Abstract
Background
The study aimed to assess the correlation between the monitoring frequency of PT-INR and the long-term prognosis in patients with mechanical heart valve (MHV) replacement after discharge.
Methods
This single-center, observational study enrolled patients who underwent MHV replacement and discharged from June 2015 to May 2018. Patients or their corresponding family members were followed with a telephone questionnaire survey in July-October 2020. Based on monitoring intervals, patients were divided into frequent monitoring (FM) group (≤ 1 month) and less frequent monitoring (LFM) group (> 1 month). The primary endpoint was the composite of thromboembolic event, major bleeding or all-cause death. The secondary endpoints were thromboembolic event, major bleeding or all-cause death, respectively.
Results
A total of 188 patients were included in the final analysis. The median follow-up duration was 3.6 years (Interquartile range: 2.6 to 4.4 years). 104 (55.3%) patients and 84 (44.7%) patients were classified into the FM group and the LFM group, respectively. The FM group had a significantly lower incidence of the primary endpoint than the LFM group (3.74 vs. 1.16 per 100 patient-years, adjusted HR: 3.31 [95% CI 1.05–10.42, P = 0.041]). Secondary analysis revealed that the risk of thromboembolic events and all-cause death were also reduced in the FM group.
Conclusions
The management of warfarin treatment in patients after MHV replacement remains challenging. Patients with less frequent monitoring of PT-INR might have worse clinical prognosis than those with frequent PT-INR monitoring.
Similar content being viewed by others
Background
Valvular heart disease (VHD) can lead to heart failure, stroke and even death if left untreated [1, 2]. VHD is one of the most common heart diseases in China, with a weighted prevalence of 3.8% [3]. Rheumatic infection is the main cause of VHD in China [3, 4]. Although the incidence of rheumatic VHD decreases gradually, the number of patients with VHD remains large due to the large and aging population in China.
Prosthetic valve replacement is an important and reliable treatment for patients with VHD [5, 6]. Bioprosthetic heart valves (BHVs) are subject to structural valve deterioration, especially in young adult patients, while mechanical heart valves (MHVs) are more durable and can last lifelong. Therefore, MHVs are widely used in patients younger than 60 years [7, 8]. However, patients should require lifelong anticoagulation with vitamin K antagonists after MHV replacement due to the high risk of thromboembolism caused by MHV [9, 10]. Warfarin is the most commonly prescribed vitamin K antagonist in China. It has several significant disadvantages including its narrow therapeutic window, potential drug-drug and drug-food interactions, unpredictable dose-response relationship, and the requirement of regular blood monitoring [11, 12]. Improper use of warfarin may cause thromboembolic or hemorrhagic complications, which are frequently fatal or result in permanent disability. Considering patients receiving MHV replacement are relatively young, these complications are most likely catastrophic to patients and increase the financial and emotional burden of their families and society. Therefore, ensuring the quality of warfarin therapy is crucial. To find an optimal balance between efficacy and safety, continuous blood monitoring is required to ensure the value of prothrombin time-the international normalized ratio (PT-INR) is within the therapeutic range [13]. In addition, the time in therapeutic range (TTR) is often calculated to assess the quality of anticoagulation therapy [14].
However, little is known about the quality of warfarin therapy after MHV replacement in China. Herein, we proposed the monitoring frequency of PT-INR as an index of patient compliance and hypothesized that the monitoring frequency of PT-INR was correlated with the long-term prognosis in patients after MHV replacement.
Methods
Study design
This single-center, observational study was approved by the medical ethics committee of the First Affiliated Hospital of Nanjing Medical University and conducted according to the Declaration of Helsinki and institutional guidelines.
Study population
We included patients who underwent MHV replacement and discharged from our institution between June 2015 and May 2018. The patients or their family members who could not be contacted via phone or declined to answer telephone questionnaires were excluded from this study. A retrospective chart review was performed for all patients. Demographic data, clinical information before the MHV replacement, and procedure-related details were collected.
Questionnaire
Between July 2020 and October 2020, a telephone follow-up questionnaire survey to patients or their family members was designed and conducted to evaluate the clinical outcomes after MHV replacement. The questionnaire contained 10 questions about the frequency of PT-INR monitoring and the occurrence of all-cause death, thromboembolic events and major bleeding (Additional File). It took 10 min or less to complete the telephone interview for all subjects. If the patients or their family members could not be reached with the initial telephone call, at least 3 additional attempts would be made. All calls were made by a single study coordinator (S.M.). During each phone call, S.M. explained the purpose of this questionnaire survey. Verbal informed consent was obtained from all patients or their family members. In order to minimize potential bias in the administration of the questionnaire and the responses from the patients or their family members, the telephone interview was based on a pre-written script.
