Abstract
Background
The risk assessment of patients with stable chest pain (SCP) to defer further cardiovascular testing is crucial, but the most appropriate risk assessment strategy remains unknown. We aimed to compare current strategies to identify low risk SCP patients.
Methods
5289 symptomatic patients who had undergone coronary artery calcium score (CACS) and coronary computed tomographic angiography scan were identified and followed. Pretest probability (PTP) of obstructive coronary artery disease (CAD) for every patient was estimated according to European Society of Cardiology (ESC)-PTP model and CACS-weighted clinical likelihood (CACS-CL) model, respectively. Based on the 2019 ESC guideline-determined risk assessment strategy (ESC strategy) and CACS-CL model-based risk assessment strategy (CACS-CL strategy), all patients were divided into low and high risk group, respectively. Area under receiver operating characteristic curve (AUC), integrated discrimination improvement (IDI) and net reclassification improvement (NRI) was used.
Results
CACS-CL model provided more robust estimation of PTP than ESC-PTP model did, with a larger AUC (0.838 versus 0.735, p < 0.0001), positive IDI (9%, p < 0.0001) and less discrepancy between observed and predicted probabilities. As a result, compared to ESC strategy which only applied CACS-CL model to patients with borderline ESC-PTP, CACS-CL strategy incorporating CACS with estimation of PTP to entire SCP patients indicated a positive NRI (19%, p < 0.0001) and a stronger association to major adverse cardiovascular events, with hazard ratios: 3.97 (95% confidence intervals: 2.75–5.72) versus 5.11 (95% confidence intervals: 3.40–7.69).
Conclusion
The additional use of CACS for all SCP patients in CACS-CL strategy improved the risk assessment of SCP patients to identify individuals at low risk.
Similar content being viewed by others
Background
In daily clinical practice, risk assessment of stable chest pain (SCP) to facilitate decision-making, such as deferral of cardiovascular testing, is important but still a challenge [1, 2]. Previous guidelines [3] recommended traditional pretest probability (PTP) models based on invasive coronary angiography (ICA), which has been demonstrated to overestimate the actual prevalence of coronary artery disease (CAD) [4,5,6,7,8]. The 2019 European Society of Cardiology (ESC) guideline [9] recommended ESC-PTP model [1], which was derived from most contemporary SCP cohorts [10, 11] and revealed robust predictive performance in external validation studies [12,13,14,15]. For patients with ESC-PTP < 5%, no further cardiovascular testing is needed and for patients with ESC-PTP > 15%, cardiovascular testing should be referred [16]. For patients with ESC-PTP between 5 and 15%, the additional analysis of other risk factors such as coronary artery calcium score (CACS) can improve the estimation of PTP [16]. However, it remains debatable whether these parameters should be limited to only patients with borderline ESC-PTP.
Recently, using data from large cohorts of symptomatic patients who underwent coronary computed tomography angiography (CCTA), a CACS-based model was developed for the estimation of PTP and the external validation and comparison conducted in the original study overwhelmingly supported the CACS-weighted clinical likelihood (CACS-CL) model [14]. According to data from the original study, low CACS-CL (< 15%) was associated with a low prevalence of obstructive CAD [14]. Although CACS-CL model has been demonstrated to provide robust prediction of obstructive CAD in patients with borderline ESC-PTP, no research has systematically compared CACS-CL model alone-based risk assessment strategy (CACS-CL strategy) and 2019 ESC guideline-determined risk assessment strategy (ESC strategy) sequentially combining two models to identify low risk patients. Moreover, unlike ESC strategy only applying CACS-based estimation of PTP to patients with borderline ESC-PTP, CACS-CL strategy may obviously increase the use of CACS.
Consequently, we aimed to validate and compare ESC-PTP and CACS-CL model in a CCTA-based SCP cohort. Moreover, we aimed to compare ESC strategy and CACS-CL strategy which both incorporated CACS with estimation of PTP and investigate whether the additional use of CACS for all SCP patients in CACS-CL strategy would be efficient in identifying low risk patients for whom further cardiovascular testing should be deferred.
Methods
Study cohort
We have previously published details of the study cohort [4, 17]. In brief, after excluded patients with acute coronary syndrome, previous CAD, insufficient image quality, missing baseline data, non-sinus rhythm, structural heart disease, heart failure and > 90 years old, 5289 consecutive patients referred to CCTA for SCP suspected of obstructive CAD were included in the final analysis in a regional cardiovascular center recognized as tertiary A level (Tianjin Chest Hospital, Tianjin, China) from December 2015 to December 2017. All patients were followed up until December 2019. This observational study complied with the Declaration of Helsinki and was approved by Tianjin Chest Hospital Ethics Committee.
