Abstract
Objective
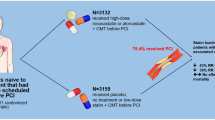
The purpose of this meta-analysis is to evaluate the role of high-intensity statin pretreatment on coronary microvascular dysfunction in patients with coronary heart disease undergoing percutaneous coronary intervention (PCI).
Methods
PubMed, Cochrane, and Embase were searched. This meta-analysis selection included randomized controlled trials (RCTs), involving high-intensity statin pretreatment as active treatment, and measurement of thrombolysis in myocardial infarction (TIMI), myocardial blush grade (MBG) or index of microvascular resistance (IMR) in coronary heart disease (CHD) patients undergoing PCI. I2 test was used to evaluate heterogeneity. Pooled effects of continuous variables were reported as Standard mean difference (SMD) and 95% confidence intervals (CI). Pooled effects of discontinuous variables were reported as risk ratios (RR) and 95% confidence intervals (CI). Random-effect or fix-effect meta-analyses were performed. The Benefit was further examined based on clinical characteristics including diagnosis and statin type by using subgroup analyses. Publication bias was examined by quantitative Egger’s test and funnel plot. We performed sensitivity analyses to examine the robustness of pooled effects.
Results
Twenty RCTs were enrolled. The data on TIMI < 3 was reported in 18 studies. Comparing with non-high-intensity statin, high-intensity statin pretreatment significantly improved TIMI after PCI (RR = 0.62, 95%CI: 0.50 to 0.78, P < 0.0001). The data on MBG < 2 was reported in 3 studies. The rate of MBG < 2 was not different between groups (RR = 1.29, 95% CI: 0.87 to 1.93, P = 0.21). The data on IMR was reported in 2 studies. High-dose statin pretreatment significantly improved IMR after PCI comparing with non-high-dose statin (SMD = -0.94, 95% CI: -1.47 to -0.42, P = 0.0004). There were no significant between-subgroup differences in subgroups based on statin type and diagnosis. Publication bias was not indicated by using quantitative Egger’s test (P = 0.97) and funnel plot. Sensitivity analyses confirmed the robustness of these findings.
Conclusions
Comparing with non-high-intensity statin, high-intensity statin pretreatment significantly improved TIMI and IMR after PCI. In the future, RCTs with high quality and large samples are needed to test these endpoints.
Similar content being viewed by others
Introduction
Percutaneous coronary intervention (PCI) has been widely used in revascularization therapy for patients with coronary heart disease, especially for acute coronary syndrome (ACS). Early removal of coronary artery stenosis or occlusion and recovery of coronary blood flow play an important role in alleviating patients' symptoms and reducing mortality, which has been unanimously recommended by national guidelines [1, 2]. However, because of Coronary microcirculation dysfunction (Coronary microvascular dysfunction, CMD), there is still a larger proportion of patients with successful opening of the narrowed or occluded epicardial coronary arteries without recovery of blood flow in the distal coronary microvessels, myocardial perfusion is not truly effective, leading to no significant relief of symptoms and increasing the risk of cardiovascular adverse events in patients with CHD [3, 4]. The pathophysiology of CMD is very complex, involving endothelial dysfunction, platelet activation, microvascular thromboembolism, and other mechanisms. How to improve the CMD after PCI treatment in patients with coronary heart disease has been paid high attention by clinical workers.
Statins are the cornerstone of drug therapy for coronary heart disease, and are competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA). It can not only inhibit the synthesis of cholesterol but also reduce the cholesterol level by increasing the low-density lipoprotein (LDL) receptor on liver cells and strengthening the endocytosis of LDL receptor-mediated [4]. In addition to the strong cholesterol-lowering effect, statins also have a variety of effects including platelet inhibition, vasodilation, inflammation inhibition, and improvement of endothelial function [5,6,7,8], which contribute to the improvement of microcirculation function [9].
