Abstract
Background
The prevalence of left ventricular (LV) diastolic dysfunction has been increasing over the past decade, and to date, effective pharmacotherapies that enhance LV diastolic function have not yet been identified. Though some data has demonstrated the beneficial effects of exercise training on LV diastolic function, little is known about the adaptations of diastolic function to daily physical activity (PA). Accordingly, our study aimed to investigate the impact of daily PA on tissue Doppler indices of LV diastolic function.
Methods
A total of 432 participants were enrolled for clinically indicated echocardiography from July 2019 to July 2020 at Peking University People’s Hospital. Participants aged ≥ 18 years were included if they had stable PA in the past six months and normal LV systolic function. A questionnaire was used to collect demographic characteristics, medical history, and daily PA. According to PA Guidelines for Americans, we identified these participants into low-intensity PA (LPA) group and moderate-high-intensity PA (MHPA) group. Propensity score matching (PSM) was performed to match potential confounding factors between the two groups. The clinical characteristics and echocardiographic parameters between LPA group and MHPA group were compared using student’s t-test, Mann-Whitney U test, and chi-square test as appropriate.
Results
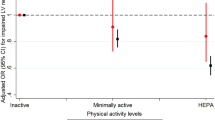
After matching potential confounding factors using PSM with a 1:3 matching ratio, our final analysis included 86 cases in the MHPA group and 214 cases in the LPA group. All demographic characteristics and comorbidities were statistically similar between the two groups. Compared to the LPA group, the MHPA group showed higher septal e’ (7.9 ± 2.9 cm/s versus 7.2 ± 2.6 cm/s, P = 0.047). Other echocardiographic parameters associated with LV diastolic function concerning lateral e’ and average E/e’, also trended towards improved LV diastolic function in the MHPA group, but failed to reach statistical significance.
Conclusions
Our study demonstrated that moderate-high-intensity daily PA was associated with improved septal e’, suggesting that moderate-high-intensity PA could potentially ameliorate LV diastolic dysfunction.
Similar content being viewed by others
Introduction
The prevalence of left ventricular (LV) diastolic dysfunction (DD) is increasing relative to the aging population with greater cardiometabolic comorbidities in recent years [1]. Current knowledge demonstrates that DD is strongly associated with impaired exercise capacity and reduced quality of life [2], ultimately resulting in the development of heart failure with preserved ejection fraction (HFpEF) and even adverse outcomes in healthy individuals [3]. However, effective pharmacotherapies have not yet been identified to enhance LV diastolic function [4, 5]. Though some data has demonstrated the beneficial effects of exercising training on LV diastolic function [6], [7,8,9], little is known about the adaptations of diastolic function to daily physical activity (PA). Echocardiography is the primary tool to identify and classify abnormal diastolic function in clinical practice [10]. Compared with traditional Doppler values, tissue Doppler indices of LV diastolic function have been proven to be more accurate, sensitive, and less load-dependent [11]. The purpose of this study was therefore to investigate the influences of comprehensive daily PA, encompassing leisure time, occupation, transportation, and household domains, on tissue Doppler indices of LV diastolic function.
Methods
Study population
From July 2019 to July 2020, 432 participants aged ≥ 18 years with stable PA in the past six months were recruited for clinically indicated echocardiography at Peking University People’s Hospital. We excluded individuals with conditions such as atrial fibrillation/flutter, cardiomyopathy, severe valvular disease, heart failure with reduced ejection fraction, myocardial infarction, pulmonary hypertension, or severe anemia. Additionally, those who either had incomplete echocardiographic data or declined to participate in the questionnaire were also excluded. Prior to their involvement, we obtained oral informed consent from all subjects.
Data collection
A questionnaire was used for collecting information concerning age, sex, height, body weight, self-reported use of alcohol, smoking, medical history, and daily PA. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Body surface area (BSA) was computed as 0.0061×height(m) + 0.0128×weight(kg)-0.1529. According to American Heart Association’s recommendations for assessing PA [12], [13], we took the international PA questionnaire (IPAQ) to evaluate recent PA, while the Compendium of PA was utilized to measure lifetime PA. The IPAQ is a globally recognized, standardized, and culturally adaptable tool used across various populations [14]. Participants completed the comprehensive self-report version of the questionnaire, which covers PA related to leisure time, occupation, transportation, household activities, as well as sedentary time. Despite the IPAQ being originally designed for individuals aged 15 to 69, its reliability in assessing PA among older adults has been adequately established [15]. Conforming to the IPAQ scoring protocol, different intensity activity levels were calculated as the metabolic equivalent (MET) and then weighted by reported minutes in every category, thereby quantifying PA expressed as MET-minutes per week. In accordance with the 2018 PA Guidelines for Americans, at least 75 min of vigorous-intensity or 150 min of moderate-intensity aerobic activities (equivalent to 600 MET-minutes) per week are recommended for significant health benefits [12, 16]. Based on these recommendations, participants who met these criteria were distributed to the moderate-high-intensity PA (MHPA) group, while those who didn’t were placed in the low-intensity PA (LPA) group. For lifetime PA, we followed a similar quantification process but weighted each category by reported hours instead of minutes, and finally normalized by age to account for accumulated activity over time.
