dwelling adults with heart failure: evidence from US NHANES 2005–2016. BMC Cardiovasc Disord. 2023;23(1):1–11.
Tamaki S, Nagai Y, Shutta R, Masuda D, Yamashita S, Seo M, et al. Combination of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as a novel predictor of cardiac death in patients with acute decompensated heart failure with preserved left ventricular ejection fraction: a multicenter study. J Am Heart Assoc. 2023;12(1):e026326.
Liu T, Wang B, Xiao S, Sun L, Zhu Z, Wang S, et al. Correlation analysis between the static and the changed neutrophil-to-lymphocyte ratio and in-hospital mortality in critical patients with acute heart failure. Postgrad Med. 2023;135(1):50–7.
Zhu X, Cheang I, Xu F, Gao R, Liao S, Yao W, et al. Long-term prognostic value of inflammatory biomarkers for patients with acute heart failure: construction of an inflammatory prognostic scoring system. Front Immunol. 2022;13:1005697.
Wang R, Wu J, Ye H, Zhang X, Wang L. Application value of systemic inflammatory indexes in the clinical evaluation of patients with heart failure with preserved ejection fraction (HFpEF). Medicina. 2022;58(10):1473.
Maeda D, Kanzaki Y, Sakane K, Tsuda K, Akamatsu K, Hourai R, et al. Prognostic value of the FAN score, a combination of the fibrosis-4 index, albumin-bilirubin score and neutrophil-lymphocyte ratio, in patients hospitalized with heart failure. Int Heart J. 2022;63(6):1121–7.
Li Z, Rong H, Wu W, Huang T, Xu J. Application value of NT-proBNP combined with NLR in evaluation of major adverse cardiac events in elderly patients with chronic heart failure. Emerg Med Int. 2022;2022:3689445.
Kocaoglu S, Alatli T. The efficiency of the HALP score and the modified HALP score in predicting mortality in patients with acute heart failure presenting to the emergency department. Age (years). 2022;73(10.18):71.50-1049.
Davison BA, Takagi K, Edwards C, Adams KF Jr, Butler J, Collins SP, et al. Neutrophil-to-lymphocyte ratio and outcomes in patients admitted for acute heart failure (as seen in the BLAST-AHF, pre-RELAX-AHF, and RELAX-AHF studies). Am J Cardiol. 2022;180:72–80.
Wang X, Fan X, Ji S, Ma A, Wang T. Prognostic value of neutrophil to lymphocyte ratio in heart failure patients. Clin Chim Acta. 2018;485:44–9.
Cooper HA, Exner DV, Waclawiw MA, Domanski MJ. White blood cell count and mortality in patients with ischemic and nonischemic left ventricular systolic dysfunction (an analysis of the Studies Of Left Ventricular Dysfunction [SOLVD]). Am J Cardiol. 1999;84(3):252–7.
Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol. 1996;27(5):1201–6.
Oikonomou E, Tousoulis D, Siasos G, Zaromitidou M, Papavassiliou AG, Stefanadis C. The role of inflammation in heart failure: new therapeutic approaches. Hellenic J Cardiol. 2011;52(1):30–40.
Reichlin T, Socrates T, Egli P, Potocki M, Breidthardt T, Arenja N, et al. Use of myeloperoxidase for risk stratification in acute heart failure. Clin Chem. 2010;56(6):944–51.
Tousoulis D, Antoniades C, Koumallos N, Stefanadis C. Pro-inflammatory cytokines in acute coronary syndromes: from bench to bedside. Cytokine Growth Factor Rev. 2006;17(4):225–33.
Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. 1992.
Hannah S, Mecklenburgh K, Rahman I, Bellingan GJ, Greening A, Haslett C, et al. Hypoxia prolongs neutrophil survival in vitro. FEBS Lett. 1995;372(2–3):233–7.
Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem cells. 2008;26(1):151–62.
Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53(1):31–47.
Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97(1):19–22.
Castro A, Bemer V, Nobrega A, Coutinho A, Truffa-Bachi P. Administration to mouse of endotoxin from gram-negative bacteria leads to activation and apoptosis of T lymphocytes. Eur J Immunol. 1998;28(2):488–95.
Mooren FC, Bloming D, Lechtermann A, Lerch MM, Volker K. Lymphocyte apoptosis after exhaustive and moderate exercise. J Appl Physiol. 2002;93(1):147–53.
Sciarretta S, Ferrucci A, Ciavarella GM, De Paolis P, Venturelli V, Tocci G, et al. Markers of inflammation and fibrosis are related to cardiovascular damage in hypertensive patients with metabolic syndrome. Am J Hypertens. 2007;20(7):784–91.
Van Soest RJ, Templeton AJ, Vera-Badillo FE, Mercier F, Sonpavde G, Amir E, et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for men with metastatic castration-resistant prostate cancer receiving first-line chemotherapy: data from two randomized phase III trials. Ann Oncol. 2015;26(4):743–9.
Wang L, Liang D, Xu X, Jin J, Li S, Tian G, et al. The prognostic value of neutrophil to lymphocyte and platelet to lymphocyte ratios for patients with lung cancer. Oncol Lett. 2017;14(6):6449–56.
Güçlü M, Ağan AF. Relationship of peripheral blood neutrophil to lymphocyteratio and irritable bowel syndrome. Turkish J Med Sci. 2017;47(4):1067–71.
Ye Z, Ai X, Fang F, Hu X, Faramand A, You C. The use of neutrophil to lymphocyte ratio as a predictor for clinical outcomes in spontaneous intracerebral hemorrhage. Oncotarget. 2017;8(52):90380.
Bisgaard A, Pihl-Jensen G, Frederiksen J. The neutrophil-to-lymphocyte ratio as disease actvity marker in multiple sclerosis and optic neuritis. Multiple Sclerosis Related Dis. 2017;18:213–7.
Abo T, Kawamura T. Immunomodulation by the autonomic nervous system: therapeutic approach for cancer, collagen diseases, and inflammatory bowel diseases. Ther Apher. 2002;6(5):348–57.
Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108(24):3006–10.
Thihalolipavan S, Morin DP. Atrial fibrillation and heart failure: update 2015. Prog Cardiovasc Dis. 2015;58(2):126–35.
Patel NJ, Patel A, Agnihotri K, Pau D, Patel S, Thakkar B, et al. Prognostic impact of atrial fibrillation on clinical outcomes of acute coronary syndromes, heart failure and chronic kidney disease. World J Cardiol. 2015;7(7):397.
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj. 2011;343:d4002.
Acknowledgements
Not applicable.
Funding
No funding has been attributed to this study.
Author information
Authors and Affiliations
Contributions
Study concept and design: M. V., N. B., S. S., D. S., S. N., M. S., B. Y., SA. E. Acquisition of data: N. B, M. V., S. S., SA. E. Analysis and interpretation of data: M. V., N. B., D. S. Drafting of the manuscript: N. B., M. V., S. S, SA. E., S. N., B. Y., M. S., D. S. Critical revision of the manuscript for valuable intellectual content: M. V., N. B., S. S., SA. E., S. N., B. Y., M. S., D. S. Statistical analysis: M. V. Administrative, technical, and material support: M. V., D. S., S. S., S. N. Supervision: D. S., M. V.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary materials.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Vakhshoori, M., Nemati, S., Sabouhi, S. et al. Neutrophil to lymphocyte ratio (NLR) prognostic effects on heart failure; a systematic review and meta-analysis. BMC Cardiovasc Disord 23, 555 (2023). https://doi.org/10.1186/s12872-023-03572-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03572-6