Abstract
Background
The role of PPAR signaling and its associated genes in the pathogenesis and progression of chronic heart failure (CHF) remains elusive.
Methods
We accessed the gene expression profile and relevant baseline information of CHF samples from the Gene Expression Omnibus (GEO) database, specifically from the GSE57338 project.
Results
From GSE57338 project, we derived the expression value of 126 PPAR-related genes. A protein-protein interaction network was then established to illustrate potential protein interactions. ClueGO analysis results revealed that these genes predominantly participate in functions such as export across plasma membrane, regulation of lipid metabolic process, fatty acid metabolism, circulatory system vascular processes, alcohol metabolism, triglyceride metabolism and regulation of lipid localization and response to nutrient. Using the cytohubba plug-in in Cytoscape, we pinpointed ACADM, PPARG and CPT2 as potential central molecules in HF pathogenesis and progression. Subsequent Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analysis delved into the potential biological role of these three genes in CHF. Immune infiltration analysis suggested that the infiltration level of neutrophils and M2 macrophages might be notably influenced by these genes, thereby playing a role in the CHF mechanism.
Conclusions
Our research provides a comprehensive insight into the significance of PPAR associated genes in CHF development. Notably, the genes ACADM, PPARG and CPT2 emerged as potential targets for clinical interventions.
Similar content being viewed by others
Introduction
Heart failure (HF) is characterized by the heart’s inability to efficiently pump the venous return blood volume due to compromised systolic and/or diastolic function. This leads to blood accumulation in the venous system and insufficient blood circulation in the arterial system. Current data suggests that the global incidence of HF ranges between 100 and 900 cases per 100,000 individuals annually [1]. HF can be categorized into acute HF and chronic HF (CHF), with CHF being the primary cause of cardiovascular-related mortalities. HF can be categorized into acute HF and chronic HF (CHF), with CHF being the primary cause of cardiovascular-related mortalities [2]. Hence, there remains a pressing need to identify effective treatments to enhance the survival prospects of CHF patients.
The peroxisome proliferator-activated receptors (PPARs) are nuclear hormone receptors that respond to fatty acids and their derivatives. As part of the ligand-activated subset of the nuclear hormone receptor family, PPARs encompass PPARα, PPARβ/δ, and PPARγ subtypes. These subtypes primarily regulate cellular metabolism and inflammation [3,4,5]. Numerous studies indicate a strong correlation between PPAR signaling and various diseases [6]. For instance, in hepatic steatosis, ETV5 modulates hepatic fatty acid metabolism through the PPAR signaling pathway, influencing disease progression [7]. The LncRNA TINCR/microRNA-107/CD36 regulatory axis operates within the context of colorectal cancer progression through the PPAR signaling pathway [8]. Additionally, in diabetic retinopathy, Fufang Xueshuantong alleviates disease progression by harnessing the PPAR signaling pathway to modify retinal hemodynamics and structure [9]. Significantly, PPAR signaling is pivotal in heart disease [4]. Evidence suggests that PGC1/PPARα signaling induces cardiomyocyte hypertrophy through YAP1 and facilitates cardiomyocyte contractility development via SF3B2 [10]. Activation of PPARγ signaling can counteract pulmonary hypertension and forestall right HF by promoting fatty acid oxidation [11]. A decline in PPARβ/δ expression in cardiac myocytes can suppress fatty acid transport and β-oxidation-related gene activity, leading to energy acquisition deficits in these cells [3]. Moreover, Wojtkowska et al. detected the PPAR expression in the aorta and left ventricle of 157 CAD patients who underwent CABG, finding that PPAR expression is not a reliable predictive factor for the development of HF in these patients post-surgery [12]. PPAR signaling is closely related to cardiac activity [13], and in this study, we will focus on the role of genes related to the PPAR signaling pathway in CHF.
