Abstract
Background
The aim of this study was to perform a retrospective analysis of patients with acute anterior wall ST-segment elevation myocardial infarction (AAW-STEMI) whose left anterior descending (LAD) artery was completely occluded and reperfused by primary percutaneous coronary intervention (PPCI) and to determine the influencing factors and prognostic value of left ventricular systolic dysfunction (LVSD) in the acute phase of acute myocardial infarction (AMI).
Methods
A total of 304 patients with AAW-STEMI were selected. The selected patients were divided into two groups: the preserved left ventricular ejection fraction (pLVEF) group (LVEF ≥ 50%, n = 185) and the reduced left ventricular ejection fraction (rLVEF) group (LVEF < 50%, n = 119). The influencing factors of LVSD and their predictive value for LVSD were analyzed. Patients were followed up by examining outpatient records and via telephone. The predictive value of LVSD for the cardiovascular mortality of patients with AAW-STEMI was analyzed.
Results
Age, heart rate (HR) at admission, number of ST-segment elevation leads (STELs), peak creatine kinase (CK) and symptom to wire-crossing (STW) time were independent risk factors for LVSD (P < 0.05). The receiver operating characteristic (ROC) analysis showed that the peak CK had the strongest predictive value for LVSD, with an area under the curve (AUC) of 0.742 (CI, 0.687 to 0.797) as the outcome. At a median follow-up of 47 months (interquartile range, 27 to 64 months), the Kaplan‒Meier survival curves up to 6-year follow-up revealed a total of 8 patients succumbed to cardiovascular disease, with 7 (6.54%) in the rLVEF group and 1 (0.56%) in the pLVEF group, respectively (hazard ratio: 12.11, [P = 0.02]). Univariate and multivariate Cox proportional hazards regression analysis demonstrated that rLVEF was an independent risk predictor of cardiovascular death in patients with AAW-STEMI discharged after PPCI (P < 0.01).
Conclusions
Age, HR at admission, number of STELs, peak CK, and STW time may be used to identify patients with a high risk of heart failure (HF) in a timely manner and initiate early standard therapy for incident LVSD in the acute phase of AAW-STEMI reperfused by PPCI. A trend toward increased cardiovascular mortality at follow-up was significantly linked to LVSD.
Similar content being viewed by others
Introduction
New-onset heart dysfunction is a common complication of acute myocardial infarction (AMI). Acute anterior wall ST-segment elevation myocardial infarction (AAW-STEMI) leads to more pronounced left ventricular systolic dysfunction (LVSD) and more adverse left ventricular (LV) remodeling than STEMI in other areas [1]. Guidelines have been presented to support that the standard treatment for patients with STEMI is primary percutaneous coronary intervention (PPCI), modern antithrombotic therapy and secondary prevention [2, 3]. Despite rapid advances in pharmacological and interventional treatment strategies, STEMI remains the leading cause of heart failure (HF) and mortality. Evidence has shown that the incidence of HF in patients with STEMI after PPCI is 4.6% at 1 month, 4.7% at 1 year, and 5.1% at 2 years [4].
LVSD can be represented by left ventricular ejection fraction (LVEF), and it is defined as LVEF < 50% [5]. Reduced LVEF (rLVEF) is well known to be associated with high AMI mortality, and it is associated with increased cardiovascular risk and a higher rate of unplanned cardiac rehospitalizations in the long term [6, 7]. Improvement of LVEF after revascularization among STEMI patients is associated with improved long-term survival. Clinical practice guidelines have provided several therapeutic recommendations for both the acute and long-term post-discharge management of AMI based on LVEF, such as angiotensin converting-enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs) and aldosterone antagonists [3]. The ongoing PARADISE-AMI trial is evaluating the safety and efficacy of angiotensin receptor II blocker-neprilysin inhibitors (ARNIs) compared with ramipril in addition to standard post myocardial infarction (MI) pharmacotherapy [8]. To further improve the outcome of AAW-STEMI patients, it is necessary to identify earlier and easily assessable predictors of poor clinical outcome. This study aimed to investigate patients whose left anterior descending (LAD) artery was completely occluded and reperfused by PPCI to determine the influencing factors of LVSD in the acute phase of AAW-STEMI and to analyze the clinical prognostic value of LVSD.
