Abstract
Background
Polypharmacy in patients with cardiovascular diseases (CVDs) has been linked to several adverse outcomes. This study aimed to investigate the pattern of medication use and prevalence of polypharmacy among CVDs patients in Iran.
Method
We used the baseline data of the Pars cohort study (PCS). The participants were asked to bring their medication bags; then, the medications were classified using the Anatomical Therapeutic Chemical classification. Polypharmacy was defined as using five or more medications concurrently. Poisson regression modeling was applied. The adjusted prevalence ratios (PR) and its 95% confidence interval (CI) were estimated.
Results
Totally, 9262 participants were enrolled in the PCS, of whom 961 had CVDs. The prevalence of polypharmacy in participants with and without CVDs was 38.9% and 7.1%, respectively. The highest prevalence of polypharmacy (51.5%) was among obese patients. Abnormal waist-hip ratio (PR: 2.79; 95% CI 1.57–4.94), high socioeconomic status (PR: 1.65; 95% CI 1.07–2.54), tobacco-smoking (PR: 1.35; 95% CI 1.00–1.81), patients with more than three co-morbidities (PR: 1.41; 95% CI 1.30–1.53), high physical activity (PR: 0.66; 95% CI 0.45–0.95), use of opiate ever (PR: 0.46; 95% CI 0.26–0.82), and healthy overweight subjects (PR: 0.22; 95% CI 0.12–0.39) were associated with polypharmacy. Cardiovascular drugs (76.1%), drugs acting on blood and blood-forming organs (50.4%), and alimentary tract and metabolism drugs (33.9%) were the most frequently used drugs. Agents acting on the renin-angiotensin system were the mostly used cardiovascular system drugs among men and those above 60 years old, while beta-blocking agents were mostly prevalent among cardiovascular system drugs in women with CVDs.
Conclusion
Given the high prevalence of polypharmacy among CVDs patients, and subsequent complications, programs to educate both physicians and patients to prevent this issue is crucial.
Similar content being viewed by others
Introduction
The aging trend of the populations around the world has led to an increasing burden of non-communicable diseases (NCDs) [1], particularly cardiovascular diseases (CVDs). CVDs are the primary cause of mortality (one-third of deaths) and morbidity globally, affecting half of all individuals over their lifetime [2, 3]. Most of these patients suffer from other NCDs such as diabetes mellitus (DM), hypertension, dyslipidemia, and chronic kidney disease [4], which require concomitant administration of several drugs to control them. Although using multiple medications in these patients is inevitable for controlling the diseases, it may lead to improper use of the medications among a significant number of the patients [5, 6].
In various studies on different populations, polypharmacy prevalence among patients varied from over 10 to 90% [7,8,9,10,11,12]. Also, cardiovascular drugs are reported to be the most prevalent type of drugs in patients with polypharmacy [13, 14]. Simultaneous use of multiple medications in these patients may lead to administration difficulties, reduce adherence levels, and contribute to dosage errors [15, 16]. Polypharmacy is also associated with adverse events such as drug interactions, longer hospital stay, more frequent falls and fractures, excessive health expenditure, and increased mortality risk [7, 8, 14]. Hence, recognizing high-risk subgroups for polypharmacy in CVDs patients is vital to decrease the above-mentioned complications and lessen the burden of CVDs-related comorbidities.
Despite the significant burden of CVDs in developing countries, the knowledge regarding polypharmacy in CVDs patients is still limited [7, 17]. In the present investigation, we aimed to measure the prevalence and characteristics of polypharmacy among patients with CVDs in a cohort study in southern Iran.