The swift advancements in bioinformatics provide researchers with a powerful tool to delve deeper into understanding diseases [14]. In our study, we sourced the expression values of 126 PPAR-related genes from the GSE57338 expression matrix. Subsequently, we crafted a protein-protein interaction network to elucidate their potential protein interactions. Results from the clueGO analysis revealed a predominant enrichment of these genes in pathways such as export across the plasma membrane, lipid metabolic process regulation, fatty acid metabolism, circulatory system vascular processes, alcohol metabolism, triglyceride metabolism, lipid localization regulation, and response to nutrients. Employing the cytohubba plug-in within Cytoscape, we discerned that ACADM, PPARG, and CPT2 could be pivotal molecules in the genesis and progression of CHF. We further investigated their potential biological implications in CHF. Immune infiltration assessments suggested that the presence of neutrophils and M2 macrophages could be markedly influenced by these three genes, thereby playing a role in the CHF mechanism.
Methods
Acquisition of public data
Data on CHF was sourced from the open-access Gene Expression Omnibus (GEO) database under the GSE57338 project (313 individuals with/without CHF). This project provides RNA-seq data for both CHF and control samples. We accessed the gene expression profile directly through the “Series Matrix File(s)” link. Before commencing the analysis, we executed data preprocessing, which encompassed probe IDs annotation, filling in missing values, and data normalization.
Acquisition of PPAR target genes
We derived the list of PPAR target genes from the PPAR gene website [15].
Protein interaction network
The protein interaction network was established with the assistance of the STRING database [14]. For visualizing the network, we employed the Cytoscape software, and its cytohubba plug-in aided in pinpointing the hub nodes [16].
Biological enrichment investigation
For biological enrichment analysis based on the input genes, we utilized the ClueGO app within the Cytoscape software [17]. The “clusterprofiler” package was employed to carry out both Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis, adhering to the set threshold [18,19,20,21].
Immune microenvironment
Immune microenvironment quantification in patients was executed using the CIBERSORT algorithm [22].
Statistical analysis
All statistical analyses were conducted utilizing R software, with a significance threshold set at 0.05. Depending on data distribution characteristics, appropriate statistical methods were chosen.
Results
Role of PPAR target genes in CHF
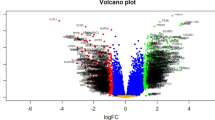
The flow chart of this study was shown in Figure S1. Using the list derived from the PPAR gene database, we extracted expression values of PPAR target genes. Figure 1 A displays the expression trends of these genes in CHF and control samples. The protein interaction network for these PPAR target genes is depicted in Fig. 1B. Our results indicated that the PPAR target genes SLC25A20, APOA1, FADS2, ACADM, ABCG2, CPT2, UGT2B4, PTGS2, SCARB1, BRCA1, CYP27A1, GHITM, VEGFA, TXNIP, SLC9A1, CAT, TNFSF10, TNIP1, APH1B, DOCK4, MLYCD, MYO18A, SYTL3, TMEM135, EXOC6B, IFIT2, PDK3, HBEGF, ACSL3 were upregulated, while HMOX1, DBI, UCP3, PLIN2, CTP1A1, UGDH, GPT, KLF10, MAP3K8, APOA5, C3, RETSAT, AP2A2, ASS1, TSC22D1, GPD1, G0S2, NAMPT, TFF2, SGK1, SAT1, CAV1, CDKN1A, SLC22A5, AK3, CTBS, DIAPH1, ECH1, IMPA2, PPARG, TIMP4, ZNF354A, ZND367, ACSL5, ANGPTL4, AP2A1, ETFDH, PDK4, VAMP8, BIRC3, CDKN2C, CSNK1G2, DCP1A, GRP180, GRAMD3, TALDO1, TNFRSF1A, FABP5 were downregulated in the CHF tissue (Table 1; Fig. 2A-D).
ClueGO analysis
Next, we delved into the potential biological impacts of these PPAR-related genes. ClueGO analysis results showed that these genes predominantly participated in pathways such as export across the plasma membrane, fatty acid metabolism, alcohol metabolism, lipid metabolic process regulation, vascular processes within the circulatory system, monocarboxylic acid metabolism, and triglyceride metabolism. Additionally, they were involved in regulating lipid localization, responding to nutrients, fatty acid metabolic process regulation, fatty acid response, cofactor biosynthesis, and adapting to oxygen levels (Fig. 3).