Definitions of baseline characteristics
Baseline clinical data including age, sex, diabetes mellitus, hypertension, hyperlipidemia, smoking, family history of premature CAD and type of SCP were prospectively collected and defined as described previously [4, 17].
PTP models and risk assessment strategies
The PTP of obstructive CAD were estimated using ESC-PTP model (age, sex and type of SCP) [9] and CACS-CL model (age, sex, type of SCP, diabetes mellitus, hypertension, hyperlipidemia, family history and CACS) [14] as previously reported, respectively.
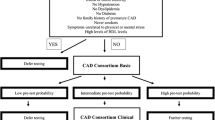
According to current guidelines, cardiovascular testing should be deferred for a low risk patient and the impact of estimation of PTP to outcome was tested by splitting patients to different risk groups according to ESC-PTP or CACS-CL model. Details of risk groups according to ESC and CACS-CL strategy were illustrated in Fig. 1 and as follows:
Flow chart. ESC European Society of Cardiology, PTP pretest probability, ESC strategy 2019 European Society of Cardiology guideline-determined risk assessment strategy, CACS-CL coronary artery calcium score-weighted clinical likelihood, CACS-CL strategy coronary artery calcium score-weighted clinical likelihood model-based risk assessment strategy, CCTA coronary computed tomographic angiography
ESC strategy: For patients with ESC-PTP between 5 and 15%, we selected CACS-CL model [14]. Thus, patients with ESC-PTP < 5% or 5% ≤ ESC-PTP ≤ 15% and CACS-CL < 15% were divided into low risk group and the other patients were classified into high risk group. CACS-CL strategy: we divided patients with CACS-CL ≥ 15% into high risk group and the other patients into low risk group.
CACS and CCTA
Procedure details of CACS and CCTA have been previously described [4, 17]. A noncontrast CACS scan was acquired to quantify CACS before every CCTA scan and CACS was determined by two blinded observers, a radiologist and a cardiologist using Agatston method [18]. The major parameters for CCTA have been previously described [4, 17, 19]: detector collimation of 2 × 128 × 0.6 mm, slice thickness of 0.6 mm, gantry rotation time of 280 ms, heart rate adaptive pitch of 0.2–0.5, tube current of 290–560 mAs/rotation and tube voltage of 80–120 kV. Three blinded observers, two radiologist and a cardiologist, evaluated the CCTA data. All segments ≥ 2 mm in diameter were analyzed and the maximal degree of coronary diameter stenosis was defined as 0%, 1–49% and ≥ 50%. Nonobstructive CAD was defined as present if a patient had at least one lesion with 1–49% diameter stenosis and no lesion with ≥ 50% diameter stenosis. Obstructive CAD was defined as present if a patient had at least one lesion with ≥ 50% diameter stenosis or any unassessable segments because of severe calcification on CCTA. All interobserver disagreements were resolved by consensus.
Follow up and endpoints
After CCTA, the local investigators made the subsequent clinical decisions for clinical management, such as further test including invasive coronary angiography ICA and clinical interventions like optimal medication treatment and coronary revascularization, based on recommendations from Coronary Artery Disease–Reporting and Data System [20], other clinical guidelines [9, 21] and the local institutional protocols [4, 17]. Contact information of all patients including telephone number, E-mail address and home address were collected before CCTA. All patients were followed up until December 2019 and follow-up information was obtained by phone call or physician interview at 6, 12, 24, 36 and 48 months after CCTA. Major adverse cardiovascular event (MACE) was defined as a composite of cardiac death and nonfatal myocardial infarction. Cardiac death was defined as any death caused by cardiac disease or for which no other cause could be found. Myocardial infarction was defined according to the Fourth Universal Definition of Myocardial Infarction [22]. The utilization of invasive procedures within 60 days after CCTA, included ICA and coronary revascularization (CR) were identified on electronic medical system. All endpoints were adjudicated via review of follow-up information and medical records by an independent clinical event committee who were blinded to other data.
Statistical analysis
All statistical analyses were performed by MedCalc (version 15.2.2) and R (version 3.2.4). Two-tailed p < 0.05 was considered statistically significant. Differences for continuous data were compared using Student’s t-test or Mann Whitney U-test as appropriate. Categorical variables were compared using χ2 test or Fisher exact test as appropriate.
