Abstract
Background
The role of intra-aortic balloon counterpulsation (IABP) in cardiogenic shock complicating acute myocardial infarction (AMI) is still a subject of intense debate. In this study, we aim to investigate the effect of IABP on the clinical outcomes of patients with AMI complicated by cardiogenic shock undergoing percutaneous coronary intervention (PCI).
Methods
From the Medical Information Mart for Intensive Care (MIMIC)-IV 2.2, 6017 AMI patients were subtracted, and 250 patients with AMI complicated by cardiogenic shock undergoing PCI were analyzed. In-hospital outcomes (death, 24-hour urine volumes, length of ICU stays, and length of hospital stays) and 1-year mortality were compared between IABP and control during the hospital course and 12-month follow-up.
Results
An IABP was implanted in 30.8% (77/250) of patients with infarct-related cardiogenic shock undergoing PCI. IABP patients had higher levels of Troponin T (3.94 [0.73–11.85] ng/ml vs. 1.99 [0.55–5.75] ng/ml, p-value = 0.02). IABP patients have a longer length of ICU and hospital stays (124 [63–212] hours vs. 83 [43–163] hours, p-value = 0.005; 250 [128–435] hours vs. 170 [86–294] hours, p-value = 0.009). IABP use was not associated with lower in-hospital mortality (33.8% vs. 33.0%, p-value = 0.90) and increased 24-hour urine volumes (2100 [1455–3208] ml vs. 1915 [1110–2815] ml, p-value = 0.25). In addition, 1-year mortality was not different between the IABP and the control group (48.1% vs. 48.0%; hazard ratio 1.04, 95% CI 0.70–1.54, p-value = 0.851).
Conclusion
IABP may be associated with longer ICU and hospital stays but not better short-and long-term clinical prognosis.
Similar content being viewed by others
Introduction
Cardiogenic shock is a life-threatening complication of acute myocardial infarction (AMI) in nearly 5-10% of patients [1]. The mortality of AMI complicated by cardiogenic shock remain unacceptably high at rates between 40 and 60% even when the patients undergo early revascularization [2,3,4]. Intra-aortic balloon pump (IABP) has been the most widely used percutaneous mechanical circulatory support (PMCS) device for several decades. The effects of IABP are believed to increase the myocardial oxygen supply/demand ratio and thus improve prognosis. Because registry studies indicated mortality benefits, former U.S. and European guidelines gave a class I.B. and class I.C. recommendation favoring IABP in patients with AMI complicated by cardiogenic shock [5,6,7]. However, the results of the largest randomized trial (the IABP-SHOCK-II [Intra-aortic Balloon Pump in Cardiogenic Shock-II study]) showed that IABP counterpulsation did not reduce 30day, 1year and 6-year mortality in cardiogenic shock complicating AMI undergoing early revascularization [8,9,10]. For this reason, the routine use of IABP in patients with infarct-related cardiogenic shock is no longer recommended by international guidelines [11, 12]. Unfortunately, the effective alternative PMCS devices for infarct-related cardiogenic shock are very limited. Therefore, the use of IABP was continued despite the paucity of survival benefit evidence based on randomized clinical trials [8,9,10, 13]. This study was designed to test the hypothesis that IABP can reduce mortality among patients with AMI complicated by cardiogenic shock undergoing percutaneous coronary intervention (PCI).
Materials and methods
Data source
This research was performed on a large critical-care database, namely, Medical Information Mart for Intensive Care (MIMIC)-IV, which comprised critical care data for patients admitted to intensive care units at the Beth Israel Deaconess Medical Center (BIDMC) [14, 15]. The latest version, MIMIC-IV 2.2, was updated in January 2023 and contained comprehensive clinical and laboratory data of patients. The date of death is determined by state and hospital records. If both exist, hospital records are used. MIMIC-IV collected state and hospital records for the date of death two years after the last patient discharge, which could lessen the impact of reporting delays in the date of death. The first author (DF) of this study passed the Protecting Human Research Participants exam (certification number: 50,924,352) to obtain the utility of the database. Data extraction from the database was done using the structured query language (SQL).
Population selection criteria
Patients with acute myocardial infarction admitted for the first time were included. Patients without infarct-related cardiogenic shock and those without percutaneous coronary intervention were excluded from the study. The flowchart of population selection is displayed in Fig. 1.