Methods
Study setting
This cross-sectional study was conducted on the baseline data of the Pars Cohort Study (PCS). PCS is an ongoing population-based prospective study that started in 2012 to determine the prevalence of NCDs risk factors in a semi-urban area, Valashahr, in Fars province, South of Iran. Valashahr has over 40,000 inhabitants, of whom nearly 10,000 people are between 40 and 75 years old. Detailed information on the studied population was published elsewhere [18]. All Valashahr residents who were between 40 and 75 years old were contacted and invited to participate in PCS. Finally, 9264 individuals agreed to participate (92% participation rate). For the current analysis, two participants were excluded due to insufficient data. Trained medical personnel (physicians and nurses) collected the data, including in-person interviews, a brief physical examination, anthropometric indices measurements, and biomedical samples analysis using standardized and calibrated equipment.
Data collection
The participants were questioned, “Has your physician told you that you have CVDs and need treatment for that?”. If the answer was “Yes”, they were categorized as having CVDs. They were asked to bring their bags of medications to the PCS center. An educated nurse listed the drugs (used at least over the past three months) and asked the patients which medications were taken at the time of the interview. Although various definitions have been proposed for polypharmacy [19], in the present investigation, polypharmacy was defined as the concurrent use of five or more different medications.
Drug classification
Aside from complementary medicines, we utilized the first level of the Anatomical Therapeutic Chemical (ATC) classification system [20] to classify the participants’ medications. Also, to categorize cardiovascular drugs, we used ATC code C.
Polypharmacy determinants
We defined the covariates as follows: age (40–49, 50–59, and 60 ≤), gender (female, male), education (literate, illiterate), marital status (married, not married), ethnicity (Fars/ non-Fars), tobacco smoking ever-use (smoker, non-smoker), central obesity based on waist to hip ratio [21] (with central obesity, without central obesity), fasting blood sugar (FBS > 110 mg/dL, FBS ≤ 110), low density lipoprotein-cholesterol (LDL˂100 mg/dL, LDL ≥ 100), high density lipoprotein-cholesterol (HDL < 45 mg/dL, HDL ≥ 45), total cholesterol (< 200 mg/dL, 200–239, and 240 ≤), triglyceride (< 150 mg/dL, 150–199, and 200 ≤), body mass index (BMI < 25; normal weight, 25–29.9; overweight, and 30 ≤ ; obese), and age at diagnosis of CVDs (< 40, 40–60, and ≥ 60).
Metabolic syndrome was defined according to the criteria suggested by Alberti et al. [22] for Asian individuals. Participants without metabolic syndrome (MetS) who were overweight were described as “healthy overweight”, and those who were overweight and had Mets simultaneously were considered as “unhealthy overweight”. The participants’ socioeconomic status (SES) was measured by applying their self-reported assets. Asset analysis was done by multiple correspondence analysis, and a latent factor was estimated. According to the quartiles of the estimated latent factor, we classified the participants into four groups (low, low-middle, middle-high, and high). Physical activity data were obtained through International Physical Activity Questionnaire (IPAQ) [23] and converted to Metabolic equivalent of task (MET) scores. Then, the participants were categorized into three groups including high (at least 3000 MET-minutes/week), moderate (at least 600 MET-minutes/week), and low (less than 600 MET-minutes/week).
We asked the participants about a list of diseases to determine comorbidities, “whether or not your doctor or health care provider has ever diagnosed each of these diseases for you?”. These conditions included hypertension, DM, jaundice, rheumatic heart disease, joint pain, back pain, anxiety, depression, insomnia, obstructive lung disease, stroke, renal failure, and cancer. Also, gastroesophageal reflux disease (GERD), irritable bowel syndrome (IBS), and functional constipation were defined according to previously described clinical criteria [24]. The participants were divided into three groups based on their comorbidities: only CVDs, two or three comorbidities, and more than three comorbidities. Furthermore, considering the date of the interview and the date of diagnosis of CVDs, we assessed the duration of CVDs for each patient (less than 2, 3 to 5 years, and 6 years and more).