We used discrimination and calibration to validate and compare ESC-PTP model and CACS-CL model [23]. Discrimination was the degree to which a model separates between positive and negative patients and manifested by the area under receiver-operator characteristic curve (AUC) and integrated discrimination improvement (IDI) [24]. Calibration was analyzed by Hosmer–Lemeshow tests which divided patients into ten groups according to deciles of PTP and calculated a chi-square statistic (H–L χ2) to determine how well model fit the actual prevalence [23].
Net reclassification improvement (NRI) was used to determine how a risk assessment strategy reclassified patients into various risk groups compared with another [24]. Kaplan–Meier curves were generated for cumulative event-free estimates survival from MACE and were compared by log-rank test. Cox proportional hazards regression models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs), which assessed association of risk groups to the time to the first MACE (or censoring). To further investigate the impact of CACS on downstream clinical management, we used the ratio for No. of additional CACS scans/No. of patients reclassified into low risk group when applying CACS-CL strategy instead of ESC strategy.
Results
Baseline characteristics grouped by CCTA result
Table 1 shows baseline characteristics of study cohort according to the presence of obstructive CAD. The cohort consisted of 5289 patients and the mean age was 56.1 years. Of these patients, 51% (2697/5289) were males and 19% (1005/5289) had obstructive CAD. Among patients with a CACS of 0, 103 had had obstructive CAD. All variables were positively associated to the presence of obstructive CAD.
Comparison of ESC-PTP and CACS-CL model
Comparison of discrimination for ESC-PTP and CACS-CL model using AUC and IDI is presented in Table 2. The AUC for CACS-CL model was significantly larger than that for ESP-PTP model (0.838 versus 0.735, p < 0.0001). Compared to ESP-PTP model, CACS-CL model demonstrated a positive IDI (9%, < 0.0001).
Figure 2 illustrated the comparison of predicted and observed probabilities of obstructive CAD by deciles of PTP. Graphically, ESC-PTP model overestimated the probability of obstructive CAD, with predicted values higher than those observed. As a result, calibration of ESC-PTP model was poor (H–L χ2 = 95.46, p < 0.0001), but CACS-CL model demonstrated less discordance (H–L χ2 = 28.74, p < 0.0001).
Model-specific PTP and observed probability by deciles of PTP. CAD coronary artery disease, ESC European Society of Cardiology, PTP pretest probability, ESC strategy 2019 European Society of Cardiology guideline-determined risk assessment strategy, CACS-CL coronary artery calcium score-weighted clinical likelihood, CACS-CL strategy coronary artery calcium score-weighted clinical likelihood model-based risk assessment strategy, CCTA coronary computed tomographic angiography
Comparison of ESC and CACS-CL strategy
According to CACS-CL strategy, 58% (3042/5289) were assigned to low risk group. Among 1479 patients with an ESC-PTP between 5 and 15%, 813 patients had a CACS-CL < 15%. Together with the 1514 patients with an ESC-PTP below 5%, ESC strategy totally classified 44% (2327/5289) into low risk group.
Table 3 shows the distribution of baseline characteristics, CAD and MACE by low and high risk group based on two strategies. In terms of both ESC and CACS-CL strategy, there were statistically significant differences for all baseline characteristics. In low and high risk group based on ESC strategy, 34% (801/2327) and 62% (1844/2962) patients had evidence of some degree of CAD on CCTA, with 7% (153/2327) and 29% (852/2962) patients having obstructive CAD, respectively. The percentages were 31% (957/3042), 75% (1688/2247), 4% (134/3042) and 39% (871/2247) for CACS-CL strategy, respectively. The distribution of CAD according to risk groups was significantly different (p < 0.0001 for two strategies, respectively). During the follow-up of 26 (interquartile range: 13 to 38) months, 211 patients experienced MACE: 24 patients died from cardiac cause and 187 patients suffered from nonfatal MI. Compared with patients in low risk group, patients in high risk group were more likely to have MACE (ESC strategy: 6% versus 1%, p < 0.0001 and CACS-CL strategy: 8% versus 1%, p < 0.0001).