Methods
Study population
Adopting a retrospective cohort research method, a total of 547 patients with AAW-STEMI reperfused by PPCI were selected in the Tianjin First Central Hospital from January 2016 to September 2021. All selected patients fulfilled the diagnostic criteria of AAW-STEMI in the “2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation “ [9]. PPCI was successfully performed to open the occluded LAD artery in a standard fashion by 2 experienced cardiologists. Thrombus aspiration, post balloon dilation, and glycoprotein IIb/IIIa receptor antagonists were given at the discretion of the physician performing the intervention. All patients were given standard drug therapy during the perioperative period of PCI. The baseline characteristics were recorded.
The inclusion criteria were as follows. (1) The symptom to admission time was within 12 h. (2) The cardiac function classification at admission was Killip I. (3) Emergency angiography confirmed complete occlusion of the LAD artery without other coronary occlusions. (4) The postprocedural thrombolysis in myocardial infarction (TIMI) grade was TIMI grade 3 flow.
The exclusion criteria were as follows. (1) Coronary angiography showing incomplete occlusion of the LAD artery. (2) Patients whose cardiac function was Killip grade 2 or above upon admission. (3) Coronary angiography showing occlusion of the left main artery. (4) The postprocedural TIMI grade was less than TIMI grade 3 flow. (5) History of old myocardial infarction or chronic heart failure. (6) Complicated with liver failure (CLIF Consortium Acute on Chronic Liver Failure score > 64) or renal failure (estimated glomerular filtration rate < 30 mL/min/1.73m2). (7) Patients who died during hospitalization. (8) Abandon treatment and discharge automatically.
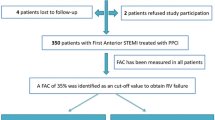
LVEF assessment by transthoracic echocardiography was preferably estimated using the Simpson biplane formula in PHLIP’S IE 33 equipment at 3–5 days after onset of STEMI. Finally, there were 304 patients meeting inclusion and exclusion criteria. They were divided into preserved LVEF (pLVEF) group (LVEF ≥ 50%) and rLVEF group (LVEF < 50%), see Fig. 1. Patients survived to hospital discharge were followed by telephone call or clinical visit for up to 6 years. The primary endpoint was cardiovascular death. Cardiovascular death was defined as the primary cause of death determined to be atherosclerotic cardiovascular disease, arrhythmia, heart failure, or sudden cardiovascular death.
Statistical analysis
Continuous variables with a normal distribution are reported as the mean ± standard deviation (\(\bar x \pm s\)). Differences between two groups were analyzed by Student’s t test. Nonnormally distributed data are presented as the median and interquartile range (IQR). Differences between two groups were analyzed by the Mann‒Whitney U test. Categorical variables are reported as frequencies and percentages, and they were analyzed using the chi square test. The univariate logistic regression analysis was used to evaluate demographic, clinical, and biochemical variables with potential meaningful factors. After screening out variables with significant differences, multivariate logistic regression analysis was performed to test the independent influencing factors for LVSD. And the forward stepwise regression method was used to carry out multi-factor analysis. The receiver operating characteristic (ROC) curve was used to evaluate the predictive value of related risk factors for LVSD. The survival rate was determined via the Kaplan‒Meier test. The survival rate between the two groups was compared with the use of the log-rank statistic. The univariate Cox proportional hazards regression analysis was used to evaluate the variables with potential predictive value. The significantly different variables in univariate Cox regression analysis were included in multivariate Cox regression analysis. The Wald regression method was used to screen the predictors of cardiovascular death. A P value of < 0.05 was considered significant.
Results
The mean age of the selected patients was 58.63 ± 11.81 years old (range 27–84 years), and 254 (83.55%) were male. Differences in the baseline and clinical outcomes between the pLVEF group and the rLVEF group are summarized in Table 1. There were statistically significant differences in age, prior ischemic stroke, STW time, HR at admission, ST-segment elevation leads (STELs) and their number, high-density lipoprotein (HDL) cholesterol levels, peak CK, N-terminal pro brain natriuretic peptide (NT-proBNP), proportion of LAD artery opening or proximal occlusion, occlusion site of LAD artery, prescription rates of Glycoprotein IIb/IIIa receptor antagonists and beta-blocker, and global registry of acute coronary events risk (GRACE) score between the two groups (P < 0.05).