Outcomes
The primary endpoint was the composite of thromboembolic event, major bleeding or all-cause death. The secondary endpoints were thromboembolic event, major bleeding or all-cause death, respectively.
Definitions
According to the criteria of the International Society on Thrombosis and Hemostasis (ISTH), major bleeding was defined as bleeding leading to a decrease in the hemoglobin level of at least 2 g/dl, transfusion of at least 2 units of packed red cells, occurring at a critical site, or resulting in death [15]. Based on the frequency of PT-INR monitoring, patients were classified into 2 major groups: frequent monitoring (FM) group with a monitoring interval ≤ 1 month, and less frequent monitoring (LFM) group with a monitoring interval > 1 month.
Statistical analysis
All of the continuous variables investigated in our study were normally distributed and presented as mean with standard deviations. Differences between groups were tested using the Student’s t test. The categorical variables were expressed as counts with percentages and compared using the Chi-Squared test or Fisher’s exact test, as appropriate. A multivariable Cox proportional hazards model was conducted from the clinical outcome measure by frequent/less frequent INR monitoring. The variables including age and gender were adjusted. Hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) were used to estimate the effect size. A P value < 0.05 was considered significantly different. The software package SPSS version 26.0 (IBM, Cooperation, New York, NY, USA) was used for statistical analysis.
Results
The flow of this study is shown in Fig. 1. Between June 2015 to May 2018, 230 patients who underwent MHV replacement were discharged from our institution, of whom 188 were analyzed. Overall, 42 patients were lost of follow-up and excluded from this study due to the following reasons: 23 patients had no telephone number recorded in their electronic health records; 16 patients could not be reached; 3 patients declined to answer the telephone questionnaire. As Fig. 2 shows, the proportion of lost to follow-up patients was 20.6%, 27.3% and 6.3%, respectively based on the year of MHV replacement from 2015 to 2018, with a significant decreasing trend (P < 0.01).
Flow of the study.
CONSORT flow diagram. Between June 2015 to May 2018, 230 patients who underwent MHV replacement were discharged from our institution, of whom 188 were finally analyzed. Overall, 42 patients lost of follow-up were excluded from this study due to the following reasons: 23 patients had no telephone number found in electronic health records; 16 patients could not be reached via phone; 3 patients declined to answer telephone questionnaires
Baseline characteristics
Of the 188 patients enrolled in this study, the mean age was 47.7 ± 7.9 years, and 94 (50%) patients were men. Regarding the surgical procedure, 71 patients (37.8%) underwent mechanical mitral valve replacement, 58 patients (30.8%) underwent mechanical aortic valve replacement, and 58 patients (30.8%) underwent both mechanical mitral and aortic valve replacement. The baseline characteristics of the 188 study subjects are shown in Table 1. Based on the PT-INR monitoring intervals, 104 patients were classified into the FM group, and another 84 patients were classified into the LFM group. Patients in the LFM group were less likely to have diabetes (5.8% vs. 0%, P = 0.03), and they had a larger left ventricular diastolic diameter (57.0 ± 11.0 mm vs. 53.7 ± 9.4 mm, P = 0.03) and smaller left ventricular ejection fraction (59.0 ± 7.55% vs. 61.6 ± 6.5%, P = 0.01). Patients who were lost to follow-up were more likely to have rheumatic heart disease (69% vs. 46%, P = 0.01) and less likely to have hypertension (5% vs. 22%, P = 0.01) than those with follow-up. No other significant differences in baseline characteristics between the patients with follow-up and patients who were lost to follow-up were found (Additional File Table 1).
Outcomes
The median follow-up duration of all 188 patients was 3.6 years (IQR: 2.6 to 4.4 years). All these patients acknowledged that they must take warfarin lifelong. Figure 3 shows that the proportion of frequent PT-INR monitoring was 38.0%, 43.8% and 77.0%, respectively based on the year of MHV replacement from 2015 to 2018, with a significant increasing trend (P < 0.01). A total of 104 (55.3%) patients were classified into the FM group, and 84 (44.7%) patients were classified into the LFM group.