Echocardiography
Echocardiography was performed and analyzed by two highly experienced and qualified sonographers using a GE Vivid E9 machine (GE Medical, Horten, Norway) with an M5S transducer. All images and analyses were reviewed by an independent cardiologist who was not aware of the patient’s clinical information and PA. In case of a discrepancy, the images were reviewed by another cardiologist. All echocardiography measurements were conducted and analyzed in accordance with the 2016 American Society of Echocardiography (ASE) recommendations [17]. M-mode measures of LV internal diameter, wall thickness of interventricular septum and LV posterior wall, and left atrial (LA) anteroposterior diameter were all collected from parasternal long-axial view. LV ejection fraction (LVEF) was computed according to the formula: (LV end-diastolic volume [LVEDV] - LV end-systolic volume)/LVEDV. LV mass (LVM) was calculated with the formula of Devereux et al, and LVM index (LVMI) was calculated as follows: LVMI = LVM/BSA [14]. The early (E) and late (A) mitral inflow velocities were both obtained with pulsed-wave Doppler in the apical four-chamber view while the peak velocity of tricuspid regurgitation (TR) was recorded in the parasternal short-axial view. Tissue Doppler imaging was performed to measure the peak early diastolic tissue velocities of the septal (septal e’) and lateral mitral annulus (lateral e’) from the apical four-chamber view.
Statistical analysis
Propensity score matching (PSM) was estimated using non-parsimonious multiple logistic regression model. To balance potential confounding factors between LPA group and MHPA group, PSM was adopted across key variables including age, gender, BMI, coronary heart disease, hypertension, diabetes mellitus, hypercholesterolemia, renal disease, and smoking history. These variables were chosen for matching due to their potential association with DD. In our propensity analysis, MHPA cases were matched in a 1:3 ratio to LPA controls with a standard caliper width of 0.2.
All continuous variables were presented as mean ± standard or as median and interquartile range for variables with skewed distribution. Categorical variables were expressed as numbers and percentages. The clinical characteristics and echocardiographic parameters between LPA group and MHPA group in matched or unmatched cohorts were compared using student’s t-test for continuous variables with normal distribution or Mann-Whitney U test for continuous variables with skewed distribution, and chi-square test for categorical variables. The correlation analysis with Spearman’s correlation coefficient was used to determine the relationships between septal e’ and various PA among the matched cohorts. All statistical analyses were performed with SPSS statistic version 23.0 (IBM Co., Chicago, IL, USA). Two-sided P values < 0.05 were considered statistically significant.
Results
A total of 432 patients were included in this study. According to PA status, we finally identified 338 participants in the LPA group and 86 participants in the MHPA group. Among the unmatched cohort (Table 1), the MHPA group demonstrated a higher E/A ratio (1.2 ± 0.5 versus 1.0 ± 0.4; P = 0.011), septal e’ (7.9 ± 2.9 versus 6.9 ± 2.4 cm/s; P = 0.002) and lateral e’ (10.4 ± 3.5 versus 9.4 ± 3.1 cm/s; P = 0.009), but a lower E/e’ ratio (9.1 ± 3.1 versus 10.0 ± 2.9; P = 0.018) compared to the LPA group. Although these discrepancies between the two groups seemed modest, they all reached statistical significance, consistently indicating better LV diastolic function in the MHPA group. However, no significant differences were found in LA, TR, LV internal diameter during diastole (LVIDd), interventricular septum dimension during diastole (IVSd), LV posterior wall thickness during diastole (LVPWd), LVEF, and LVMI between the two groups. It was worth noting that the LPA group was significantly older and had a greater percentage of female participants compared to the MHPA group. Besides, hyperlipidemia was more common in the LPA group. These factors have been currently known as potential risk factors for DD. Other demographic data were largely consistent between the two groups.