On multivariate analysis, LVSD was used as the dependent variable, and the significantly different indicators on univariate analysis in Table 1 were used as the independent variables. The result showed that age, HR at admission, number of STELs, STW time and peak CK were independent risk factors for LVSD, see Table 2. The simple linear regressions between LVEF and the independent variables are presented in Fig. 2. The ROC analysis showed that the peak CK had the strongest predictive value for LVSD, and the AUC value was 0.742 (CI, 0.687 to 0.797). At a cutoff value of 29.11 U/cL, peak CK exhibited 80.67% diagnostic sensitivity and 57.84% specificity. The AUC values of other related independent variables were less than 0.70, see Fig. 3.
In subgroup analysis, patients with HR ≥ 80 bpm at admission were more likely to develop LVSD than patients with HR < 70 bpm (P < 0.05). Peak CK was positively correlated with the number of STELs. Opening or proximal occlusion of LAD artery generated a significant increase in CK than middle or distal occlusion (P < 0.05). Peak CK was higher in younger AAW-STEMI patients whose age were less than 50 years [41.61(25.08,58.28) vs. 30.66(20.34,47.20) U/cL, P < 0.01]. The STW time was approximately 30 min longer in the ≥ 60 years group than in the < 60 years group (P < 0.05). The average first medical contact to wire-crossing (FTW) time was 77.11 min, the fastest time was 19 min, the longest time was 237 min, the proportion of patients with less than 90 min was 75.24%, and 37.79% achieved the currently advocated FTW of < 60 min.
There were 15 patients lost to follow up, the rate was 4.93%. Except for 2 patients with non-cardiovascular death, a total of 8 patients dead of cardiovascular disease after a median follow-up of 47 months (interquartile range: 27 to 64 months): 7 (6.54%) patients in the rLVEF group and 1 (0.56%) patient in the pLVEF group, respectively (hazard ratio: 12.11; 95% confidence interval: 1.49 to 98.70 [P = 0.02]). The total number of exposed persons was 821, and the cardiovascular mortality rate was 0.97 per 100 person-years. Univariate Cox regression analysis was performed for each suspected confounding factor. Prior ischemic stroke, GRACE score and rLVEF were adverse predictors for cardiovascular death in patients with AAW-STEMI reperfused by PPCI (P < 0.05). These three indicators from univariate analysis were included in multivariate Cox regression analysis, demonstrating that rLVEF was an independent predictor of cardiovascular death, see Table 3. The Kaplan‒Meier survival curves up to 6-year follow-up are shown in Fig. 4.
Discussion
LVSD is often the consequence of an irreversible loss of viable mass following STEMI, occasionally in combination with loss of contractility in ischemic, but still viable myocardium (hibernating myocardium) [10]. LVEF is one of the most general indicators of LVSD and is widely available in clinical settings. Evaluation of LVEF after STEMI treated with PPCI is well established as an important predictor of clinical outcome [11]. By using the Kaplan‒Meier method, we found significant differences in the survival rate between the rLVEF group and pLVEF group. A lower baseline LVEF and larger infarct size have been associated with failure of LV recovery [12]. Timely identification of subjects at risk for HF development using a multimodality approach and early initiation of guideline-directed HF therapy in these patients can decrease the HF burden [13]. Previous studies had confirmed that age, delayed presentation, previous history of myocardial infarction, atrial fibrillation, hyperlipidaemia, renal impairment, anterior localization of AMI and proximal lesion location were the risk factors of LVSD in STEMI patients [14,15,16,17,18]. And some markers, such as peak CK, B-type natriuretic peptide, fasting blood glucose, stress marker total thiol groups, and fibrosis marker HE-4 had been confirmed to be associated with rLVEF after STEMI [19,20,21]. In this study, we found that age, HR at admission, number of STELs, peak CK, and STW time may be used to identify the high risk of HF timely for patients with anterior wall STEMI whose LAD artery was completely occluded and reperfused by PPCI. Moreover, these risk factors are common clinical variables which will make the clinical application of the research results more widespread. We will then briefly discuss each of these risk factors, emphasizing the importance of identifying them and initiating early standard therapy.