Figure 3 exhibitions Kaplan–Meier curves comparing survival probabilities in high and low risk groups. High risk group according to ESC strategy and CACS-CL strategy demonstrated a significantly higher risk of MACE, respectively (log-rank test: p < 0.0001 for ESC strategy and p < 0.0001 for CACS-CL strategy). The association of risk groups determined by CACS-CL strategy (high versus low) with MACE was stronger (HR for ESC strategy: 3.97, 95% CI: 2.75 to 5.72, p < 0.0001 and HR for CACS-CL strategy: 5.11, 95% CI: 3.40 to 7.69, p < 0.0001).
Kaplan–Meier curves comparing high and low risk groups determined by four strategies. ESC European Society of Cardiology, PTP pretest probability, ESC strategy 2019 European Society of Cardiology guideline-determined risk assessment strategy, CACS-CL coronary artery calcium score-weighted clinical likelihood, CACS-CL strategy coronary artery calcium score-weighted clinical likelihood model-based risk assessment strategy, CCTA coronary computed tomographic angiography
Table 4 is the reclassification table to compare ESC strategy and CACS-CL strategy. Among the 4284 negative patients, compared to ESC strategy, CACS-CL strategy reclassified 801 from high to low risk group, but 67 from low to high. Of the 1005 positive patients, 32 were reclassified to high risk group but 13 to low. As a result, the NRI comparing CACS-CL strategy to ESC strategy was 17% in negative, 2% in positive, and 19% in all (p < 0.0001).
In total, 1143 patients had ICA based on CCTA and 328 patients underwent CR. Compared with low risk patients, high risk patients had more ICA (ESC strategy: 31% (924/2962) versus 9% (219/2327), p < 0.0001; CACS-CL strategy: 39% (882/2247) versus 9% (261/3042), p < 0.0001) and CR (ESC strategy: 8% (249/2962) versus 3% (79/2327), p < 0.0001; CACS-CL strategy: 11% (244/2247) versus 3% (84/3042), p < 0.0001).
The impact of CACS on ESC and CACS-CL strategy
ESC strategy classified 49% (2110/4284) negative patients into high risk group, for which further cardiovascular testing were recommend according to current guidelines. The application of CACS-CL strategy instead of ESC strategy would result in a prominently change of diagnostic strategy: 38% (801/2110) of these patients were reclassified into low risk group, for which no further cardiovascular testing was recommend. Moreover, during follow-up, only 2 nonfatal MI occurred among the 801 patients. Based on ESC strategy, 28% of the 5289 patients with borderline ESC-PTP would require further CACS scan, with an additional (5289–1479 = 3810) CACS scans required if following the CACS-CL strategy. In a word, the replacement of ESC strategy by CACS-CL strategy would avoid 38% unnecessary cardiovascular testing and avoid an unnecessary cardiovascular testing at the expense of 3810/801≈5 additional CACS scans.
Discussion
In this CCTA-based analysis of patients with SCP suggestive of CAD, compared to ESC-PTP model, CACS-CL model revealed a larger AUC, a positive IDI and less discrepancy between observed and predicted probabilities. Both ESC strategy and CACS-CL strategy classified a proportion of patients into low risk groups with low prevalence of CAD and MACE, but the additional use of CACS in CACS-CL strategy improved the identification of patients who may derived minimal benefit from further cardiovascular testing.
Although the concept of PTP has been recognized as the cornerstone of SCP clinical management for decades [3, 25], numerous external validation studies suggested that the traditional invasive ICA-based approaches to PTP revealed significant overestimation of the actual prevalence of CAD [4,5,6, 26], which accounted, in part, for the substantial number of unnecessary cardiovascular testing [27,28,29]. To improve the estimation of PTP, Knuuti et al. developed the ESC-PTP model [1] based on data from 3 contemporary cohorts with a total of 22 366 SCP patients referred for CCTA [10, 11, 26] and the diagnostic performance of ESC-PTP model has been validated externally [12,13,14,15]. Moreover, ESC guideline suggested a novel strategy incorporating CACS with estimation of PTP, especially for patients with borderline ESC-PTP [9].
Although the diagnostic and prognostic value of CACS have been established [30, 31], extensive literature has repeatedly demonstrated the strong interplay between CACS and risk factors for predicting the presence of obstructive CAD and for future MACE [17, 32,33,34]. In conformity with previous findings comparing CACS-based PTP models to other models [4, 6], we demonstrated that CACS-CL model offered precise estimation of PTP and prediction of MACE. As far as we know, this is the first comparative description of two different strategies which both included CACS-CL model: in contrast to ESC strategy, CACS-CL strategy applied CACS scan to all SCP patients. A recent study with a shorter follow-up selected CAD Consortium extended model (CCEM), which also incorporated clinical variables plus CACS, for further risk assessment in patients with borderline ESC-PTP [35]. However, the CCEM was a traditional approach to estimate pretest probability (PTP) based on ICA [36] and has been demonstrated to obviously overestimate the actual prevalence of CAD according to the calibration plots [14].