Identification of the hub nodes and their biological role
Using the STRING database and Cytoscape software, we built a protein network centered on the PPAR target genes (Fig. 4A). The cytohubba software pinpointed the top 10 pivotal nodes as HMOX1, CAT, UCP3, MLYCD, ACADM, CPT2, GPT, VEGFA, PPARG, and PTGS2 (Fig. 4B), with ACADM, PPARG, and CPT2 emerging as the top three crucial nodes (Fig. 4C). Correlation analysis showed that there is a significant expression correlation between ACADM, PPARG, and CP2 pairwise (Figure S2, ACADM-PPARG: cor = -0.309, P < 0.001; ACADM-CPT2: cor = 0.427, P < 0.001; PPARG-CPT2: cor = -0.143, P = 0.011).
Protein interaction network of PPAR target genes differentially expressed between CHF and control samples
Notes: A: Protein interaction network of these PPAR target genes differentially expressed between CHF and control samples; B: The top ten important nodes of the protein interaction network identified by cytoHubba plug-in of cytoscape software; C: The top three important nodes of the protein interaction network identified by cytoHubba plug-in of cytoscape software – ACADM, PPARG, CPT2.
The biological role of ACADM, PPARG and CPT2
Subsequently, we delved into the biological functions of ACADM, PPARG, and CPT2. Our findings revealed that ACADM played a significant role in ribonucleoside monophosphate metabolism, energy derivation from organic compound oxidation, cellular respiration, cell-substrate adherens junctions, mitochondrial inner membrane structuring, mitochondrial matrix processes, electron transfer activity, actin binding, coenzyme binding, thermogenesis, non-alcoholic fatty liver disease, and carbon metabolism (Fig. 5A); CPT2 was predominantly involved in small molecule catabolism, carboxylic and organic acid catabolic processes, integral components of the mitochondrial membrane, mitochondrial inner membrane dynamics, mitochondrial matrix, flavin adenine dinucleotide binding, NAD binding, coenzyme binding, carbon metabolism, herpes simplex virus 1 infection, and the degradation of valine, leucine, and isoleucine (Fig. 5B); PPARG was chiefly associated with striated muscle cell differentiation, energy derivation by organic compound oxidation, muscle system processes, contractile fiber components, myofibrils, muscle structural constituents, coenzyme and actin binding, non-alcoholic fatty liver disease, the citrate cycle (TCA cycle), and carbon metabolism (Fig. 5C).
Effect of ACADM, PPARG and CPT2 CHF microenvironment
We employed the CIBERSORT algorithm to assess the tissue microenvironment of CHF. The data suggested that patients exhibiting elevated ACADM expression displayed increased levels of CD8 + T cells, CD4 + naïve T cells, and Tregs, but decreased levels of resting CD4 + memory T cells, M2 macrophages, and neutrophils (Fig. 6A). Similarly, those with elevated PPARG expression manifested higher counts of naïve CD4 + T cells, Tregs, and resting NK cells, but a diminished count of M2 macrophages (Fig. 6B); Patients with augmented CPT2 expression showed increased levels of naïve CD4 + T cells and resting mast cells (Fig. 6C).
Immune microenvironment analysis using CIBERSORT algorithm
Notes: A: Immune infiltration analysis of ACADM, * = P < 0.05, ** = P < 0.01, *** = P < 0.001; B: Immune infiltration analysis of PPARG, * = P < 0.05, ** = P < 0.01, *** = P < 0.001; C: Immune infiltration analysis of CPT2, ** = P < 0.01, *** = P < 0.001
Discussion
HF is a prevalent cardiovascular ailment characterized by cardiac insufficiency due to compromised systolic and/or diastolic heart function. Current data estimates that about 37.7 million individuals globally are affected by HF, with CHF standing out as the primary cardiovascular-related cause of mortality [1]. The significant threat CHF poses to human health amplifies the worldwide healthcare burden. Consequently, identifying effective treatments to enhance disease prognosis remains imperative.