In the FM group, all-cause death occurred in 1 patient, ischemic stroke in 0 patients, and major bleeding in 4 patients. In the LFM group, all-cause death occurred in 7 patients, ischemic stroke in 6 patients, and major bleeding in 3 patients. The main cause of death in the LFM group was major bleeding, mainly intracranial hemorrhage, and recurrent heart failure. The only death in the FM group was a complicated case: a 32-year-old male who had a history of kidney transplantation and received regular hemodialysis due to the deterioration of renal function. He underwent aortic valve replacement after diagnosed with infectious endocarditis. After the MHV replacement, he lived on with hemodialysis until death caused by intracranial hemorrhage. Characteristics of patients with ischemic stroke, major bleeding, or all-cause death occurred during follow-up are summarized in Additional File Table 1.
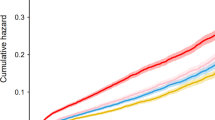
The incidences of primary and secondary clinical outcome in the LFM and FM group were shown in Table 2. Based on multivariable Cox analysis, the LFM group remained to have a significantly higher incidence of the primary endpoint event (3.74 vs. 1.16 per 100 patient-years, HR: 3.31 [95% CI 1.05–10.42, P = 0.041]) and all-cause death (2.18 vs. 0.29 per 100 patient-years, HR: 8.33 [95% CI 1.01–66.67, P = 0.049]) after adjustment of age and sex. Figure 4 shows the Kaplan-Meier curves of primary outcome event free survival in the LFM and FM group.
Discussion
This telephone-based questionnaire survey demonstrated that the frequency of PT-INR monitoring was clinically unsatisfactory, with only 55.3% of the patients having a monitoring interval of less than 1 month. In addition, patients with less frequent monitoring of PT-INR had a poorer prognosis than those with frequent monitoring.
Patients with MHV replacement are recommended to have their PT-INR values monitored at least once a month [16, 17]. Therefore, we defined frequent monitoring as having a monitoring interval ≤ 1 month. Based on our study, less frequent monitoring of PT-INR was significantly associated with increased risk of the primary composite endpoints and all-cause deaths independent of age and gender. The risk of ischemic stroke was also significantly increased when PT-INR was less frequently monitored. There was no significant difference in the incidence of major bleeding between the FM and LFM groups. However, the death rate due to major bleeding was as high as 50% in our study. Therefore, frequent PT-INR monitoring and appropriate warfarin dose adjustment are crucial for better clinical prognosis in patients after MHV replacement. In some complicated cases, we propose that the PT-INR monitoring interval ≤ 1 month may not be frequent enough. Notably, nearly 75% of the patients in our study had at least one patient-related risk factor (mitral or tricuspid valve replacement, previous thromboembolism, atrial fibrillation, mitral stenosis of any degree, or left ventricular ejection fraction < 35%) listed in the 2021 ESC/EACTS guidelines [18]. We speculate that frequent monitoring of PT-INR not only ensures timely adjustment of warfarin dose but also optimizes other medical treatments and improves the general conditions of the patients.
In our study, the overall proportion of patients with frequent monitoring was 55.3%, and it decreased significantly as postoperative time was prolonged. This reflected that patient compliance was becoming worse over time. In patients with MHV replacement, medical compliance has a particularly large impact on their outcomes after being discharged [19, 20]. The reasons for patients having a less frequent monitoring of PT-INR might be the following: (1) Low spatial accessibility of health services: many patients live in remote regions away from hospitals, which makes it inconvenient for them to have PT-INR tests; (2) Insufficient patient awareness: patients may not truly understand the importance of frequent monitoring of PT-INR and dose adjustment of warfarin, or their level of attention might decline over time. In recent years, advances in technology have made it possible for patients to reach their doctors through smartphone social media apps or telemedicine platforms. However, in the past, patients heavily relied on telephone to contact their doctors. Developing a closer relationship with their doctors may positively impact patients’ compliance. Telemonitoring has been widely used in clinical practices for follow-up [21, 22]. Internet-based management could be an alternative for patients in remote regions. Zhu et al. reported that internet-based warfarin management was superior to the conventional method, as it could reduce anticoagulation complications in patients who received long-term warfarin treatment after MHV replacement [23]. To improve the quality of warfarin therapy, we proposed the following suggestions: (1) Intensify patient education: to emphasize the importance of frequent monitoring of PT-INR and appropriate warfarin dose adjustment; (2) Encourage the use of home-based self-monitoring of PT-INR and internet-based management of warfarin dose adjustment, especially for patients living in remote regions (3) Improve the capacity of long-term postoperative management for patients with MHV replacement by primary healthcare providers.