In the present study, compared to ESC strategy, CACS-CL strategy seemed to be associated with greater effectiveness in identifying patients at low risk for whom further cardiovascular testing should be deferred, resulting from the superiority for the diagnosis of CAD and prediction of MACE. More importantly, the replacement of ESC strategy by CACS-CL strategy would avoid an unnecessary cardiovascular testing at the expense of (5289–1479)/801 = 5 additional CACS scans. Greater emphasis should be placed on this management decision paradigm incorporating CACS-based estimation of PTP to all SCP patients, because of low radiation exposure and scan costs, no need for provocation, vessel puncture or contrast, few contraindications, and little difficulty in operation and interpretation of CACS scan [37]. It also bears mentioning that comparison of cost-effectiveness between this attractive risk assessment strategy and sequential instrument incorporating CACS-based PTP estimation only to subgroups of SCP patients is needed in the future.
Limitations of the study
Several limitations needed attention. First, the present study was subjected to the observational design. The clinical decision before and after CCTA were made by local physicians and the substantial selection biases should not be ignored, such as a significant number of low risk patients not being included, as well as local referral pattern bias of CTA versus functional testing or ICA. Thus, the generalizability of the present findings in patients who did not undergo CCTA need further study. Second, the ESC guideline also recommended other new predictors, such as changes on rest or exercise electrocardiogram (ECG) and LV dysfunction exercise to improve estimation of PTP. However, CACS provided the most incremental diagnosis and prognosis information above traditional cardiovascular risk factors [6, 36]. Third, we defined unassessable segments as positive ones because further testing should usually be referred for nonconclusive CCTA. Fourth, this analysis focused on the presence of coronary diameter stenosis ≥ 50%. Evaluation of high risk CAD, such as left main disease or 3-vessel disease with maximal degree of coronary diameter stenosis ≥ 70% would be helpful to identify patients who may derive maximal benefit from CR [38,39,40]. However, our data also supported the potential of CACS-CL strategy to optimize the downstream utilization of invasive procedures. Fifth, although both the ESC [1] and CACS-CL [14] strategy included patients presenting with dyspnea, this study only focused on SCP. Thus, the conclusions should not be extrapolated to patients with dyspnea or acute chest pain, or asymptomatic patients.
Conclusions
In conclusion, CACS-CL model provided precise estimation of PTP, resulting in the superiority of CACS-CL strategy in identifying patients at low risk who may derive minimal benefit from further cardiovascular testing. Moreover, compared to ESC strategy, CACS-CL strategy might have more potential to defer unnecessary cardiovascular testing at a low expense. For risk assessment of patients presenting with SCP suggestive of obstructive CAD, the cost-effectiveness of CACS-CL strategy which obviously increase the use of CACS scan needs to be comprehensive validated and compared with other strategies in the future.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to the ongoing project but are available from the corresponding author on reasonable request.
Abbreviations
- SCP:
-
Stable chest pain
- PTP:
-
Pretest probability
- ICA:
-
Invasive coronary angiography
- CAD:
-
Coronary artery disease
- CACS:
-
Coronary artery calcium score
- CCTA:
-
Coronary computed tomography angiography
- CACS-CL:
-
CACS-weighted clinical likelihood
- CACS-CL strategy:
-
CACS-CL model alone-based risk assessment strategy
- ESC strategy:
-
2019 ESC guideline-determined risk assessment strategy
- AUC:
-
Area under receiver-operator characteristic curve
- IDI:
-
Integrated discrimination improvement
- H–L χ2 :
-
Hosmer–Lemeshow chi-square statistic
- NRI:
-
Net reclassification improvement
- HR:
-
Hazard ratio
- CI:
-
Confidence intervals
- MACE:
-
Major adverse cardiovascular event
- CCEM:
-
CAD Consortium extended model
References
Knuuti J, Ballo H, Juarez-Orozco LE, Saraste A, Kolh P, Rutjes AWS, Juni P, Windecker S, Bax JJ, Wijns W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J. 2018;39(35):3322–30.
Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T, Diercks DB, Gentile F, Greenwood JP, Hess EP, Hollenberg SM, Jaber WA, Jneid H, Joglar JA, Morrow DA, O’Connor RE, Ross MA, Shaw LJ. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78(22):e187–285.
Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Guidelines ESCCFP, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 34(38):2949–3003.
Zhou J, Liu Y, Huang L, Tan Y, Li X, Zhang H, Ma Y, Zhang Y. Validation and comparison of four models to calculate pretest probability of obstructive coronary artery disease in a Chinese population: a coronary computed tomographic angiography study. J Cardiovasc Comput Tomogr. 2017;11(4):317–23.
Baskaran L, Danad I, Gransar H, Hartaigh BO, Schulman-Marcus J, Lin FY, Pena JM, Hunter A, Newby DE, Adamson PD, Min JK. A comparison of the updated Diamond-Forrester, CAD Consortium, and CONFIRM history-based risk scores for predicting obstructive coronary artery disease in patients with stable chest pain: the SCOT-HEART Coronary CTA Cohort. J Am Coll Cardiol Imging. 2019;12(72):1392–400.
Genders TSS, Coles A, Hoffmann U, Patel MR, Mark DB, Lee KL, Steyerberg EW, Hunink MGM, Douglas PS, Consortium CAD, the PI. The external validity of prediction models for the diagnosis of obstructive coronary artery disease in patients with stable chest pain: insights from the PROMISE trial. J Am Coll Cardiol Imging. 2018;11(3):437–446.
Ferreira AM, Marques H, Tralhao A, Santos MB, Santos AR, Cardoso G, Dores H, Carvalho MS, Madeira S, Machado FP, Cardim N, de Araujo GP. Pre-test probability of obstructive coronary stenosis in patients undergoing coronary CT angiography: comparative performance of the modified diamond-Forrester algorithm versus methods incorporating cardiovascular risk factors. Int J Cardiol. 2016;222:346–51.
Adamson PD, Fordyce CB, McAllister DA, Udelson JE, Douglas PS, Newby DE. Identification of patients with stable chest pain deriving minimal value from coronary computed tomography angiography: an external validation of the PROMISE minimal-risk tool. Int J Cardiol. 2018;252:31–4.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77.
Reeh J, Therming CB, Heitmann M, Hojberg S, Sorum C, Bech J, Husum D, Dominguez H, Sehestedt T, Hermann T, Hansen KW, Simonsen L, Galatius S, Prescott E. Prediction of obstructive coronary artery disease and prognosis in patients with suspected stable angina. Eur Heart J. 2019;40(18):1426–35.
Foldyna B, Udelson JE, Karády J, Banerji D, Lu MT, Mayrhofer T, Bittner DO, Meyersohn NM, Emami H, Genders TSS, Fordyce CB, Ferencik M, Douglas PS, Hoffmann U. Pretest probability for patients with suspected obstructive coronary artery disease: re-evaluating Diamond-Forrester for the contemporary era and clinical implications: insights from the PROMISE trial. Eur Heart J Cardiovasc Imaging. 2019;20(5):574–81.
Malhotra S, Batal O, Douglas P, Soman P. Accurate prediction of myocardial perfusion abnormality by the European society of Cardiology pretest probability estimates of coronary artery disease. Circ Cardiovasc Imaging. 2020;13(10): e011342.
Bing R, Singh T, Dweck MR, Mills NL, Williams MC, Adamson PD, Newby DE. Validation of European Society of Cardiology pre-test probabilities for obstructive coronary artery disease in suspected stable angina. Eur Heart J Qual Care Clin Outcomes. 2020;6(4):293–300.
Winther S, Schmidt SE, Mayrhofer T, Botker HE, Hoffmann U, Douglas PS, Wijns W, Bax J, Nissen L, Lynggaard V, Christiansen JJ, Saraste A, Bottcher M, Knuuti J. incorporating coronary calcification into pre-test assessment of the likelihood of coronary artery disease. J Am Coll Cardiol. 2020;76(21):2421–32.
Winther S, Schmidt SE, Rasmussen LD, Juárez Orozco LE, Steffensen FH, Bøtker HE, Knuuti J, Bøttcher M. Validation of the European Society of Cardiology pre-test probability model for obstructive coronary artery disease. Eur Heart J. 2021;42(14):1401–11.
Saraste A, Barbato E, Capodanno D, Edvardsen T, Prescott E, Achenbach S, Bax JJ, Wijns W, Knuuti J. Imaging in ESC clinical guidelines: chronic coronary syndromes. Eur Heart J Cardiovasc Imaging. 2019;20(11):1187–97.