Age
The number of individuals who develop HF after AMI undergoing PCI increases with age [22]. In an observational study of elderly patients with first MI, 75% of survivors developed HF in the subsequent 5 years [23]. In young patients, only nearly one-third presented with LV dysfunction post-MI, and LVEF recovery occurred in more than 40% [24]. The following reasons can explain this phenomenon. The mechanical contractility of the heart, function of the conduction system, heart reserve capacity and tolerance to ischemia or hypoxia are reduced in elderly individuals. In addition, the severity and extent of coronary artery disease increase with age. Patients with multivessel coronary artery disease have diminished coronary flow reserve and more severe myocardial damage after AMI. For older patients, we need to pay more attention to the seriousness of STEMI.
Heart rate at admission
Accelerated HR is an important external manifestation of sympathetic nerve excitement, which is one of the primary internal mechanisms for the occurrence and development of HF after AMI. In the early stage of AMI, increased HR further promotes myocardial oxygen consumption and shortens myocardial perfusion time, which can directly cause the expansion of the infarction size. As the stroke volume decreases and coronary perfusion decreases, HR increases in an attempt to regain cardiac output and maintain sufficient perfusion of the heart [25]. The formation of a vicious cycle leads to a rapid deterioration of heart function. A study showed that prehospital HR ≥ 100 bpm in STEMI was independently associated with larger infarct size, reduced LVEF and an increased risk of all-cause mortality and HF [26]. Therefore, HR at admission serves as an easily obtainable and powerful tool to identify STEMI patients at high risk.
Number of ST-segment elevation leads
Electrocardiography has several advantages including simplicity, non-invasive and low cost. It not only has the ability to estimate the location and range of a myocardial ischemic area, but also can be used to predict possible adverse events and complications, playing a central role in the diagnosis of STEMI in an emergency setting. Blood is supplied by LAD artery to the left anterior wall, the anterior lateral wall, and the front two-thirds of the ventricular septum. When LAD artery is blocked, those area will be damaged or even necrosis, and the corresponding anterior wall leads will show typical “damage type” changes, namely ST segment elevation. Anterior myocardial infarctions lead to more pronounced LVSD and more adverse LV remodeling compared with myocardial infarction in other areas [27].When occlusion occurs near the proximal of LAD artery, in addition to lesions at the base of left ventricle, the anterior wall, left lateral wall and ventricular septum will also be involved, causing elevation of the ST-segment of anterior (V1 ~ V4) and lateral (I, aVL, and V5 ~ V6) [28, 29]. When the occlusion site is at distal segment of LAD artery, the ST segment elevation is more obvious in V4-V6 leads, but not in V1-V2 or aVL leads [30]. Similarly, the greater the number of ST elevation leads that appear, the wider the myocardial injury and infarct range will be. The number of leads with ST-segment elevation was associated with LV remolding occurrence [31]. For patients especially with extensive anterior wall combined with high sidewall ST-segment elevation, we need to be aware of the risk of their illness.
Peak creatine kinase
CK has certain value for the early diagnosis and severity of AMI patients. CK mainly exists in skeletal muscle and myocardium, and also exists in brain tissue. It plays an important role in catalyzing cell energy conversion and regulating cell electrophysiological activities. When MI occurs, myocardial cells rupture and necrosis, and then CK will be released into the blood. CK will increase within 6 h, and reach peak at about 24 h, and then return to normal, generally lasting for 3–4 days. A large infarct size is associated with progressive LV remodeling. Patients with higher peak CK are more likely to experience left ventricular remodeling after STEMI and present more often with a lower LVEF [32, 33]. Patients with AAW-STEMI are at increased risk for higher CK [34]. Peak CK was higher in younger patients (< 50 years) in this study. This may be due to decreased protein synthesis and lower levels of CK in the aged cardiomyocytes of elderly individuals [35]. In addition, because of the lack of ischemic preconditioning and less collateral circulation, the tolerance of cardiomyocytes to acute ischemia was reduced in younger patients. Monitoring peak CK is necessary for all patients with STEMI.