Given the discrepant risk factors between the two groups, PSM was further performed and 1:3 balanced cohorts were generated with 86 cases in the MHPA group and 214 cases in the LPA group. The matching was based on specific variables detailed in the Methods section. Post-matching, all demographic characteristics and comorbidities were statistically comparable between the two groups (Table 2). Within the matched cohorts, the MHPA group exhibited a higher septal e’ (7.9 ± 2.9 cm/s versus 7.2 ± 2.6 cm/s, P = 0.047) than the LPA group. Other echocardiographic parameters, such as lateral e’ and average E/e’ related to LV diastolic function, demonstrated a tendency towards improved LV diastolic function in the MHPA group, though they did not achieve statistical significance.
Notably, the MHPA group engaged in significantly more moderate-high-intensity and lifetime PA, coupled with less low-intensity PA and fewer sedentary periods compared to the LPA group (Table 3). Moreover, the MHPA group reported significantly more PA in working, transportation, and leisure domains relative to the LPA group (Table 3). The differences in PA between the two groups after matching mirrored the results obtained before (Table S1). We further executed a Spearman correlation analysis to examine the relationship between various types of PA and septal e’ within the matched cohorts. Our findings indicated that enhanced septal e’ was significantly correlated with higher levels of moderate-high-intensity and lifetime PA, but inversely related to low-intensity PA and sedentary time (Table 4). Interestingly, moderate-high-intensity PA exhibited a more profound correlation with septal e’ (r = 0.185, P = 0.001) compared to lifetime PA (r = 0.132, P = 0.022).
Discussion
The current study sought to evaluate the effects of daily PA on tissue Doppler indices of LV diastolic function in a cross-sectional population. Our primary finding indicated that moderate-high-intensity PA correlated with improved septal e’, suggestive of enhanced LV diastolic function.
Although several exercise studies have reported on diastolic indices, the impact of exercise or PA on diastolic function remains controversial. The Ex-DHF trial revealed that exercise training significantly improves diastolic E/e’ index in HFpEF patients, which is the first randomized multicenter trial to determine the benefits of exercise training on LV diastolic function [18]. However, a recent meta-analysis of randomized controlled trials in HFpEF patients reported that exercise training enhances exercise capacity without improvement in diastolic function [19]. In our study, we also did not identify a significant change in E/e’. Nonetheless, we discovered that moderate-high-intensity PA was associated with an improved diastolic septal e’ index. This result aligns with a previous work examining LV diastolic function in response to lifelong exercise [20]. The aforementioned divergent results might be attributed to several factors, including variations in the intensity, duration, and types of exercise training or PA, different observed indices of LV diastolic function, as well as the natural heterogeneity of abnormal diastolic function.
DD typically results from impaired LV relaxation and increased LV chamber stiffness. Furthermore, elevated LV filling pressure is the strongest evidence in favor of well-developed DD [21]. For this reason, cardiac catheterization to measure LV filling pressures has been perceived as the gold standard for the assessment of DD [19]. The term “LV filling pressures” is often synonymous with pulmonary capillary wedge pressure (PCWP) [10]. One previous study explored the correlation between echocardiographic indices and PCWP in healthy volunteers, concluding that septal e’ shows a stronger correlation with PCWP (r = 0.81, P < 0.001) than either lateral e’ (r = 0.63, P < 0.05) or E/e’(r = 0.14, P > 0.05) [22]. Another study showed that septal e’ provides more consistently obtainable and less variable measurements than lateral e’ [23]. Collectively, these findings suggest that septal e’ may be a more reliable index of diastolic function. Likewise, in patients post-myocardial infarction, Ricardo et al proved that septal e’ has the strongest echocardiographic relation to exercise capacity, assessed via oxygen consumption (r = 0.42, P < 0.001) [24]. These findings might help explain why, in our cohort of subjects, moderate-high-intensity PA was associated only with improved septal e’, but not with other diastolic function indices. Nevertheless, the underlying pathophysiologic mechanism explaining the close association between septal e’ and diastolic function remains unknown. While we can conclude from our data that PA is associated with an improved diastolic septal e’ index suggesting improved diastolic function, it would be inaccurate to say that this is conclusive evidence of improved diastolic function as no one single non-invasive index hitherto is a perfect marker of diastolic function.