In our research, we spotlighted HMOX1, CAT, UCP3, MLYCD, ACADM, CPT2, GPT, VEGFA, PPARG, and PTGS2 as potential central molecules in CHF’s evolution. These genes also wield significant influence over essential biological functions. HMOX1, for instance, offers protection against ischemic damage by stabilizing the hypoxia-inducible factor, HIF-1α [23]. Furthermore, HMOX1 exerts a protective effect against ischemia/reperfusion injury in cardiomyocytes [24]. Notably, studies show that HF can elevate cardiac UCP3 protein levels, which then instigates mitochondrial uncoupling, subsequently diminishing cardiac efficiency post high-frequency feeding [25]. CPT2, with its oxidative bioenergetic role, when deficient, can inhibit long-chain fatty acid oxidation, ushering in cardiac hypertrophy [26]. Within the dorsal aorta, VEGFA signaling can stimulate processes in endothelial, arterial, and hematopoietic stem cells [27]. Additionally, Qbestatine has been found to alleviate water retention in CHF by suppressing AQP2 expression in kidneys via PPARG signaling [28]. Research by Xing et al. has indicated that managing PTGS2 expression levels can mitigate symptoms of pulmonary hypertension [29]. Such findings underscore the significance of these identified genes in cardiovascular disorders, emphasizing their potential as therapeutic targets.
Immune infiltration analysis revealed that neutrophil and M2 macrophage infiltration might play a role in the progression of CHF. Research has demonstrated that elevated levels of neutrophils and persistent neutrophil activation are primary factors contributing to the excessive inflammation observed in acute HF and the long-term outcomes of CHF [30]. Hendrik B. Sager et al. identified macrophage proliferation in CHF following myocardial infarction [31]. Building on this, Michael Horckmans et al. elucidated that after a myocardial infarction, cardiomyocyte neutrophil depletion facilitates HF development and heart repair through the macrophage shift from the M1 to M2 phenotype [32]. Such insights highlight the potential of neutrophils and M2 macrophages as therapeutic targets in CHF management.
The clueGO analysis results indicated that the genes were primarily associated with processes such as export across the plasma membrane, regulation of lipid metabolism, fatty acid metabolism, vascular activities in the circulatory system, alcohol metabolism, triglyceride metabolism, lipid localization regulation, and nutrient response. It’s well-documented that a failing heart exhibits metabolic anomalies, predominantly favoring fatty acid metabolism [33]. Crucially, essential fatty acid metabolism plays a pivotal role in hemodynamic stress-induced myocardial phospholipid remodeling and is closely linked to cardiac contractile dysfunction [34]. Furthermore, the downregulation of genes involved in fatty acid metabolism has been tied to post-infarction HF in rats [35]. In terminal HF stages, a decline in myocardial lipid content paired with heightened ketone body utilization from lipid metabolism by cardiac cells has been observed [36]. Vascular biological processes are also intricately tied to HF. Research has shown that while myocardial angiogenesis can trigger myocardial hypertrophy, a hypertrophic response in the myocardium can also induce angiogenesis [37]. It’s well-established that alcohol consumption has a significant correlation with cardiovascular diseases [38]. The myocardium lacks alcohol dehydrogenase, making it susceptible to the direct toxic effects of ethanol and acetaldehyde on cardiac myocytes. Moreover, alcohol can suppress the activities of tricarboxylic acid cycle enzymes in mitochondria, impacting the energy uptake in cardiomyocytes, which can ultimately diminish their contractility [39].
Our study does have certain limitations. Firstly, aside from the GSE57338 cohort, the sample size in other CHF cohorts is quite limited, making it challenging to corroborate our findings in additional cohorts. Secondly, real-world validation of our results remains essential.
Data Availability
The original data of GSE57338 can be obtained from https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE57338. All data are available from the corresponding author on reasonable request.