In our study, most of the baseline characteristics were balanced between patients with follow-up and patients who were lost to follow-up. Therefore, the impact of lost to follow-up on the conclusions might not be significant. However, the high rate of lost to follow-up in patients of 2015–2017 does reflect the shortcomings of the current follow-up system, which requires the joint efforts of both doctors and patients to improve it.
To the best of our knowledge, this is the first study to investigate warfarin monitoring frequency and its correlation with clinical outcomes after MHV replacement in Mainland China. However, there are some limitations to the study: (1) This study was a telephone-based questionnaire survey, so it was not feasible to obtain all the PT-INR values, which made it impossible to compare the difference in PT-INR levels between the 2 groups. Theoretically, frequent testing can ensure timely adjustment of warfarin dose to achieve a higher level of TTR and a better clinical outcome. (2) The sample size of this study is small. Whether the conclusion can be generalized to a larger sample size needs to be further verified. Prospective cohort studies or even randomized controlled studies that allow detailed recording of each PT-INR detection during the study are more convincing. In addition, we were unable to obtain statistically significant results regarding the relationship between anticoagulation intensity and embolic risk in patients with different locations of replaced valves due to limited statistical power. (3) Although we attempted every available method to reach out to the patients, the rate of lost to follow-up was unfortunately high. It indicates the unfavorable real-world conditions in our center. Clearly, this serves as a wake-up call for us to enhance our efforts in monitoring patients post-discharge to reduce the rate of lost to follow-up. (4) It remains uncertain that the use of one month as a criterion to determine whether the PT-INR is monitored frequently is ready for widespread implementation, as its feasibility may be affected by factors such as the level of economic development, proximity to hospitals, and other relevant considerations. (5) In addition, the effect of CYP2C9 and VKORC1 gene polymorphisms on the metabolism of warfarin should also be considered in future studies [24, 25].
Conclusion
The management of warfarin treatment in patients after MHV replacement remains challenging. Less frequent monitoring of PT-INR might be associated with worse clinical prognosis compared with frequent PT-INR monitoring. Large-scale efforts are urgently needed to reduce warfarin related adverse consequences in patients after MHV replacement in China.
Data Availability
The data generated and analyzed during the current study available from the Prof. Mingfang Li on reasonable request.
Abbreviations
- BHV:
-
Bioprosthetic heart valve
- FM:
-
Frequent monitoring
- LFM:
-
Less frequent monitoring
- MHV:
-
Mechanical heart valve
- PT-INR:
-
Prothrombin time-the international normalized ratio
- TTR:
-
Time in therapeutic range
- VHD:
-
Valvular heart disease
References
Gill H, Chehab O, Allen C, Patterson T, Redwood S, Rajani R, et al. The advantages, pitfalls and limitations of guideline-directed medical therapy in patients with valvular heart disease. Eur J Heart Fail. 2021;23(8):1325–33.
DesJardin JT, Chikwe J, Hahn RT, Hung JW, Delling FN. Sex differences and similarities in Valvular Heart Disease. Circ Res. 2022;130(4):455–73.
Yang Y, Wang Z, Chen Z, Wang X, Zhang L, Li S, et al. Current status and etiology of valvular heart disease in China: a population–based survey. BMC Cardiovasc Disord. 2021;21(1):339.
Huang X, Dhruva SS, Yuan X, Bai X, Lu Y, Yan X, et al. Characteristics, interventions and outcomes of patients with valvular heart disease hospitalised in China: a cross-sectional study. BMJ Open. 2021;11(11):e052946.
Members WC, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):e25–197.
Iung B, Delgado V, Rosenhek R, Price S, Prendergast B, Wendler O, et al. Contemporary presentation and management of valvular heart disease: the EURObservational Research Programme Valvular Heart Disease II Survey. Circulation. 2019;140(14):1156–69.
David T. How to decide between a bioprosthetic and mechanical valve. Can J Cardiol. 2021;37(7):1121–3.
Head SJ, Celik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J. 2017;38(28):2183–91.
Menichelli D, Poli D, Antonucci E, Cammisotto V, Testa S, Pignatelli P, et al. The Italian Federation of Anticoagulation Clinics Fcsa. Comparison of anticoagulation quality between acenocoumarol and warfarin in patients with mechanical prosthetic heart valves: insights from the Nationwide PLECTRUM Study. Molecules. 2021;26(5):1425.