At present, the effects of statin with different dose intensity on the improvement of coronary microcirculation in patients with CHD after PCI have been studied, but the results are controversial. Studies have reported that high-intensity statin before PCI can significantly improve the dysfunction of coronary microcirculation after PCI compared with low-dose statin [10]. However, other studies have shown that high-intensity statins do not improve CMD in patients with CHD after PCI compared with low-dose statins [11, 12]. The differences in research results may be related to the design factors. Therefore, the purpose of this study was to perform a meta- analysis based on the collection of relevant RCTs to evaluate whether high-intensity statins are more effective in improving coronary microcirculation in patients with CHD after PCI than the low-dose intensive statins.
Methods
The meta-analysis was conducted with conforming to Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) guidelines. (Registration in PROSPERO: CRD42020184732) (Additional file 1: Table S1).
Search strategy
A comprehensive search of PubMed, Cochrane, and Embase databases was conducted for all published papers comparing the effects of high-intensity and non-high-intensity statin pretreatment statin on microcirculatory function in patients with CHD after PCI, without restriction of language. The retrieval time is up to March, 2020. Retrieval keywords include Statin, Percutaneous intervention, and Randomized Controlled trial. The literature types were all RCT. This study also carried out manual retrieval of the references of relevant papers.
Inclusion and exclusion criteria
Inclusion criteria
Population: patients with CHD, including ACS and stable angina.
Treatment measures of high-intensity statin group: High-intensity statins were given before PCI. As we know, high-intensity statins were defined as atorvastatin 40-80 mg/day, Rosuvastatin 20-40 mg/day, and simvastatin 80 mg/day [13].
Treatment measures of non-high-intensity statin group: Low dose, no statins or placebo were administered before PCI.
The endpoints: Related indexes of coronary microcirculation after PCI such as Thrombolysis in myocardial infarction(TIMI)、Myocardial blush grade(MBG) and index of microvascular resistance(IMR) [14].
The type of study: Peer-reviewed RCT studies.
Exclusion criteria
(1)Non-RCTs, (2)head-to-head comparisons of different statins, (3)studies without PCI as a part of the protocol, (4)studies with revascularization as an exclusion criterion, (5)unavailable data on CMD, and (6)duplicate articles were excluded.
Study selection and data extraction
The literature was screened separately by two authors. In case of any disagreement, discuss with the third author to reach a consensus on inclusion. According to the inclusion and exclusion criteria, the two authors initially selected the relevant articles by reading the titles and abstracts respectively. After reading the full text of the preliminarily selected articles and clarifying the included articles, the basic data of each study and relevant endpoints of the study were collected, including the first author's last name, research published time, countries, the number of population, diagnosis, the patient's age, sex ratio, statin type, and dosage, studies the endpoints, etc.
Quality assessment
The two authors used the Cochrane Collaboration Risk Assessment tool to evaluate the quality of the included studies [15], and if there were differences in the evaluation process, they discussed with the third author to reach consensus. Specific quality assessment items include the following kinds of bias risk assessment: risk of selective bias, risk of implementation bias, risk of measurement bias, risk of follow-up bias, risk of reporting bias, and other bias risks. For each biased item, low risk, high risk, and ambiguity were used for evaluation.
Statistical analyses
The meta-analysis used STATA 15.1 (STATA Corp. College Station, TX, USA) and Revman 5.3 (Nordic Cochrane Centre, Denmark) for statistical analysis. I2 test was used to evaluate heterogeneity, when I2 ≤ 50%, it indicated good homogeneity among various tests, and fixed effect model was used for analysis. If I2 > 50% indicates heterogeneity among trials, random-effects model was used for analysis. Pooled effects of continuous variables were reported as Standard mean difference (SMD) and 95% CI. Pooled effects of discontinuous variables were reported as risk ratios (RR) and 95%CI. Statistical significance was accepted at p < 0.05. Publication bias was examined by quantitative Egger’s test and funnel plot when the number of included studies reaches 10 or more [16]. Benefit was further examined based on clinical characteristics including diagnosis and statin type by using subgroup analyses. Statistically significant subgroup effect was accepted at p < 0.01 [17]. Finally, we performed sensitivity analyses to examine the robustness of pooled effects.
Results
Literature screening results
A total of 1126 related articles were retrieved from PubMed, Cochrane and Embase databases, and 28 duplicate articles were excluded. The remaining 1098 articles were first read by title and abstract, and then the full text of potentially related articles was read to determine whether the inclusion criteria were fit, and finally, 20 RCTS were included [10,11,12, 18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. The specific inclusion process is shown in Fig. 1.