Abbreviations
- chronic heart failure:
-
CHF
- Gene Expression Omnibus:
-
GEO
- Heart failure:
-
HF
- peroxisome proliferator-activated receptors:
-
PPARs
- Gene Ontology:
-
GO
- Kyoto Encyclopedia of Genes and Genomes:
-
KEGG
References
Ziaeian B, Fonarow GC. Epidemiology and aetiology of Heart Failure. Nat Rev Cardiol. 2016;13(6):368–78. https://doi.org/10.1038/nrcardio.2016.25. Epub 2016/03/05.
Dick SA, Epelman S. Chronic Heart Failure and inflammation: what do we really know? Circ Res. 2016;119(1):159–76. https://doi.org/10.1161/circresaha.116.308030. Epub 2016/06/25.
Magadum A, Engel FB. Pparβ/∆: Linking Metabolism to Regeneration. Int J Mol Sci (2018) 19(7). Epub 2018/07/13. https://doi.org/10.3390/ijms19072013.
Wang S, Dougherty EJ, Danner RL. Pparγ Signaling and Emerging opportunities for Improved therapeutics. Pharmacol Res. 2016;111:76–85. https://doi.org/10.1016/j.phrs.2016.02.028. Epub 2016/06/09.
Zhang X, Young HA. Ppar and Immune System–what do we know? Int Immunopharmacol. 2002;2(8):1029–44. https://doi.org/10.1016/s1567-5769(02)00057-7. Epub 2002/09/28.
Wagner N, Wagner KD. The Role of Ppars in Disease. Cells (2020) 9(11). Epub 2020/11/01. https://doi.org/10.3390/cells9112367.
Mao Z, Feng M, Li Z, Zhou M, Xu L, Pan K, et al. Etv5 regulates hepatic fatty acid metabolism through Ppar Signaling Pathway. Diabetes. 2021;70(1):214–26. https://doi.org/10.2337/db20-0619. Epub 2020/10/24.
Zhang X, Yao J, Shi H, Gao B, Zhang L. Lncrna Tincr/Microrna-107/Cd36 regulates cell proliferation and apoptosis in Colorectal Cancer Via Ppar Signaling Pathway based on Bioinformatics Analysis. Biol Chem. 2019;400(5):663–75. https://doi.org/10.1515/hsz-2018-0236. Epub 2018/12/07.
Sun HH, Chai XL, Li HL, Tian JY, Jiang KX, Song XZ, et al. Fufang Xueshuantong alleviates Diabetic Retinopathy by activating the Ppar Signalling Pathway and Complement and Coagulation cascades. J Ethnopharmacol. 2021;265:113324. https://doi.org/10.1016/j.jep.2020.113324. Epub 2020/09/06.
Murphy SA, Miyamoto M, Kervadec A, Kannan S, Tampakakis E, Kambhampati S, et al. Pgc1/Ppar drive cardiomyocyte maturation at single cell Level Via Yap1 and Sf3b2. Nat Commun. 2021;12(1):1648. https://doi.org/10.1038/s41467-021-21957-z. Epub 2021/03/14.
Legchenko E, Chouvarine P, Borchert P, Fernandez-Gonzalez A, Snay E, Meier M et al. Pparγ Agonist Pioglitazone Reverses Pulmonary Hypertension and Prevents Right Heart Failure Via Fatty Acid Oxidation. Sci Transl Med (2018) 10(438). Epub 2018/04/27. https://doi.org/10.1126/scitranslmed.aao0303.
Wojtkowska I, Tysarowski A, Seliga K, Siedlecki JA, Juraszyński Z, Marona M et al. Ppar Gamma Expression Levels During Development of Heart Failure in Patients with Coronary Artery Disease after Coronary Artery Bypass-Grafting. PPAR research (2014) 2014:242790. Epub 2014/11/06. https://doi.org/10.1155/2014/242790.
Ehara N, Ono K, Morimoto T, Kawamura T, Abe M, Hasegawa K. The possible role of peroxisome proliferator-activated receptor Gamma in Heart Failure. Experimental and Clinical Cardiology. 2004;9(3):169–73. Epub 2004/10/01.