Outcomes and covariates
The extraction variables included age, gender, diagnosis of STEMI, diagnosis of chronic total occlusion (CTO), history (hypertension, diabetes, tobacco, prior myocardial infarction, prior chronic kidney disease), arterial blood gas on arrival (pH, partial pressure of oxygen [PaO2], partial pressure of carbon dioxide [PaCO2], lactate), baseline serum creatinine, hemoglobin, total cholesterol (T.C.), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), troponin T, and mechanical ventilation. The primary outcome was one-year (long-term) mortality. The secondary outcomes included in-hospital (short-term) mortality, 24-hour urine volumes, length of intensive care unit (ICU) stay, and length of hospital stay. Patients with cardiogenic shock complicating AMI undergoing PCI were distributed to IABP versus control. The start and end times of IABP were also extracted to calculate the IABP duration and compare it with the PCI procedure time.
Statistical analyses
Skewness and kurtosis tests were used to test the normality of continuous variables. Normally distributed continuous variables were compared using Student t-tests and were expressed as mean ± S.D. Skewed distributed continuous variables were compared using a two-sample Wilcoxon rank-sum (Mann-Whitney) test and were expressed as median (interquartile range). Categorical variables were compared using Pearson chi-square tests and were expressed as numbers (percentages). Survival probability throughout one year after admission was characterized using Kaplan–Meier survival estimates, with the log-rank test used to compare the two groups.
Identifying independent clinical and laboratory risk factors at baseline related to death was performed using Cox proportional hazards regression modeling. All variables considered clinically relevant and related to mortality on univariable analysis (defined by P < 0.10) were further analyzed in a stepwise multivariable model.
STATA (version 17.0, USA) software was used for the statistical analysis. All calculated p-value were 2-sided, and p-value < 0.05 were considered statistically significant.
Results
In our study, 6017 patients with AMI were extracted, and 250 patients with AMI complicated by cardiogenic shock undergoing PCI were included, and the associated flow chart is displayed in Fig. 1. The cardiogenic shock occurred in 12.4% (749/6017) of patients with AMI. An IABP was implanted in 30.8% (77/250) of patients. The baseline characteristics of patients treated with and without IABP are presented in Table 1. Patients managed with IABP had a higher level of Troponin T (3.94 [0.73–11.85] ng/ml vs. 1.99 [0.55–5.75] ng/ml, p-value = 0.02). Other baseline characteristics were similar between the groups. IABP placement was performed after PCI in 85.7% of patients. The mean IABP duration was 69.6 h in all patients managed with IABP.
The clinical outcomes of patients treated with and without IABP are displayed in Table 2. In-hospital (short-term) and 1-year (long-term) mortality was similar in the patients between the two groups (33.8% vs. 33.0%, p-value = 0.90; 48.1% vs. 48.0%, p-value = 0.99). 24-hour urine volumes on the first day were similar in the patients receiving an IABP and those who did not (2100 [1455–3208] ml vs. 1915 [1110–2815] ml, p-value = 0.25). Patients managed with IABP were more likely to have a longer length of ICU and hospital stays (124 [63–212] hours vs. 83 [43–163] hours, p-value = 0.005; 250 [128–435] hours vs. 170 [86–294] hours, p-value = 0.009). The cumulative 1-year (long-term) survival of patients managed with and without IABP was similar (p-value = 0.85). Time-to-survival curves through 1 year are shown in Fig. 2.
Time-to-survival curves through 1 year
Time-to-survival curves through 1 year for all-cause mortality. The cumulative 1-year (long-term) survival of patients managed with and without IABP was characterized using Kaplan-Meier estimates. The p-value was calculated by the log-rank test. IABP indicates intra-aortic balloon pump
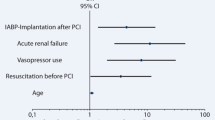
Stepwise multivariable modeling revealed increasing age, mechanical ventilation, chronic total occlusion, and baseline arterial lactate as independent risk factors for long-term mortality (Table 2). IABP treatment was not predictive of survival (HR 1.04, 95% CI 0.70–1.54, p-value = 0.851). The timing of IABP implantation and duration of IABP treatment was also not predictive of long-term outcomes.
Time-to-survival curves through 1 year for all-cause mortality. The cumulative 1-year (long-term) survival of patients managed with and without IABP was characterized using Kaplan-Meier estimates. The p-value was calculated by the log-rank test. IABP indicates intra-aortic balloon pump.