Characteristics and quality assessment of included studies
A total of 3165 patients were included in the study, including 1535 in the high-intensity statin group and 1630 in the control group. Eighteen studies reported postoperative TIMI, three reported postoperative MBG, and two reported postoperative IMR. In terms of population inclusion, 11 studies were included in the diagnosis of ST-elevation myocardial infarction (STEMI), 2 studies were included in the diagnosis of stable coronary artery disease (SCAD), 2 studies were included in the diagnosis of unstable angina (UA), only 1 study was included in the diagnosis of coronary artery disease (CAD), 1 study was included in the diagnosis of non-ST-elevated myocardial infarction (NSTEMI), 2 studies were included in the diagnosis of non-ST-elevation acute coronary syndrome(NSTE-ACS), only 1 study was included in the diagnosis of ACS. And in terms of statins, there were 17 studies using atorvastatin, 2 studies using rosuvastatin, and only 1 study using simvastatin. The study characteristics and patient characteristics of the 20 studies are shown in Tables 1 and 2.
The risk of bias was assessed in 20 studies. In terms of selection bias, randomization was described in detail and correctly in 14 studies. 2 studies describe the correct method of allocating concealment. As for the blind method, patients and evaluators were blinded in 5 studies, only evaluators were blinded in 5 studies, and patients and evaluators were not blinded in 4 studies. In terms of follow-up bias, 5 studies described the number of missing persons and the causes, which were similar between groups. With regard to reporting bias, seven studies had published research proposals. There was no other risk bias was mentioned in the 20 studies (Figs. 2 and 3).
Results of the included studies
TIMI grading
Eighteen studies reported postoperative TIMI < 3. Heterogeneity analysis suggested that there was no significant heterogeneity among randomized controlled trials (I2 = 16%, P = 0.26). The results of fixed-effect model analysis showed that 7.0% of patients (101/1453) in the high-intensity statin treatment group had postoperative TIMI c. In the control group, patients with postoperative TIMI < 3 accounted for 12.0% (186/1551). The results showed that high-intensity statin pretreatment significantly improved postoperative TIMI grading (RR = 0.62, 95%CI: 0.50 to 0.78, P < 0.0001) (Fig. 4).
MBG grading
Three studies reported postoperative MBG < 2. Heterogeneity analysis suggested that there was no significant heterogeneity among randomized controlled trials (I2 = 25%, P = 0.26). The results of fixed-effect model analysis showed that 25.1% of patients (42/167) in the high-intensity statin group received postoperative MBG < 2, and 19.5% (34/174) in the control group received postoperative MBG < 2. There was no significant effect on the two groups(RR = 1.29, 95% CI: 0.87 to 1.93, P = 0.21) (Fig. 5).
IMR
Two studies reported results of IMR immediately after surgery. Heterogeneity analysis suggested significant heterogeneity among randomized controlled trials (I2 = 61%, P = 0.11). The results of fixed-effect model analysis showed that the high-intensity statin group significantly reduced postoperative IMR compared with the control group (SMD = -0.94, 95% CI: -1.47 to -0.42, P = 0.0004) (Fig. 6).
Subgroup analysis
As mentioned above, subgroup difference test was conducted according to different statin types, and statistical results as shown in Fig. 7 showed that there was no subgroup effect between different statin types (P = 0.66). It showed that different statin types did not affect the intervention. According to different clinical manifestations for subgroup difference test, the statistical results (P = 0.27) showed that there was no subgroup effect among patients with different clinical manifestations, indicating that there was no significant difference in intervention effects among different clinical manifestations (Fig. 8).
Publication bias detection
Similarly, funnel plot and Egger check method are used in this study to detect publication bias. As shown in Fig. 9, funnel plot is basically symmetric. In addition, the Egger check results (P = 0.97) also indicate that there was no obvious publication bias.
Sensitivity analysis
As shown in Figs. 10, 11 and 12, in the sensitivity analysis of TIMI, MBG, and IMR, none of the trials excluded from the meta-analysis significantly changed the results, indicating the robustness of the results (Figs. 10, 11 and 12).