Symptoms to wire-crossing time
Larger infarct size is associated with greater LV dysfunction, adverse cardiac remodeling, and HF over time. Shortening the STW time is very important to reduce the risk of HF in patients, especially those with AAW-STEMI [36]. STW time includes the time from onset of symptoms to first medical contact (STF) and FTW time. Older patients over 60 years had longer STW time than those under 60 years. It is considered that it may be the cognitive and socioeconomic factors of older patients contributing further to the delay. The proportion of FTW time achieved the currently advocated FTW of < 60 min is lower relatively. Several reasons could explain the prolonged FTW time, including the complicated treatment process in the emergency department for STEMI patients during the COVID-19 pandemic, the extremely low ratio of patients admitted directly to the catheterization laboratory, the occupancy of the catheter room and the hesitation of the patient’s family on the PCI operation. STW time is an independent negative influencing factor of LVEF in patients with anterior wall STEMI in the acute phase. It is still necessary to strengthen the publicity of AMI knowledge among the public to emphasize the importance of timely consultation for patients with chest pain. All efforts should be extended to shorten the STW time by educating the public to activate emergency medical services early to bypass the emergency department and allow timely PPCI for the best outcome [36].
Recommendations for early treatment and follow-up
STEMI is associated with cardiomyocyte loss, fibrosis, and cardiac remodeling, which together represent the main pathophysiological mechanisms that comprise the clinical picture of HF. Timely reperfusion therapy can save the ischemic myocardium and reduce the loss of viable mass. Medication following reperfusion therapy is also critical to improving prognosis. In this study, early application of ACEIs/ARBs or ARNIs and β-blockers failed to improve the LVEF level in the acute phase. However, it has been confirmed that the early application of ACEIs/ARBs or ARNIs and β-blockers can inhibit myocardial remodeling and improve long-term prognosis [37]. Improvement in LVEF commonly begins within 3 days in patients who are revascularized with myocardial stunning and improved function of viable myocardium as the mechanisms, and the main improvement in LVEF occurs within 1 month [38, 39]. The first month following primary reperfusion is a critical period during which the greatest degree of cardiac remodeling occurs [40]. Early prediction of LVSD and timely application of recombinant human brain natriuretic peptide, levosimendan, aldosterone antagonist or sodium-glucose cotransporter type 2 inhibitors may reduce the occurrence of HF after AMI [41,42,43,44]. LVSD occurred in more than one-third of patients with AAW-STEMI reperfused by PPCI in our study. Closer follow-up is recommended for patients with LVSD to benefit from early optimization of HF treatment, thereby reducing or delaying cardiogenic death.
Limitations
Several limitations should be acknowledged. First, the present study was a single-center, retrospective, observational study, and unexpected and unmeasured confounders may have affected the outcomes. Second, we studied patients whose cardiac function was Killip grade 1 because we observed that this population accounted for the majority of patients who presented within 12 hours of the onset of AAW-STEMI. In contrast to the conventional treatment for Killip class 1 cardiac function, treatment for HF was initiated for patients with Killip grade 2 or above upon admission. And we selected patients who had improved after hospitalization and were discharged from the hospital, excluding those who died during hospitalization. Therefore, this patient subgroup was not entirely representative of the overall cohort. Third, in multivariable Cox regression analysis, there were only 8 events of cardiovascular death. Although the number of events was small, the difference in cardiovascular mortality between pLVEF group and rLVEF group was extremely significant. Fourth, LVEF calculated by transthoracic echocardiography was a crude estimation of the true performance. Ideally, cardiac MRI would better define the extent of cardiac damage. Fifth, due to the lack of dynamic measurements of LVEF, we did not analyze and discuss the effect of changes in LVEF on prognosis during follow-up. Sixth, the rate of prescribing optimal drug therapy after STEMI was lower than expected in our cohort. Last, the sample size of the present study might be relatively small, and the proportion of very elderly patients in this study was lower.
Conclusions
Our findings suggested that age, HR at admission, number of STELs, peak CK, and STW time were independent risk factors for LVSD in the acute phase in patients with AAW-STEMI whose LAD artery was completely occluded and underwent PPCI. HR at admission and STW time are factors that we can intervene in. All these predictors may be used to identify timely the risk of LVSD and initiate early standard therapy in these patients to decrease the occurrence of HF as much as possible. Incident LVSD in the acute phase after AAW-STEMI was significantly associated with long-term adverse outcomes. For patients with LVSD, closer follow-up is recommended to help these patients benefit from early optimization.