Discussion
In this study, patients with AMI complicated by cardiogenic shock remain at a high risk of mortality (approximately 50%) regardless of whether they receive IABP treatment. Early studies indicate a mortality benefit for several indications, including AMI complicated by cardiogenic shock, to support coronary artery bypass graft (CABG) or high-risk PCI, as well as following thrombolysis [16,17,18]. However, much debate has been on whether IABP counterpulsation benefits patients with cardiogenic shock. Indeed, the multicenter open-label IABP-SHOCK II trial’s only adequately powered randomized trial failed to show any mortality benefit for IABP over the control or any advantages for secondary outcomes [8,9,10]. Based on IABP-SHOCK, current guidelines recommend not using IABP routinely [11, 12]. In the present study, IABP was not associated with survival benefits, which is consistent with the results of the IABP-SHOCK-II trial. However, in this study, patients managed with IABP were more likely to have higher troponin T levels, indicating that these patients were admitted later and had larger infarct sizes. In addition, in the present study, IABP use was seemly greater in patients with higher risk characteristics, including those with STEMI (83.1% vs. 74.6%), CTO (16.9% vs. 11.0%), a history of hypertension (37.7% vs. 27.7%), a history of diabetes (44.1% vs. 33.5%), a history of tobacco (11.7% vs. 5.7%), prior MI (15.6% vs. 12.7%), and higher arterial lactate (2.4 mmol/liter vs. 2.1 mmol/liter) and in patients supported by mechanical ventilation (63.6% vs. 51.4%) but those characteristics without significantly statistical differences (p-value < 0.05; Table 1). In stepwise multivariable Cox regression analysis, age, mechanical ventilation, CTO, and baseline lactate were associated with higher long-term mortality (Table 3). Moreover, the use of IABP was associated with longer ICU and hospital stays (124 [63–212] vs. 83 [43–163], p-value = 0.005; 250 [128–435] vs. 170 [86–294], p-value = 0.009). Therefore, in this study, the baseline characteristics were not remarkably dissimilar between patients with IABP and controls. The short-term and long-term mortality was similar between patients managed with IABP and control, which may not be enough evidence to suggest that patients with infarct-related cardiogenic shock can benefit from IABP treatment.
In the present study, approximately one-third of patients with AMI complicated by cardiogenic shock managed with IABP. Indeed, however, the application of IABP in cardiogenic shock was significantly influenced by the results of IABP-SHOCK. Based on IABP-SHOCK, the use of IABP has decreased dramatically since 2012, despite increasing rates of infarct-related cardiogenic shock [19]. Despite several pathophysiological studies suggesting a hemodynamic gain under IABP for cardiogenic shock [20, 21], only one randomized trial, IABP-SHOCK, indicates no improvement was observed in measured hemodynamic parameters [22]. Under some clinical situations, the ventricular-arterial uncoupling may cause the IABP’s inefficiency and affect IABP performance via complex mechanisms [23]. IABP was widely used clinically, mainly based on its underlying functional idea to increase left ventricular output and coronary perfusion by diastolic augmentation and afterload reduction. It is probably unreasonable that there were no differences in median diastolic pressure between patients managed with IABP and controls in the IABP-SHOCK II trial (55 [46–67] mmHg vs. 55 [45–65]mmHg) [8]. In addition, it is important to remember that patients without adequate myocardial reperfusion may benefit from the increased coronary perfusion pressure obtained by IABP, whereas this effect of IABP would not translate into a therapeutic advantage in patients with enough flow [23]. Hence, denying the benefits of IABP based on just one powered randomized trial is likely unreasonable. At the very least, no study found that IABP increased significant adverse events and mortality. Indeed, Impella Support, more advanced and expensive PMCS devices, was not associated with a mortality benefit compared with IABP [24,25,26]. In addition, Impella, compared with IABP, was associated with a higher rate of major bleeding events in randomized controlled trial [24]. In this regard, the continued use of IABP in patients with AMI complicated by cardiogenic shock is not unreasonable.
IABP seems to have other benefits besides increasing coronary perfusion and left ventricular output. A cohort study found that IABP is probably associated with improving cerebral blood flow, particularly in patients with impaired left cardiac function [27]. This study implicated that IABP is probably associated with improving neurological outcomes. In a clinical trial [28], IABP was found to have synergistic effects with extracorporeal membrane oxygenation (ECMO). Furthermore, it was found that IABP, in conjunction with ECMO, can increase in-hospital survival in patients with cardiogenic shock more efficiently than ECMO alone [29]. Studies have also found that IABP can increase renal blood flow [30]. However, the use of IABP in this study was not associated with a significant increase in 24-hour urine volumes compared to controls, suggesting that IABP is unlikely to improve renal perfusion significantly. What is certain, however, is that IABP does not cause kidney injury.