Discussion
A total of 20 randomized controlled trials were included in this study. TIMI grading was reported in 18 RCTs, MBG was reported in 3 RCTs, and IMR was reported in 2 RCTs. Firstly, the meta-analysis showed that compared with non-high-intensity statins, high-intensity statins significantly improved TIMI grading and significantly reduced IMR after PCI in patients with CHD. There was no significant difference in postoperative MBG between the two groups. Secondly, subgroup differences were detected for TIMI grading according to clinical diagnosis and statin types in the study, and the results showed that there were no subgroup differences in benefits between different clinical diagnoses and different statin types. Thirdly, sensitivity analysis was conducted in this study, the results showed no significant changes in the meta-analysis results, indicating the robustness of the results. Fourthly, this study detected potential publication bias through funnel plot and quantitative Egger inspection method, and the results showed that there was no publication bias.
Microcirculation is not only an important determinant of effective restoration of myocardial perfusion after PCI to reduce myocardial cell damage and improve cardiac function in patients with CHD, but also an important factor affecting the prognosis of patients [34].
Statins are competitive inhibitors of HMG-CoA reductase [4]. High-intensity statins significantly reduce LDL_C levels, further reducing the risk of ischemic events in patients with CHD. Based on this, national guidelines suggest that patients with CHD should be initiated early with high-intensity statins [1, 2, 35]. In addition to the strong cholesterol-lowering effect, statins also have a variety of effects including platelet inhibition, vasodilation, inflammation inhibition, and improvement of endothelial function [5,6,7,8], which contribute to the improvement of microcirculation function [9].
Due to technical reasons, it is still not possible to directly observe the blood flow of coronary microvessels in the human body. Therefore, most of the previous clinical studies on statin's improvement of CMD mainly used TIMI grading to indirectly reflect the situation of coronary microcirculation perfusion. A meta-analysis of 15 randomized controlled trials showed that high-intensity statins significantly improved post-operative TIMI grading (OR = 0.61, 95% CI: 0.46–0.80, p = 0.0005) [36]. A total of 20 RCTS were included in this meta-analysis, 18 of which reported TIMI results. The meta- analysis showed that compared with the control group, TIMI grading was significantly higher in the high-intensity statins group after PCI than in the control group (RR = 0.62, 95%CI: 0.50 to 0.78, P < 0.0001). This is consistent with previous findings [36], suggesting that high-dose and high-intensity statins are beneficial for improving microcirculatory perfusion. However, TIMI grading is related to the operator's operation and observation experience, which cannot accurately reflect the microcirculation function. Therefore, indicators such as MBG and IMR were included in this study for comprehensive evaluation, which is of more reference value for us to evaluate the effect of high-intensity statin on microcirculation. IMR is a simple and specific index to reflect the function of coronary microcirculation proposed by Fearon and other scholars in recent years. It has a good correlation with the actual microvascular resistance and can be measured by the guide wire with pressure/thermal sensor. It also has the advantages of good repeatability and is not affected by factors such as heart rate and blood pressure. IMR has been shown to have good prognostic value in both ACS and stable angina patients, and is considered to be a reliable indicator for the evaluation of coronary microvascular function [14]. The meta-analysis showed that although MBG results were similar in the two groups after PCI, high-intensity statins significantly reduced IMR(SMD = -0.94, 95% CI: -1.47 to -0.42, P < 0.0004). It also indicates that high-intensity statin can better improve the CMD in patients with CHD after PCI compared with low-dose statin.
Current guidelines recommend that patients with CHD should be treated early with high-intensity statins. However, it is not clear when statins will be given. The results showed that compared with non-high-intensity statin treatment, high-intensity statin treatment effectively improved microcirculation dysfunction after PCI, suggesting that high-intensity statin pretreatment can bring additional benefits to patients, and we should actively give high-intensity statin treatment as early as possible before operation in the absence of contraindications.
The meta-analysis has several advantages. First of all, the study focused on comparing the effects of high-intensity statin with non-high-intensity statin treatment on microcirculation function in patients with CHD after PCI. Different from previous studies, TIMI, MBG, and IMR were included in this meta-analysis to evaluate the effects of high-intensity statin pretreatment on coronary microcirculation. Secondly, only RCTs were included in this meta-analysis to minimize the influence of confounding factors on the results and improve the reliability of the results. Finally, subgroup analysis, sensitivity analysis, and publication bias analysis were performed on the results of this study, which all indicated the robustness of the results.