Zhou J, Chen Y, Zhang Y, Wang H, Tan Y, Liu Y, Huang L, Zhang H, Ma Y, Cong H. Epicardial fat volume improves the prediction of obstructive coronary artery disease above traditional risk factors and coronary calcium score. Circ Cardiovasc Imaging. 2019;12(1): e008002.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32.
Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, Nieman K, Pontone G, Raff GL. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8(5):342–58.
Narula J, Chandrashekhar Y, Ahmadi A, Abbara S, Berman DS, Blankstein R, Leipsic J, Newby D, Nicol ED, Nieman K, Shaw L, Villines TC, Williams M, Hecht HS. SCCT 2021 expert consensus document on coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography. J Cardiovasc Comput Tomogr. 2021;15(3):192–217.
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165.
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–64.
Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, McGinn T, Guyatt G. Discrimination and calibration of clinical prediction models: users’ guides to the medical literature. JAMA. 2017;318(14):1377–84.
Pencina MJ, Agostino Sr. RB, Agostino Jr. RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72 (discussion 207-12).
Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64(18):1929–49.
Cheng VY, Berman DS, Rozanski A, Dunning AM, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJ, Delago A, Gomez M, Hadamitzky M, Hausleiter J, Karlsberg RP, Kaufmann P, Lin FY, Maffei E, Raff GL, Villines TC, Shaw LJ, Min JK. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM). Circulation. 2011;124(22):2423–32.
Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291–300.
Greenwood JP, Ripley DP, Berry C, McCann GP, Plein S, Bucciarelli-Ducci C, Dall’Armellina E, Prasad A, Bijsterveld P, Foley JR, Mangion K, Sculpher M, Walker S, Everett CC, Cairns DA, Sharples LD, Brown JM. Effect of care guided by cardiovascular magnetic resonance, myocardial perfusion scintigraphy, or NICE guidelines on subsequent unnecessary angiography rates: the CE-MARC 2 randomized clinical trial. JAMA. 2016;316(10):1051–60.
Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, Forbes J, Hunter A, Lewis S, MacLean S, Mills NL, Norrie J, Roditi G, Shah ASV, Timmis AD, van Beek EJR, Williams MC. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924–33.
Agha AM, Pacor J, Grandhi GR, Mszar R, Khan SU, Parikh R, Agrawal T, Burt J, Blankstein R, Blaha MJ, Shaw LJ, Al-Mallah MH, Brackett A, Cainzos-Achirica M, Miller EJ, Nasir K. The Prognostic value of CAC zero among individuals presenting with chest pain: a meta-analysis. J Am Coll Cardiol Imging. 2022;15(10):1745–57.
Grandhi GR, Mszar R, Cainzos-Achirica M, Rajan T, Latif MA, Bittencourt MS, Shaw LJ, Batlle JC, Blankstein R, Blaha MJ, Cury RC, Nasir K. Coronary calcium to rule out obstructive coronary artery disease in patients with acute chest pain. J Am Coll Cardiol Imging. 2022;15(2):271–80.
Mortensen MB, Dzaye O, Bødtker H, Steffensen FH, Bøtker HE, Jensen JM, Rønnow Sand NP, Maeng M, Warnakula Olesen KK, Sørensen HT, Kanstrup H, Blankstein R, Blaha MJ, Nørgaard BL. Interplay of risk factors and coronary artery calcium for CHD risk in young patients. J Am Coll Cardiol Imging. 2021;14(12):2387–96.
Mortensen MB, Gaur S, Frimmer A, Bøtker HE, Sørensen HT, Kragholm KH, Niels Peter SR, Steffensen FH, Jensen RV, Mæng M, Kanstrup H, Blaha MJ, Shaw LJ, Dzaye O, Leipsic J, Nørgaard BL, Jensen JM. Association of age with the diagnostic value of coronary artery calcium score for ruling out coronary stenosis in symptomatic patients. JAMA Cardiol. 2022;7(1):36–44.