Managing infarct-related cardiogenic shock is challenging, despite the rapid development of primary PCI. Cardiologists face a significant conundrum because there are no evidence-based alternative interventions for patients with AMI complicated by cardiogenic shock, who have rapidly deteriorating hemodynamics and bad clinical outcomes. As a result, we urgently require a more advanced strategy to improve the poor clinical outcomes of infarct-related cardiogenic shock patients. Before it, IABP seemed to be the only intervention available for patients at most institutions; it seemly could explain why the IABP was still used in many hospitals despite the current guidelines recommend not to use it routinely. However, it is well known that IABP is associated with more complications (major limb ischemia, severe bleeding, balloon leak, death directly due to IABP insertion or failure) and higher medical costs [31]. Whether to use IABP in patients with myocardial infarction complicated by cardiogenic shock is probably based on the operator’s clinical experience in most hospitals. From this study, we prefer the current guideline recommendation not to use IABP routinely [11, 12].
Our study’s primary limitation is that it is a retrospective observational study without randomization. The results of this study are insufficient to conclude that IABP will benefit patients with AMI complicated by cardiogenic shock. However, in our study, only 14.3% of patients received treatment of IABP before the percutaneous coronary intervention, so the timing of the use of IABP probably impacted the findings. If the IABP was done before PCI, possible outcomes could be better. In addition, due to the lack of some data, indicators such as hemodynamic parameters focused on by some researchers were not included.
Conclusion
In summary, even when patients with AMI complicated by cardiogenic shock receive percutaneous coronary intervention, the 1-year mortality rate remains unacceptably high (about 50%). The results of this study are consistent with IABP-SHOCK in that IABP is not a predictor of both short- and long-term survival. IABP patients tended to have longer length of ICU and hospital stays. To improve the clinical outcomes of patients with AMI complicated by cardiogenic shock, more advanced evidence-based PMCS devices are urgently required.
Data Availability
All data generated or analyzed during this study are included in this published article. After completing relevant training and registration, the raw data are available in the physionet (https://physionet.org/content/mimiciv) [14, 15].
References
Vallabhajosyula S, Verghese D, Henry TD, Katz JN, Nicholson WJ, Jaber WA, Jentzer JC. Contemporary Management of Concomitant Cardiac arrest and cardiogenic shock complicating myocardial infarction. Mayo Clin Proc. 2022;97(12):2333–54.
Aissaoui N, Puymirat E, Tabone X, Charbonnier B, Schiele F, Lefèvre T, Durand E, Blanchard D, Simon T, Cambou JP, et al. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST-MI french nationwide registries. Eur Heart J. 2012;33(20):2535–43.
Thiele H, Akin I, Sandri M, de Waha-Thiele S, Meyer-Saraei R, Fuernau G, Eitel I, Nordbeck P, Geisler T, Landmesser U, et al. One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 2018;379(18):1699–710.
Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, et al. PCI strategies in patients with Acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377(25):2419–32.
Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to revise the 1999 guidelines for the management of patients with Acute myocardial infarction). Circulation. 2004;110(5):588–636.
Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the management of ST-Segment Elevation Acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2008;29(23):2909–45.
Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31(20):2501–55.
Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–96.
Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, de Waha A, Richardt G, Hennersdorf M, Empen K, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382(9905):1638–45.
Thiele H, Zeymer U, Thelemann N, Neumann FJ, Hausleiter J, Abdel-Wahab M, Meyer-Saraei R, Fuernau G, Eitel I, Hambrecht R, et al. Intraaortic balloon pump in cardiogenic shock complicating Acute myocardial infarction: long-term 6-Year outcome of the Randomized IABP-SHOCK II Trial. Circulation. 2019;139(3):395–403.
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. : 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165.
Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):197–215.
Prondzinsky R, Lemm H, Swyter M, Wegener N, Unverzagt S, Carter JM, Russ M, Schlitt A, Buerke U, Christoph A, et al. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38(1):152–60.
Johnson A et al. “MIMIC-IV” (version 2.2). PhysioNet (2023), https://doi.org/10.13026/6mm1-ek67.
Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, Pollard TJ, Hao S, Moody B, Gow B, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. 2023;10(1):1.
Ohman EM, Nanas J, Stomel RJ, Leesar MA, Nielsen DW, O’Dea D, Rogers FJ, Harber D, Hudson MP, Fraulo E, et al. Thrombolysis and counterpulsation to improve survival in myocardial infarction complicated by hypotension and suspected cardiogenic shock or heart failure: results of the TACTICS trial. J Thromb Thrombolysis. 2005;19(1):33–9.
Perera D, Stables R, Thomas M, Booth J, Pitt M, Blackman D, de Belder A, Redwood S. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trial. JAMA. 2010;304(8):867–74.
Patel MR, Smalling RW, Thiele H, Barnhart HX, Zhou Y, Chandra P, Chew D, Cohen M, French J, Perera D, et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA. 2011;306(12):1329–37.