However, the study also has limitations. First, the characteristics of the included population in each study may be different, which may lead to the existence of bias and heterogeneity. Although the subgroup difference analysis of clinical diagnosis and statin type in this study showed consistency of results, while the study could not exclude the influence of other factors on the results, including the age, complications, ethnic groups, different statin dosage before PCI in non-high-intensity group, and so on. Second, some of the experiments included in the study did not use the blind method, and some of the studies did not mention whether to use the blind method in the literature, which may have an impact on the research results. Third, the number of RCTs on IMR and MBG is small. Therefore, higher-quality studies will be needed to verify the results through more reliable and rigorous methods in the future.
Conclusions
Compared with non-high-intensity statin treatment, high-intensity statin significantly improved TIMI grading and IMR in patients with CHD after PCI, and effectively improved microcirculation dysfunction. More studies with larger samples and higher quality are still needed to verify the results In the future.
Availability of data and materials
All data and material used for this study are available through the corresponding author upon reasonable request.
References
Members WC, Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines[J]. J Am Coll Cardiol. 2022;79(2):e21–e129.
Chinese Society of Cardiology of Chinese Medical Association, Editorial Board of Chinese Journal of Cardiology. 2019 Chinese Society of Cardiology (CSC) guidelines for the diagnosis and management of patients with ST-segment elevation myocardial infarction. Zhonghua xin xue guan bing za zhi. 2019;47(10):66.
Arnold SV, Jang JS, Tang F, et al. Prediction of residual angina after percutaneous coronary intervention. Eur Heart J Qual Care Clin Outcomes. 2015;1(1):23–30.
Konijnenberg LSF, Damman P, Duncker DJ, et al. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc Res. 2020;116(4):787–805.
LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282(24):2340–6.
Sanguigni V, Pignatelli P, Lenti L, et al. Short-term treatment with atorvastatin reduces platelet CD40 ligand and thrombin generation in hypercholesterolemic patients. Circulation. 2005;111(4):412–9.
Schönbeck U, Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation. 2004;109(21_suppl_1):II-18–II−26.
Ray KK, Cannon CP. The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J Am Coll Cardiol. 2005;46(8):1425–33.
Wassmann S, Faul A, Hennen B, et al. Rapid effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition on coronary endothelial function. Circ Res. 2003;93(9):e98–103.
He GX, Tan W. High-dose atorvastatin pretreatment could diminishes microvascular impairment in patients undergoing elective percutaneous coronary intervention. J Geriatr Cardiol. 2013;10(4):355.
Hahn JY, Kim HJ, Choi YJ, et al. Effects of atorvastatin pretreatment on infarct size in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. 2011;162(6):1026–33.
Ko YG, Won H, Shin DH, et al. Efficacy of early intensive rosuvastatin therapy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention (ROSEMARY study). Am J Cardiol. 2014;114(1):29–35.
Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165.
Fearon WF, Kobayashi Y. Invasive assessment of the coronary microvasculature: the index of microcirculatory resistance. Circ Cardiovasc Interv. 2017;10(12):e005361.
Dondo Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Lau J, Ioannidis JPA, Terrin N, et al. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600.
Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Glob Health. 2019;7(2):192–8.
Post S, Post MC, van den Branden BJ, et al. Early statin treatment prior to primary PCI for acute myocardial infarction: REPERATOR, a randomized placebo-controlled pilot trial. Catheter Cardiovasc Interv. 2012;80(5):756–65.
Shimomura M, Oyama J, Takeuchi M, et al. Acute effects of statin on reduction of angiopoietin-like 2 and glyceraldehyde-derived advanced glycation end-products levels in patients with acute myocardial infarction: a message from SAMIT (Statin for Acute Myocardial Infarction Trial). Heart Vessels. 2016;31(10):1583–9.
Liu H, Yang Y, Yang S, et al. Administration of a loading dose of atorvastatin before percutaneous coronary intervention prevents inflammation and reduces myocardial injury in STEMI patients: a randomized clinical study. Clin Ther. 2013;35(3):261–72.