Besides, left atrial volume index (LAVI) has been recognized as one of comprehensive measurements of diastolic function. However, our study primarily investigated the association between PA and LV diastolic function, rather than the correlation between left atrium size and LV diastolic function. While exercise can enhance LV diastolic function, it also contributes to an enlarged left atrium [25]— a paradoxical situation since such enlargement typically indicates DD. Due to this complexity, we did not include LAVI in our study to avoid potential confusion in interpreting its changes.
In this study, we also demonstrated a modest association between moderate-high-intensity PA and septal e’ (r = 0.185, P = 0.001). Conversely, both low-intensity PA and sedentary time were inversely related, thereby substantiating that a sedentary lifestyle contributes to cardiovascular disease. Given that diastolic function is affected by numerous factors such as age, gender, hypertension, diabetes, and PA [26, 27], it seems reasonable to attribute the moderate correlation coefficients between PA and the diastolic septal e’ index to these varying factors.
Most trials have focused on the effectiveness of center-based supervised exercise on LV diastolic function [19, 28]. Few home-based exercise trials exist, and those that do mainly focus on intensive exercise or recreational activities rather than comprehensive PA [29]. The low adherence rates to these existing exercise protocols have raised significant concerns in long-term exercise intervention studies [20]. Indeed, PA is primarily derived from four common domains concerning occupational, domestic, transportation, and leisure time [12]. It is obvious that an increase in PA in one domain cloud be offset by a decreased activity in another. By integrating PA into various domains according to an individual’s lifestyle, we can significantly boost adherence to exercise protocols. In our study, the MHPA group had markedly more PA in work, transportation, and leisure domains compared to the LPA group. Therefore, emphasis should not solely be placed on leisure PA; the levels of PA in other domains also warrant consideration given their potential contributions to improved diastolic function.
To our knowledge, this study is the first to investigate the impacts of comprehensive daily PA on diastolic function. In this research, PA was quantified by calculating the amount of different intensity activity levels in four domains, measured in MET, and weighted by the reported minutes of PA conducted each week. We observed that 75 minutes of vigorous-intensity or 150 minutes of moderate-intensity PA (equivalent to 600 MET-minutes) per week is associated with an improved diastolic septal e’ index. This novel finding supports and strengthens the assertions of the 2018 PA Guidelines for Americans. Importantly, it was noted that the MHPA group engaged in more lifetime PA and had less sedentary time, factors that may partly contribute to improved diastolic function [30].
Despite these findings, the literature thus far cannot elucidate the mechanisms by which PA improves LV diastolic function. Owing to the known beneficial effects on reducing peripheral vascular resistance and arterial stiffening, it is hypothesized that PA could enhance LV diastolic function by decreasing LV afterload and diastolic filling pressure [31]. Further evidence suggested that PA may play a cardioprotective role in preventing DD by reversing endothelial dysfunction, oxidative stress, and insulin resistance, thereby restoring mitochondrial abnormality and reducing cardiac fibrosis [32,33,34]. Nonetheless, establishing a direct cause-and-effect relationship between PA and DD necessitates more extensive mechanistic studies.
Several potential limitations of this study should be acknowledged. Firstly, the relatively small sample size was a significant limitation and could have restricted our ability to detect meaningful group differences. The evaluation of LV diastolic function via echocardiography relies on multiple indices, and the solitary enhanced septal e’ in the MHPA group was not sufficient to affirm the effectiveness of PA on LV diastolic function. Thus, larger-scale trials are necessary to clarify the role of PA in enhancing LV diastolic function. Secondly, self-reported PA gathered from questionnaires may be subject to recall and social desirability biases. As electronic devices advance, device-measured PA will provide more accurate and valid data for assessing PA. Thirdly, the cross-sectional study design precludes assessment of longitudinal changes in diastolic function adaptations to PA, and we cannot establish the direction of the association or causation. Lastly, though we applied PSM to adjust covariates, the possibility of residual confounding cannot be entirely excluded.