Statistical analysis
Frequency, mean, and standard deviation (SD) were calculated to describe the variables, where appropriate. Prevalence ratio (PR) and its 95% confidence interval (CI) were assessed using Poisson distribution. Considering the standard world population (WHO 2000–25), the age-standardized prevalence of polypharmacy was estimated. Chi-square and Mann–Whitney U tests were used for univariate analyses. To determine independent correlates of polypharmacy prevalence, Poisson regression modeling was applied; variables with a univariate p value of less than 0.3 were included in the multivariable modeling as potentially independent variables. In the final analysis, the model was proportionated using a backward elimination technique. p values less than 0.05 were considered statistically significant. STATA software (Release 11, College Station, TX:Stata Corp LLC) was used to analyze the data.
Results
Of 9262 participants, 961 were CVDs patients, including 404 (42.0%) men and 557 (58%) women with the mean age of 58.5 ± 9.8. The prevalence of polypharmacy was 374/961 (38.9%, 95% CI 35.8%, 42.0%). The overall age- and gender-standardized prevalence of polypharmacy was 36.0% (95% CI 32.7%, 39.2%). The estimated age-standardized prevalence of polypharmacy was 29.6% (95% CI 25.1%, 34.1%) for males and 40.6% (95% CI 35.9%, 45.4%) for females.
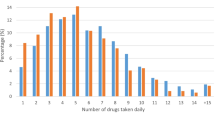
Among the participants with CVDs, the highest estimated prevalence of polypharmacy (51.5%; 95% CI 44.9%, 58.1%) was among obese patients, and the lowest prevalence belonged to patients without central obesity (16.6%; 95% CI 10.6%, 25.1%). In all variables, the prevalence of polypharmacy was significantly higher in patients with CVDs compared to those without CVDs (Table 1). Also, Fig. 1 displays the percentage of concurrently used drugs among patients with and without CVDs, and Fig. 2 shows number of concurrently cardiovascular drugs used by patients with CVDs.
Overall, more than half of the patients (59.4%) suffered from a minimum of three comorbidities (p < 0.001). According to Table 2, the top three comorbidities with a significantly higher prevalence of polypharmacy compared to patients without those comorbidities were DM (62.9%; 95% CI 55.5%, 69.7%), obstructive lung disease (55.8%; 95% CI 43.9%, 67.1%), and hypertension (53.6%; 95% CI: 47.8%, 58.3%).
According to Table 3, high levels of physical activity (Adjusted PR: 0.66; 95% CI 0.56, 0.78), opiate ever used (Adjusted PR: 0.46; 95% CI 0, 26, 0.82), and being healthy overweight (Adjusted PR: 0.22; 95% CI 0.12, 0.39) were associated with the lower prevalence of polypharmacy.
Cardiovascular drugs (76.1%), drugs acting on blood and blood-forming organs (50.4%), and alimentary tract and metabolism drugs (33.9%) were the most frequently used drugs among the participants with CVDs. Cardiovascular drugs (83.0%) and drugs acting on blood and blood-forming organs (55.1%) had the highest prevalence of use among the elderly. These two drug classes also had equally high prevalence rates among females and males, about 75.0% for cardiovascular drugs and 50.0% for drugs acting on blood and blood-forming organs (Table 4). According to Table 5, agents acting on the renin-angiotensin system (Class C09) were the mostly used cardiovascular system drugs among men and those above 60 years old, while beta blocking agents (Class C07) were the most common drugs used among cardiovascular system drugs in women.
Discussion
This study found that more than one-third of CVDs patients are likely to suffer from polypharmacy. The prevalence of polypharmacy was nearly 1.5-fold higher among females. Having a higher SES, being physically inactive, using tobacco, not using opiates, number of chronic comorbidities, being unhealthy overweight, and having an abnormal waist-hip ratio have been shown to be associated with increased prevalence of polypharmacy in patients with CVDs.