Bergstrom G, Persson M, Adiels M, Bjornson E, Bonander C, Ahlstrom H, Alfredsson J, Angeras O, Berglund G, Blomberg A, Brandberg J, Borjesson M, Cederlund K, de Faire U, Duvernoy O, Ekblom O, Engstrom G, Engvall JE, Fagman E, Eriksson M, Erlinge D, Fagerberg B, Flinck A, Goncalves I, Hagstrom E, Hjelmgren O, Lind L, Lindberg E, Lindqvist P, Ljungberg J, Magnusson M, Mannila M, Markstad H, Mohammad MA, Nystrom FH, Ostenfeld E, Persson A, Rosengren A, Sandstrom A, Sjalander A, Skold MC, Sundstrom J, Swahn E, Soderberg S, Toren K, Ostgren CJ, Jernberg T. Prevalence of subclinical coronary artery atherosclerosis in the general population. Circulation. 2021;144(12):916–29.
Zhou J, Li C, Cong H, Duan L, Wang H, Wang C, Tan Y, Liu Y, Zhang Y, Zhou X, Zhang H, Wang X, Ma Y, Yang J, Chen Y, Guo Z. Comparison of different investigation strategies to defer cardiac testing in patients with stable chest pain. J Am Coll Cardiol Imging. 2022;15(1):91–104.
Genders TS, Steyerberg EW, Hunink MG, Nieman K, Galema TW, Mollet NR, de Feyter PJ, Krestin GP, Alkadhi H, Leschka S, Desbiolles L, Meijs MF, Cramer MJ, Knuuti J, Kajander S, Bogaert J, Goetschalckx K, Cademartiri F, Maffei E, Martini C, Seitun S, Aldrovandi A, Wildermuth S, Stinn B, Fornaro J, Feuchtner G, De Zordo T, Auer T, Plank F, Friedrich G, Pugliese F, Petersen SE, Davies LC, Schoepf UJ, Rowe GW, van Mieghem CA, van Driessche L, Sinitsyn V, Gopalan D, Nikolaou K, Bamberg F, Cury RC, Battle J, Maurovich-Horvat P, Bartykowszki A, Merkely B, Becker D, Hadamitzky M, Hausleiter J, Dewey M, Zimmermann E, Laule M. Prediction model to estimate presence of coronary artery disease: retrospective pooled analysis of existing cohorts. BMJ. 2012;344: e3485.
Rozanski A, Berman DS. Coronary artery calcium scanning in symptomatic patients: ready for use as a gatekeeper for further testing? J Nucl Cardiol. 2017;24(3):835–8.
Yang Y, Chen L, Yam Y, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng VY, Chinnaiyan K, Cury R, Delago A, Dunning A, Feuchtner G, Hadamitzky M, Hausleiter J, Karlsberg RP, Kaufmann PA, Kim YJ, Leipsic J, LaBounty T, Lin F, Maffei E, Raff GL, Shaw LJ, Villines TC, Min JK, Chow BJ. A clinical model to identify patients with high-risk coronary artery disease. J Am Coll Cardiol Img. 2015;8(4):427–34.
Jang JJ, Bhapkar M, Coles A, Vemulapalli S, Fordyce CB, Lee KL, Udelson JE, Hoffmann U, Tardif JC, Jones WS, Mark DB, Sorrell VL, Espinoza A, Douglas PS, Patel MR. Predictive model for high-risk coronary artery disease. Circ Cardiovasc Imaging. 2019;12(2): e007940.
Shan D, Yang J, Chen Y. Noninvasive cardiac imaging technologies in detecting coronary artery disease: from research to clinical practice. Cardiol Plus. 2020;5(1):13.
Acknowledgements
None.
Funding
This work was supported by Key Natural Fund Projects of Tianjin Science and Technology Commission (21JCZDJC01060), the National Natural Science Foundation of China (62206197), Applied and Basic Research by Multi-input Foundation of Tianjin (21JCYBJC00820) and Tianjin Health Research Project (TJWJ2022QN067).
Author information
Authors and Affiliations
Contributions
JM made contributions to the collection, analysis and interpretation of data and writing the manuscript. JZ made contributions to the conception and design of the work as well as collection, analysis and interpretation of data and provided the fund supports. HJ and KR made contributions to the collection, analysis and interpretation of data. All of the authors have approved the submitted version and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This observational study complied with the Declaration of Helsinki. The ethics committee of Tianjin Chest Hospital approved to carry out the study within its facilities. Informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Meng, J., Jiang, H., Ren, K. et al. Comparison of risk assessment strategies incorporating coronary artery calcium score with estimation of pretest probability to defer cardiovascular testing in patients with stable chest pain. BMC Cardiovasc Disord 23, 53 (2023). https://doi.org/10.1186/s12872-023-03076-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03076-3