Data Availability
The data will become available immediately following publication and there is no end date. The datasets used in the study are available from the corresponding author on reasonable request.
Abbreviations
- AAW-STEMI:
-
Acute anterior wall ST-segment elevation myocardial infarction
- ACEIs:
-
Angiotensin converting-enzyme inhibitors
- AMI:
-
Acute myocardial infarction
- ARBs:
-
Angiotensin receptor blockers
- ARNIs:
-
Angiotensin receptor II blocker-neprilysin inhibitors
- AUC:
-
Area under the curve
- CK:
-
Creatine kinase
- FTW:
-
First medical contact to wire-crossing
- GRACE:
-
Global registry of acute coronary events risk
- HDL:
-
High-density lipoprotein
- HF:
-
Heart failure
- HR:
-
Heart rate
- IQR:
-
Interquartile range
- LAD:
-
Left anterior descending
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
- LVSD:
-
Left ventricular systolic dysfunction
- MI:
-
Myocardial infarction
- NT-proBNP:
-
N-terminal pro brain natriuretic peptide
- pLVEF:
-
Preserved left ventricular ejection fraction
- PPCI:
-
Primary percutaneous coronary intervention
- rLVEF:
-
Reduced left ventricular ejection fraction
- ROC:
-
Receiver operating characteristic
- STELs:
-
ST-segment elevation leads
- STF:
-
Symptoms to first medical contact
- STW:
-
Symptom to wire-crossing
- TIMI:
-
Thrombolysis in myocardial infarction
References
Masci PG, Ganame J, Francone M, et al. Relationship between location and size of myocardial infarction and their reciprocal influences on post-infarction left ventricular remodelling. Eur Heart J. 2011;32(13):1640–8.
Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-Elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary intervention and the 2013 ACCF/AHA Guideline for the management of ST-Elevation myocardial infarction. J Am Coll Cardiol. 2016;67(10):1235–50.
Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77.
Kelly DJ, Gershlick T, Witzenbichler B, et al. Incidence and predictors of heart failure following percutaneous coronary intervention in ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. Am Heart J. 2011;162(4):663–70.
Akhtar MM, Lorenzini M, Pavlou M, et al. Association of Left ventricular systolic dysfunction among carriers of truncating variants in Filamin C with frequent ventricular arrhythmia and end-stage heart failure. JAMA Cardiol. 2021;6(8):891–901.
Spitzer E, Frei M, Zaugg S, et al. Rehospitalizations following primary percutaneous coronary intervention in patients with ST-Elevation myocardial infarction: results from a Multi-Center Randomized Trial. J Am Heart Assoc. 2017;6(8):1–10.
Syyli N, Hautamaki M, Antila K, et al. Left ventricular ejection fraction adds value over the GRACE score in prediction of 6-month mortality after ACS: the MADDEC study. Open Heart. 2019;6(1):e001007.
Jering KS, Claggett B, Pfeffer MA, et al. Prospective ARNI vs. ACE inhibitor trial to DetermIne Superiority in reducing heart failure events after myocardial infarction (PARADISE-MI): design and baseline characteristics. Eur J Heart Fail. 2021;23(6):1040–8.
Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev Esp Cardiol (Engl Ed). 2021;74(6):544.
Almeida AG, Carpenter JP, Cameli M, et al. Multimodality imaging of myocardial viability: an expert consensus document from the European Association of Cardiovascular Imaging (EACVI). Eur Heart J Cardiovasc Imaging. 2021;22(8):e97–e125.
Bulluck H, Zheng H, Chan MY, et al. Independent predictors of Cardiac Mortality and hospitalization for heart failure in a multi-ethnic Asian ST-segment Elevation Myocardial Infarction Population treated by primary percutaneous coronary intervention. Sci Rep. 2019;9(1):10072.
Ndrepepa G, Mehilli J, Martinoff S, et al. Evolution of left ventricular ejection fraction and its relationship to infarct size after acute myocardial infarction. J Am Coll Cardiol. 2007;50(2):149–56.