Conclusions
The present study revealed that moderate-high-intensity PA, originating from occupational, leisure, and transportation domains, correlated positively with enhanced septal e’. This implied that engaging in moderate-high-intensity PA could potentially ameliorate LV diastolic function. For a more comprehensive understanding, larger-scale studies utilizing objective assessment methods for PA are needed to be further studied in the future.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- LV:
-
Left ventricular
- DD:
-
Diastolic dysfunction
- HFpEF:
-
Heart failure with preserved ejection fraction
- PA:
-
Physical activity
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- IPAQ:
-
International physical activity questionnaire
- MET:
-
Metabolic equivalent
- MHPA:
-
Moderate-high-intensity physical activity
- LPA:
-
Low-intensity physical activity
- ASE:
-
American Society of Echocardiography
- LVEF:
-
Left ventricular ejection fraction
- LVEDV:
-
Left ventricular end-diastolic volume
- LVM:
-
Left ventricular mass
- LVMI:
-
Left ventricular mass index
- TR:
-
Tricuspid regurgitation
- PSM:
-
Propensity score matching
- LVIDd:
-
Left ventricular internal diameter during diastole
- IVSd:
-
Left ventricular posterior wall thickness during diastole
- LVPWd:
-
Left ventricular posterior wall thickness during diastole
- PCWP:
-
Pulmonary capillary wedge pressure
- LAVI:
-
Left atrial volume index
References
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 update: a Report from the American Heart Association. Circulation. 2020;141:e139–e596.
Grewal J, McCully RB, Kane GC, Lam C, Pellikka PA. Left ventricular function and exercise capacity. JAMA. 2009;301:286–94.
Playford D, Strange G, Celermajer DS, Evans G, Scalia GM, Stewart S, et al. Diastolic dysfunction and mortality in 436 360 men and women: the National Echo Database Australia (NEDA). Eur Heart J Cardiovasc Imaging. 2021;22:505–15.
Parikh KS, Sharma K, Fiuzat M, Surks HK, George JT, Honarpour N, et al. Heart Failure with preserved ejection Fraction Expert Panel Report: current controversies and implications for clinical trials. JACC Heart Fail. 2018;6:619–32.
Ghionzoli N, Gentile F, Del Franco AM, Castiglione V, Aimo A, Giannoni A et al. Current and emerging drug targets in Heart Failure treatment. Heart Fail Rev 2021.
O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic Heart Failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50.
Pearson MJ, Mungovan SF, Smart NA. Effect of exercise on diastolic function in Heart Failure patients: a systematic review and meta-analysis. Heart Fail Rev. 2017;22:229–42.
Nolte K, Schwarz S, Gelbrich G, Mensching S, Siegmund F, Wachter R, et al. Effects of long-term endurance and resistance training on diastolic function, exercise capacity, and quality of life in asymptomatic diastolic dysfunction vs. Heart Failure with preserved ejection fraction. ESC Heart Fail. 2014;1:59–74.
Asrar Ul Haq M, Goh CY, Levinger I, Wong C, Hare DL. Clinical utility of exercise training in Heart Failure with reduced and preserved ejection fraction. Clin Med Insights Cardiol. 2015;9:1–9.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by Echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314.
Chetrit M, Cremer PC, Klein AL. Imaging of Diastolic Dysfunction in Community-based epidemiological studies and randomized controlled trials of HFpEF. JACC Cardiovasc Imaging. 2020;13:310–26.
Strath SJ, Kaminsky LA, Ainsworth BE, Ekelund U, Freedson PS, Gary RA, et al. Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the American Heart Association. Circulation. 2013;128:2259–79.
Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr., Tudor-Locke C, et al. 2011 Compendium of Physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–81.
Wittekind SG, Edwards NM, Khoury PR, McCoy CE, Dolan LM, Kimball TR, et al. Association of Habitual Physical Activity with Cardiovascular Risk factors and target organ damage in adolescents and young adults. J Phys Act Health. 2018;15:176–82.
Tomioka K, Iwamoto J, Saeki K, Okamoto N. Reliability and validity of the International Physical Activity Questionnaire (IPAQ) in elderly adults: the Fujiwara-Kyo Study. J Epidemiol. 2011;21:459–65.
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. Phys Activity Guidelines Americans JAMA. 2018;320:2020–8.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14.
Edelmann F, Bobenko A, Gelbrich G, Hasenfuss G, Herrmann-Lingen C, Duvinage A, et al. Exercise training in Diastolic Heart Failure (Ex-DHF): rationale and design of a multicentre, prospective, randomized, controlled, parallel group trial. Eur J Heart Fail. 2017;19:1067–74.
Fukuta H, Goto T, Wakami K, Kamiya T, Ohte N. Effects of exercise training on cardiac function, exercise capacity, and quality of life in Heart Failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Heart Fail Rev. 2019;24:535–47.
Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick-Ranson G, Palmer MD, et al. Impact of lifelong exercise dose on left ventricular compliance and distensibility. J Am Coll Cardiol. 2014;64:1257–66.
von Bibra H, St John Sutton M. Diastolic dysfunction in Diabetes and the metabolic syndrome: promising potential for diagnosis and prognosis. Diabetologia. 2010;53:1033–45.