Wei Y, Chen X, Ren X, Wang B, Zhang Q, Bu H, et al. Identification of Mx2 as a Novel Prognostic Biomarker for Sunitinib Resistance in Clear Cell Renal Cell Carcinoma. Front Genet. 2021;12:680369. https://doi.org/10.3389/fgene.2021.680369. Epub 2021/07/27.
Fang L, Zhang M, Li Y, Liu Y, Cui Q, Wang N, Ppargene. A Database of Experimentally Verified and Computationally Predicted Ppar Target Genes. PPAR research (2016) 2016:6042162. Epub 2016/05/06. https://doi.org/10.1155/2016/6042162.
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. Cytohubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC systems biology (2014) 8 Suppl 4(Suppl 4):S11. Epub 2014/12/19. https://doi.org/10.1186/1752-0509-8-s4-s11.
Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, et al. Cluego: a Cytoscape Plug-in to Decipher functionally grouped Gene Ontology and Pathway Annotation Networks. Bioinf (Oxford England). 2009;25(8):1091–3. https://doi.org/10.1093/bioinformatics/btp101. Epub 2009/02/25.
Yu G, Wang LG, Han Y, He QY. Clusterprofiler: an R Package for comparing Biological themes among Gene Clusters. OMICS. 2012;16(5):284–7. https://doi.org/10.1089/omi.2011.0118. Epub 2012/03/30.
Kanehisa M, Goto S. Kegg: Kyoto Encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. https://doi.org/10.1093/nar/28.1.27. Epub 1999/12/11.
Kanehisa M. Toward understanding the origin and evolution of Cellular organisms. Protein Science: A Publication of the Protein Society. 2019;28(11):1947–51. https://doi.org/10.1002/pro.3715. Epub 2019/08/24.
Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. Kegg for Taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51(D1):D587–d92. https://doi.org/10.1093/nar/gkac963. Epub 2022/10/28.
Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling Tumor Infiltrating Immune Cells with Cibersort. Methods in molecular biology (Clifton, NJ) (2018) 1711:243 – 59. Epub 2018/01/19. https://doi.org/10.1007/978-1-4939-7493-1_12.
Dunn LL, Kong SMY, Tumanov S, Chen W, Cantley J, Ayer A, et al. Hmox1 (Heme Oxygenase-1) protects against ischemia-mediated Injury Via stabilization of Hif-1α (hypoxia-Inducible Factor-1α). Arterioscler Thromb Vasc Biol. 2021;41(1):317–30. https://doi.org/10.1161/atvbaha.120.315393. Epub 2020/11/20.
Yoshida T, Maulik N, Ho YS, Alam J, Das DK. H(Mox-1) constitutes an adaptive response to effect antioxidant cardioprotection: a study with transgenic mice heterozygous for targeted disruption of the Heme Oxygenase-1 gene. Circulation. 2001;103(12):1695–701. https://doi.org/10.1161/01.cir.103.12.1695. Epub 2001/03/29.
Boudina S, Han YH, Pei S, Tidwell TJ, Henrie B, Tuinei J, et al. Ucp3 regulates Cardiac Efficiency and mitochondrial coupling in High Fat-Fed mice but not in leptin-deficient mice. Diabetes. 2012;61(12):3260–9. https://doi.org/10.2337/db12-0063. Epub 2012/08/23.
Pereyra AS, Harris KL, Soepriatna AH, Waterbury QA, Bharathi SS, Zhang Y, et al. Octanoate is differentially metabolized in liver and muscle and fails to rescue Cardiomyopathy in Cpt2 Deficiency. J Lipid Res. 2021;62:100069. https://doi.org/10.1016/j.jlr.2021.100069. Epub 2021/03/25.
Leung A, Ciau-Uitz A, Pinheiro P, Monteiro R, Zuo J, Vyas P, et al. Uncoupling Vegfa functions in Arteriogenesis and hematopoietic stem cell specification. Dev Cell. 2013;24(2):144–58. https://doi.org/10.1016/j.devcel.2012.12.004. Epub 2013/01/16.