Jenca D, Melenovsky V, Stehlik J, et al. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail. 2021;8(1):222–37.
Hendrickson MJ, Arora S, Vaduganathan M, et al. Prevalence and prognostic implications of reduced left ventricular ejection fraction among patients with STEMI in India. ESC Heart Fail. 2022;9(6):3836–45.
Rajic D, Jeremic I, Stankovic S, et al. Oxidative stress markers predict early left ventricular systolic dysfunction after acute myocardial infarction treated with primary percutaneous coronary intervention. Adv Clin Exp Med. 2018;27(2):185–91.
Khaled S, Shalaby G. Severe left ventricular dysfunction earlier after Acute myocardial infarction treated with primary percutaneous coronary intervention: predictors and In-Hospital Outcome- A Middle Eastern Tertiary Center Experience. J Saudi Heart Assoc. 2022;34(4):257–63.
Noaman S, Goh CY, Vogrin S, et al. Comparison of short-term clinical outcomes of proximal versus nonproximal lesion location in patients treated with primary percutaneous coronary intervention for ST-elevation myocardial infarction: the PROXIMITI study. Catheter Cardiovasc Interv. 2019;93(1):32–40.
Reinstadler SJ, Stiermaier T, Eitel C, et al. Impact of Atrial Fibrillation during ST-Segment-Elevation myocardial infarction on infarct characteristics and prognosis. Circ Cardiovasc Imaging. 2018;11(2):e006955.
de Abreu M, Mariani J, Guridi C, et al. Association between biochemical markers and left ventricular dysfunction in the ST-elevation acute myocardial infarction]. Arch Cardiol Mex. 2014;84(4):243–9.
Wang H, Zhang Y, Shen Z, et al. Prognostic value of fasting glucose on the risk of heart failure and left ventricular systolic dysfunction in non-diabetic patients with ST-segment elevation myocardial infarction. Front Med. 2021;15(1):70–8.
Kilci H, Altinbilek E, Cetinkal G, et al. Relation of a novel fibrosis marker and post-myocardial infarction left ventricular ejection fraction in revascularized patients. Biomark Med. 2021;15(17):1651–8.
Kim DW, Her SH, Park HW, et al. Incremental age-related one-year MACCE after acute myocardial infarction in the drug-eluting stent era (from KAMIR-NIH registry). J Geriatr Cardiol. 2018;15(9):574–84.
Torabi A, Rigby AS, Cleland JG. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;55(1):79–81.
Wu WY, Biery DW, Singh A, et al. Recovery of left ventricular systolic function and clinical outcomes in young adults with myocardial infarction. J Am Coll Cardiol. 2020;75(22):2804–15.
Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117(5):686–97.
Nepper-Christensen L, Lonborg J, Ahtarovski KA. Importance of elevated heart rate in the very early phase of ST-segment elevation myocardial infarction: results from the DANAMI-3 trial. Eur Heart J Acute Cardiovasc Care. 2019;8(4):318–28.
Kim DH, Park CB, Jin ES, et al. Predictors of decreased left ventricular function subsequent to follow-up echocardiography after percutaneous coronary intervention following acute ST-elevation myocardial infarction. Exp Ther Med. 2018;15(5):4089–96.
Taglieri N, Saia F, Alessi L, et al. Diagnostic performance of standard electrocardiogram for prediction of infarct related artery and site of coronary occlusion in unselected STEMI patients undergoing primary percutaneous coronary intervention. Eur Heart J Acute Cardiovasc Care. 2014;3(4):326–39.
Eskola MJ, Nikus KC, Holmvang L, et al. Value of the 12-lead electrocardiogram to define the level of obstruction in acute anterior wall myocardial infarction: correlation to coronary angiography and clinical outcome in the DANAMI-2 trial. Int J Cardiol. 2009;131(3):378–83.
Engelen DJ, Gorgels AP, Cheriex EC, et al. Value of the electrocardiogram in localizing the occlusion site in the left anterior descending coronary artery in acute anterior myocardial infarction. J Am Coll Cardiol. 1999;34(2):389–95.