Liu W, Zou Z, Jiang H, et al. Clinical effect of preoperative high-dose atorvastatin against no-reflow after PCI. Exp Ther Med. 2017;13(1):97–102.
Lee BK, Koo BK, Nam CW, et al. Does pre-treatment with high dose atorvastatin prevent microvascular dysfunction after percutaneous coronary intervention in patients with acute coronary syndrome? Korean Circ J. 2016;46(4):472–80.
Su Q, Li L, Liu Y, et al. Effect of intensive atorvastatin therapy on periprocedural PDCD4 expression in CD4+ T lymphocytes of patients with unstable angina undergoing percutaneous coronary intervention. Cardiology. 2014;127(3):169–75.
Kim EK, Hahn JY, Song YB, et al. Effects of high-dose atorvastatin pretreatment in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: a cardiac magnetic resonance study. J Korean Med Sci. 2015;30(4):435–41.
Kim JS, Kim J, Choi D, et al. Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: the STATIN STEMI trial. JACC: Cardiovasc Interv. 2010;3(3):332–9.
Liu Z, Joerg H, Hao H, et al. Efficacy of high-intensity atorvastatin for Asian patients undergoing percutaneous coronary intervention. Ann Pharmacother. 2016;50(9):725–33.
Yang J, Liu C, Zhang L, et al. Intensive atorvastatin therapy attenuates the inflammatory responses in monocytes of patients with unstable angina undergoing percutaneous coronary intervention via peroxisome proliferator-activated receptor γ activation. Inflammation. 2015;38(4):1415–23.
Jia X, Fu X, Zhang J, et al. Intensive cholesterol lowering with statin improves the outcomes of percutaneous coronary intervention in patients with acute coronary syndrome. Chin Med J. 2009;122(6):659–64.
Shehata M, Fayez G, Nassar A. Intensive statin therapy in NSTE-ACS patients undergoing PCI: clinical and biochemical effects. Tex Heart Inst J. 2015;42(6):528–36.
Shehata M, Samir A, Dardiri M. Prognostic impact of intensive statin therapy on N-terminal pro-BNP level in non-ST-segment elevation acute myocardial infarction patients. J Interv Cardiol. 2017;30(6):514–21.
Chen M, Li H, Wang Y. Protection by atorvastatin pretreatment in patients undergoing primary percutaneous coronary intervention is associated with the lower levels of oxygen free radicals. J Cardiovasc Pharmacol. 2013;62(3):320–4.
García-Méndez RC, Almeida-Gutierrez E, Serrano-Cuevas L, et al. Reduction of no reflow with a loading dose of atorvastatin before primary angioplasty in patients with acute ST Myocardial Infarction. Arch Med Res. 2018;49(8):620–9.
Takano H, Ohba T, Yamamoto E, et al. Usefulness of rosuvastatin to prevent periprocedural myocardial injury in patients undergoing elective coronary intervention. Am J Cardiol. 2013;111(12):1688–93.
Heusch G. Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res Cardiol. 2019;114(6):45.
Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407.
Xiao Y, He S, Zhang Z, et al. Effect of High-Dose Statin Pretreatment for Myocardial Perfusion in Patients Receiving Percutaneous Coronary Intervention (PCI): A Meta-Analysis of 15 Randomized Studies. Med Sci Monit. 2018;24:9166.
Acknowledgements
We acknowledge the study participants and all those who contributed to this work.
Funding
No funding was received for this paper.
Author information
Authors and Affiliations
Contributions
Bihan Huang and Xueying Han are the co-first authors of this paper in no particular order. They have made equally important contributions to the design, statistical analysis, and writing of this paper. Yun Pan and Dongdong Chen performed the literature search, data extraction, and quality assessment. Dongdong Chen revised the manuscript critically.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. PRSIMA 2020 Checklist
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, B., Han, X., Pan, Y. et al. A systematic review and meta-analysis of the effect of high-intensity statin on coronary microvascular dysfunction. BMC Cardiovasc Disord 23, 370 (2023). https://doi.org/10.1186/s12872-023-03402-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03402-9