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the heart rhythm society in collaboration with the Society of thoracic surgeons. Circulation. 2019;140(2):e125–51.
Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, Crowther M, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165(10):1095–106.
Fareed J, Thethi I, Hoppensteadt D. Old versus new oral anticoagulants: focus on pharmacology. Annu Rev Pharmacol Toxicol. 2012;52:79–99.
Dorgalaleh A, Favaloro EJ, Bahraini M, Rad F. Standardization of Prothrombin Time/International Normalized ratio (PT/INR). Int J Lab Hematol. 2021;43(1):21–8.
Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–9.
Schulman S, Kearon C, Subcommittee on control of anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–4.
Grzymala-Lubanski B, Svensson PJ, Renlund H, Jeppsson A, Själander A. Warfarin treatment quality and prognosis in patients with mechanical heart valve prosthesis. Heart. 2017;103(3):198–203.
Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis: american college of chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e152S–84S.
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2021;60(4):727–800.
Oh EM, Lee OS, Jang BM, Park S, Cho EJ, Kim KS, et al. Effect of post-operative anticoagulation management in patients who have undergone On-X mechanical heart valve replacement surgery on post-discharge warfarin therapy. J Clin Pharm Ther. 2020;45(4):767–73.
Van Damme S, Van Deyk K, Budts W, Verhamme P, Moons P. Patient knowledge of and adherence to oral anticoagulation therapy after mechanical heart-valve replacement for congenital or acquired valve defects. Heart Lung. 2011;40(2):139–46.
Prigent A, Gentina T, Launois S, Meurice JC, Pia d’Ortho M, Philippe C, et al. Telemonitoring in continuous positive airway pressure-treated patients with obstructive sleep apnoea syndrome: an algorithm proposal. Rev Mal Respir. 2020;37(7):550–60.
Ding H, Jayasena R, Chen SH, Maiorana A, Dowling A, Layland J, et al. The Effects of telemonitoring on patient compliance with self-management recommendations and outcomes of the innovative telemonitoring enhanced care program for chronic heart failure: randomized controlled trial. J Med Internet Res. 2020;22(7):e17559.
Zhu Z, Li C, Shen J, Wu K, Li Y, Liu K, et al. New internet-based warfarin anticoagulation management approach after mechanical heart valve replacement: prospective, multicenter, randomized controlled trial. J Med Internet Res. 2021;23(8):e29529.
Amorosi CJ, Chiasson MA, McDonald MG, Wong LH, Sitko KA, Boyle G, et al. Massively parallel characterization of CYP2C9 variant enzyme activity and abundance. Am J Hum Genet. 2021;108(9):1735–51.
Mark A, Rishavy KW, Hallgren L, Wilson S, Singh KW, Runge. Kathleen L Berkner. Warfarin alters vitamin K metabolism: a surprising mechanism of VKORC1 uncoupling necessitates an additional reductase. Blood. 2018;131(25):2826–35.
Acknowledgements
The authors are grateful to the following doctors for their contribution in this study. Miss Shuyang Ma (Nanjing Medical University) as the study coordinator who made all the telephone calls to the patients. Mr. Larry Jiadong Li (Nanjing Medical University) who completed the language polishing of this manuscript.
Funding
This work was supported by National Natural and Science Foundation of China [grant numbers 82270329].
Author information
Authors and Affiliations
Contributions
Data was collected by Le Geng, Jiaxi Gu and Minghui Li. Le Geng and Mingfang Li made substantial contributions to the design of the work. Data was interpreted by Hong Liu, Haoliang Sun, and Buqing Ni. Le Geng was responsible for the first draft of the manuscript which was revised by Mingfang Li. Weidong Gu, Yongfeng Shao and Minglong Chen made substantial revisions to the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This single-center, observational study was approved by the medical ethics committee of the First Affiliated Hospital of Nanjing Medical University (Number: 2014-SR-113) and conducted according to the Declaration of Helsinki and institutional guidelines. Verbal informed consent was approved by the medical ethics committee of the First Affiliated Hospital of Nanjing Medical University and was obtained from all patients or their family members.
Consent for publication
Not applicable.
Competing interests
The authors confirm that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Geng, L., Gu, J., Li, M. et al. Frequency of prothrombin time-international normalized ratio monitoring and the long-term prognosis in patients with mechanical valve replacement. BMC Cardiovasc Disord 23, 322 (2023). https://doi.org/10.1186/s12872-023-03293-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03293-w