Kasprzak M, Fabiszak T, Kozinski M, et al. Diagnostic performance of selected baseline Electrocardiographic Parameters for Prediction of Left ventricular remodeling in patients with ST-Segment Elevation myocardial infarction. J Clin Med. 2021;10(11):1–15.
Lund GK, Stork A, Muellerleile K, et al. Prediction of left ventricular remodeling and analysis of infarct resorption in patients with reperfused myocardial infarcts by using contrast-enhanced MR imaging. Radiology. 2007;245(1):95–102.
Garcia G, de la Chao JM, Mirebeau-Prunier D, et al. Metabolomic Approach in STEMI-Patients undergoing left ventricular remodeling. Int J Mol Sci. 2019;20(2):1–11.
Nienhuis MB, Ottervanger JP, de Boer MJ, et al. Prognostic importance of creatine kinase and creatine kinase-MB after primary percutaneous coronary intervention for ST-elevation myocardial infarction. Am Heart J. 2008;155(4):673–9.
Ruiz-Meana M, Bou-Teen D, Ferdinandy P, et al. Cardiomyocyte ageing and cardioprotection: consensus document from the ESC working groups cell biology of the heart and myocardial function. Cardiovasc Res. 2020;116(11):1835–49.
Meisel SR, Kleiner-Shochat M, Abu-Fanne R, et al. Direct admission of patients with ST-Segment-Elevation myocardial infarction to the catheterization Laboratory Shortens Pain-to-balloon and door-to-balloon time intervals but only the Pain-to-balloon interval impacts short- and long-term mortality. J Am Heart Assoc. 2021;10(1):e018343.
Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the management of Heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the heart failure society of America. J Card Fail. 2017;23(8):628–51.
Solomon SD, Glynn RJ, Greaves S, et al. Recovery of ventricular function after myocardial infarction in the reperfusion era: the healing and early afterload reducing therapy study. Ann Intern Med. 2001;134(6):451–8.
Sjoblom J, Muhrbeck J, Witt N, et al. Evolution of left ventricular ejection fraction after acute myocardial infarction: implications for implantable cardioverter-defibrillator eligibility. Circulation. 2014;130(9):743–8.
Daubert MA, White JA, Al-Khalidi HR, et al. Cardiac remodeling after large ST-elevation myocardial infarction in the current therapeutic era. Am Heart J. 2020;223:87–97.
Sangaralingham SJ, Burnett JC, McKie PM, et al. Rationale and design of a randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy of B-type natriuretic peptide for the preservation of left ventricular function after anterior myocardial infarction. J Card Fail. 2013;19(8):533–9.
Wu X, Wu J, Yan X, et al. Enhancement of myocardial function and reduction of injury with levosimendan after percutaneous coronary intervention for acute myocardial infarction: a pilot study. Cardiology. 2014;128(2):202–8.
Dahal K, Hendrani A, Sharma SP, et al. Aldosterone Antagonist Therapy and Mortality in Patients with ST-Segment Elevation Myocardial Infarction without Heart failure: a systematic review and Meta-analysis. JAMA Intern Med. 2018;178(7):913–20.
Tripolt NJ, Kolesnik E, Pferschy PN, et al. Impact of EMpagliflozin on cardiac function and biomarkers of heart failure in patients with acute MYocardial infarction-the EMMY trial. Am Heart J. 2020;221:39–47.
Acknowledgements
Not applicable.
Funding
This work was supported in part by Science and Technology Project of Tianjin Municipal Health Commission (ZC20059), and Tianjin Key Medical Discipline (specially) Construction Project (TJYXZDXK-054B). The funding bodies played on role in design of the study, collection, analysis, and interpretation of the data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
JYL, LCZ, GSZ, CBW, YXY, HQ, XW and XT were involved in investigation and data collection. JYL and LCZ drafted and corrected the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of Tianjin First Central Hospital (2020N216KY). Our study was a retrospective and observational study, and we got the informed consent from all participants who were followed up.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest associated with this research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jing, Y., Lu, C., Guo, S. et al. Influencing factors and prognostic value of left ventricular systolic dysfunction in patients with complete occlusion of the left anterior descending artery reperfused by primary percutaneous coronary intervention. BMC Cardiovasc Disord 23, 344 (2023). https://doi.org/10.1186/s12872-023-03341-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03341-5