Nan Tie E, Dinh D, Chan W, Clark DJ, Ajani AE, Brennan A, Dagan M, Cohen N, Oqueli E, Freeman M, et al. Trends in Intra-Aortic Balloon Pump Use in cardiogenic shock after the SHOCK-II Trial. Am J Cardiol. 2023;191:125–32.
Patterson T, Perera D, Redwood SR. Intra-aortic balloon pump for high-risk percutaneous coronary intervention. Circ Cardiovasc Interv. 2014;7(5):712–20.
Unverzagt S, Buerke M, de Waha A, Haerting J, Pietzner D, Seyfarth M, Thiele H, Werdan K, Zeymer U, Prondzinsky R. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev. 2015;2015(3). Cd007398.
Prondzinsky R, Unverzagt S, Russ M, Lemm H, Swyter M, Wegener N, Buerke U, Raaz U, Ebelt H, Schlitt A, et al. Hemodynamic effects of intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP shock trial. Shock. 2012;37(4):378–84.
Bendjelid K. IABP and cardiogenic shock: a heartbreaking story. Am Heart J. 2018;199:178–80.
Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after Acute myocardial infarction. J Am Coll Cardiol. 2017;69(3):278–87.
Karami M, Eriksen E, Ouweneel DM, Claessen BE, Vis MM, Baan J, Beijk M, Packer EJS, Sjauw KD, Engstrom A, et al. Long-term 5-year outcome of the randomized IMPRESS in severe shock trial: percutaneous mechanical circulatory support vs. intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2021;10(9):1009–15.
Bochaton T, Huot L, Elbaz M, Delmas C, Aissaoui N, Farhat F, Mewton N, Bonnefoy E. Mechanical circulatory support with the Impella® LP5.0 pump and an intra-aortic balloon pump for cardiogenic shock in acute myocardial infarction: the IMPELLA-STIC randomized study. Arch Cardiovasc Dis. 2020;113(4):237–43.
Pfluecke C, Christoph M, Kolschmann S, Tarnowski D, Forkmann M, Jellinghaus S, Poitz DM, Wunderlich C, Strasser RH, Schoen S, et al. Intra-aortic balloon pump (IABP) counterpulsation improves cerebral perfusion in patients with decreased left ventricular function. Perfusion. 2014;29(6):511–6.
Ma P, Zhang Z, Song T, Yang Y, Meng G, Zhao J, Wang C, Gu K, Peng J, Jiang B, et al. Combining ECMO with IABP for the treatment of critically ill adult heart failure patients. Heart Lung Circ. 2014;23(4):363–8.
Zeng P, Yang C, Chen J, Fan Z, Cai W, Huang Y, Xiang Z, Yang J, Zhang J, Yang J. Comparison of the efficacy of ECMO with or without IABP in patients with cardiogenic shock: a Meta-analysis. Front Cardiovasc Med. 2022;9:917610.
Sloth E, Sprogøe P, Lindskov C, Hørlyck A, Solvig J, Jakobsen C. Intra-aortic balloon pumping increases renal blood flow in patients with low left ventricular ejection fraction. Perfusion. 2008;23(4):223–6.
Ferguson JJ 3rd, Cohen M, Freedman RJ Jr, Stone GW, Miller MF, Joseph DL, Ohman EM. The current practice of intra-aortic balloon counterpulsation: results from the Benchmark Registry. J Am Coll Cardiol. 2001;38(5):1456–62.
Acknowledgements
The authors are grateful to the original study group for providing data for the current analysis.
Funding
There is no funding to report.
Author information
Authors and Affiliations
Contributions
DF and HC designed the study. DF and DY conducted data collection and data analysis. DF and DY wrote the manuscript. WM, JX, and YZ analyzed and interpreted the result. All authors reviewed this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The collection of patient information and creation of the research resource was reviewed by the Institutional Review Board at the Beth Israel Deaconess Medical Center, which granted a waiver of informed consent and approved the data-sharing initiative. The first author of this article completed the required CITI Data or Specimens Only Research training and gained the credential. The data agreement for the project had been signed. Hence, the ethical approval statement and the need for informed consent were waived for this article. All methods were carried out following relevant guidelines and regulations. All methods in our study were performed under the Declaration of Helsinki.
Consent for publication
Not applicable.
Conflict of interest
The authors report no conflicts of interest in this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fang, D., Yu, D., Xu, J. et al. Effects of intra-aortic balloon pump on in-hospital outcomes and 1-year mortality in patients with acute myocardial infarction complicated by cardiogenic shock. BMC Cardiovasc Disord 23, 425 (2023). https://doi.org/10.1186/s12872-023-03465-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03465-8