Firstenberg MS, Levine BD, Garcia MJ, Greenberg NL, Cardon L, Morehead AJ, et al. Relationship of echocardiographic indices to pulmonary capillary wedge pressures in healthy volunteers. J Am Coll Cardiol. 2000;36:1664–9.
Khan S, Bess RL, Rosman HS, Nordstrom CK, Cohen GI, Gardin JM. Which echocardiographic doppler left ventricular diastolic function measurements are most feasible in the clinical echocardiographic laboratory? Am J Cardiol. 2004;94:1099–101.
Fontes-Carvalho R, Sampaio F, Teixeira M, Rocha-Goncalves F, Gama V, Azevedo A, et al. Left ventricular diastolic dysfunction and E/E’ ratio as the strongest echocardiographic predictors of reduced exercise capacity after acute Myocardial Infarction. Clin Cardiol. 2015;38:222–9.
Park JH, Kim KH, Rink L, Hornsby K, Cho JY, Cho GY, et al. Left atrial enlargement and its association with left atrial strain in university athletes participated in 2015 Gwangju Summer Universiade. Eur Heart J Cardiovasc Imaging. 2020;21:865–72.
Beladan CC, Botezatu S, Popescu BA. Reversible left ventricular diastolic dysfunction-overview and clinical implications. Echocardiography. 2020;37:1957–66.
Pfeffer MA, Shah AM, Borlaug BA. Heart Failure with preserved ejection Fraction in Perspective. Circ Res. 2019;124:1598–617.
Mueller S, Winzer EB, Duvinage A, Gevaert AB, Edelmann F, Haller B, et al. Effect of high-intensity interval training, moderate continuous training, or Guideline-based physical activity advice on peak oxygen consumption in patients with Heart Failure with preserved ejection fraction: a Randomized Clinical Trial. JAMA. 2021;325:542–51.
Zwisler AD, Norton RJ, Dean SG, Dalal H, Tang LH, Wingham J, et al. Home-based cardiac rehabilitation for people with Heart Failure: a systematic review and meta-analysis. Int J Cardiol. 2016;221:963–9.
Matta S, Chammas E, Alraies C, Abchee A, AlJaroudi W. Association between sedentary lifestyle and diastolic dysfunction among outpatients with normal left ventricular systolic function presenting to a Tertiary Referral Center in the Middle East. Clin Cardiol. 2016;39:269–75.
Alves AJ, Ribeiro F, Goldhammer E, Rivlin Y, Rosenschein U, Viana JL, et al. Exercise training improves diastolic function in Heart Failure patients. Med Sci Sports Exerc. 2012;44:776–85.
Bostick B, Aroor AR, Habibi J, Durante W, Ma L, DeMarco VG, et al. Daily exercise prevents diastolic dysfunction and oxidative stress in a female mouse model of western diet induced obesity by maintaining cardiac heme oxygenase-1 levels. Metabolism. 2017;66:14–22.
Li S, Liang M, Gao D, Su Q, Laher I. Changes in Titin and Collagen Modulate effects of Aerobic and Resistance Exercise on Diabetic Cardiac function. J Cardiovasc Transl Res. 2019;12:404–14.
Bei Y, Wang L, Ding R, Che L, Fan Z, Gao W, et al. Animal exercise studies in cardiovascular research: current knowledge and optimal design-A position paper of the Committee on Cardiac Rehabilitation, Chinese medical doctors’ Association. J Sport Health Sci. 2021;10:660–74.
Acknowledgements
The authors would like to recognize the participants, without whom this study would not have been possible. Thank you for your contributions to science.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
SY contributed to the study conceptualization, design, and revising the manuscript. LS contributed to data collection, data analysis, data interpretation, and drafting of the manuscript. XY and YP contributed to data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was approved by the Ethics Committee of Peking University People’s Hospital with the code number: 2023PHB247-001. We certify that the study was performed in accordance with the 1964 Declaration of Helsinki and later amendments. Informed consent was obtained from all the participants prior to the enrollment of this study.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Su, L., Yan, X., Pan, Y. et al. Cross-sectional associations between questionnaire-measured physical activity and tissue doppler indices of left ventricular diastolic function. BMC Cardiovasc Disord 23, 527 (2023). https://doi.org/10.1186/s12872-023-03559-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03559-3