Bao LZ, Shen M, Qudirat H, Shi JB, Su T, Song JW, et al. Obestatin ameliorates Water Retention in Chronic Heart Failure by Downregulating Renal Aquaporin 2 through Gpr39, V2r and Pparg Signaling. Life Sci. 2019;231:116493. https://doi.org/10.1016/j.lfs.2019.05.049. Epub 2019/06/04.
Xing Y, Zhao S, Wei Q, Gong S, Zhao X, Zhou F et al. A Novel Piperidine Identified by Stem Cell-Based Screening Attenuates Pulmonary Arterial Hypertension by Regulating Bmp2 and Ptgs2 Levels. Eur Respir J (2018) 51(4). Epub 2018/02/17. https://doi.org/10.1183/13993003.02229-2017.
Kain V, Halade GV. Role of Neutrophils in Ischemic Heart Failure. Pharmacol Ther (2020) 205:107424. Epub 2019/10/20. https://doi.org/10.1016/j.pharmthera.2019.107424.
Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, et al. Proliferation and recruitment contribute to myocardial macrophage expansion in Chronic Heart Failure. Circul Res. 2016;119(7):853–64. https://doi.org/10.1161/circresaha.116.309001. Epub 2016/07/23.
Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, et al. Neutrophils orchestrate Post-myocardial Infarction Healing by Polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38(3):187–97. https://doi.org/10.1093/eurheartj/ehw002. Epub 2017/02/06.
Yamamoto T, Sano M. Deranged Myocardial Fatty Acid Metabolism in Heart Failure. Int J Mol Sci (2022) 23(2). Epub 2022/01/22. https://doi.org/10.3390/ijms23020996.
Le CH, Mulligan CM, Routh MA, Bouma GJ, Frye MA, Jeckel KM, et al. Delta-6-Desaturase links polyunsaturated fatty acid metabolism with Phospholipid Remodeling and Disease Progression in Heart Failure. Circ Heart Fail. 2014;7(1):172–83. https://doi.org/10.1161/circheartfailure.113.000744. Epub 2013/11/29.
Rosenblatt-Velin N, Montessuit C, Papageorgiou I, Terrand J, Lerch R. Postinfarction Heart Failure in rats is Associated with Upregulation of Glut-1 and downregulation of genes of fatty acid metabolism. Cardiovasc Res. 2001;52(3):407–16. https://doi.org/10.1016/s0008-6363(01)00393-5. Epub 2001/12/12.
Bedi KC Jr., Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and increased myocardial ketone utilization in Advanced Human Heart Failure. Circulation. 2016;133(8):706–16. https://doi.org/10.1161/circulationaha.115.017545. Epub 2016/01/29.
Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and Cardiac Hypertrophy: maintenance of cardiac function and causative roles in Heart Failure. Circ Res. 2014;114(3):565–71. https://doi.org/10.1161/circresaha.114.300507. Epub 2014/02/01.
Biddinger KJ, Emdin CA, Haas ME, Wang M, Hindy G, Ellinor PT, et al. Association of Habitual Alcohol Intake with Risk of Cardiovascular Disease. JAMA Netw Open. 2022;5(3):e223849. https://doi.org/10.1001/jamanetworkopen.2022.3849. Epub 2022/03/26.
Bing RJ. Cardiac Metabolsim: its contributions to Alcoholic Heart Disease and myocardial failure. Circulation. 1978;58(6):965–70. https://doi.org/10.1161/01.cir.58.6.965. Epub 1978/12/01.
Acknowledgements
Not Applicable.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
KZ and TW collected the data and performed the analysis. ZG wrote the manuscript. CK designed this work. All listed authors must have made a significant scientific contribution to the research in the manuscript approved its claims and agreed to be an author.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author reports no conflicts of interest in this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ke, ZP., Tao, WQ., Zhao, G. et al. Role of PPAR-related genes in chronic heart failure: evidence from large populations. BMC Cardiovasc Disord 23, 552 (2023). https://doi.org/10.1186/s12872-023-03554-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03554-8