The prevalence of polypharmacy in our study was higher than that in a study from Ethiopia among CVDs patients [9]. On the other hand, some studies reported a higher prevalence of polypharmacy than our results. For instance, in Qatar and Oman 75.5% and 76.3% polypharmacy prevalence rates were observed, respectively [7, 25]. Also, previous reports from developed countries, Switzerland (11.8%), and United Kingdom (22.8%) indicated a lower prevalence of polypharmacy [26, 27]. One reason for the discrepancies could be the designs of the studies and the studied populations; the study in Oman was conducted at a tertiary care hospital, the study from Ethiopia evaluated outpatients, and our study was a population-based one. Furthermore, the prevalence of chronic diseases like DM and hypertension, as the risk factors of CVDs, in Arabic countries was much higher than that in our population, [28], resulting in higher polypharmacy rates.
It was shown that the female gender was associated with a higher prevalence of polypharmacy. Al-Dahshan et al. discovered the same pattern in CVDs patients [25]. However, some studies have reported no impact of gender on polypharmacy [7, 8]. Sechana et al. have reported an opposite result, indicating that polypharmacy was higher in the male gender [29]. As to the higher prevalence of polypharmacy in females, being more concerned about their health status than men may lead to more health-seeking behaviors, higher rates of adherence to medication therapy, and self-medication [30, 31]. Another explanation may be the higher life expectancy and longer time period of living with chronic diseases [32] which subsequently contribute to higher medication use. Thus, a higher prevalence of polypharmacy was seen in the females in our study.
Compared to low SES, our results demonstrated that higher SES levels lead to a higher rate of polypharmacy. Such findings have also been reported by others [14]. In contrast, some studies have found no difference based on SES [7, 9, 25]. In the city where our study was conducted, specialized medical services are not available, so individuals with higher SES may have better accessibility and affordability to utilize diagnostic and treatment services in neighboring cities, which leads to receiving more medications than the others.
We found that physical inactivity, existence of unhealthy overweight, abnormal waist-hip ratio, and tobacco smoking were associated with a higher prevalence of polypharmacy in CVDs patients. These conditions are contributing factors in the development of NCDs [33] and metabolic syndrome which leads to higher odds of having CVDs [34], confirming the notion that individuals with more comorbidities are at higher risk of suffering from polypharmacy. Also, one possible reason is that these conditions make it harder to control the underlying diseases, which leads to more medication use. Another finding of our study was that opiate use led to a lower prevalence of polypharmacy. It may stem from lower adherence to prescribed therapies in individuals with substance abuse, such as opiates [35]. Another possible justification could be the sedative and strong analgesic effect of opiates, which lead to delays in the diagnosis of diseases in these subgroups.
As addressed in previous studies, we also investigated the relationship between the number of comorbidities and polypharmacy [8, 36]. It is important to emphasize that the higher number of comorbidities correlates with an increase in the prevalence of polypharmacy. This can be explained by multiple medications prescription to control several diseases. Over-prescription and polypharmacy can contribute to an increased risk of adverse drug-drug and drug-disease interactions [37]. One possible solution could be the use of polypills, multiple drugs in one tablet [38], which should be further investigated in future studies.
According to our results, the most commonly used drugs in the CVDs patients were those acting on the cardiovascular system (Class C), followed by blood and blood-forming organs medications (Class B) and those acting on the alimentary tract and metabolism (Class A). Al-Hashar et al. [7] in Oman and Al-Amin et al. [8] in Bangladesh have shown that among CVDs patients, drugs related to the alimentary tract and metabolism were the most frequently used after cardiovascular medications. One possible explanation for this may be the use of different medicines in patients with CVDs which makes it inevitable to use antacid drugs to control the GI upset caused by simultaneous consumption of other medications [39].
Among cardiovascular system drugs, we showed that agents acting on the renin-angiotensin system and beta blocking agents (BB) were the mostly used drugs by CVDs patients. BBs were the topmost used drug in females with a subtle difference. A study from Ethiopia reported that diuretics and angiotensin-converting-enzyme inhibitors were the most frequently used drugs among CVDs patients [9]. Agents acting on the renin-angiotensin system have several benefits like reducing blood pressure and proteinuria, being drug of choice for hypertension in DM, and decreasing cardiovascular mortality and morbidity [40]. Also, BBs are used in a wide range of cardiovascular diseases [41]. Although they are not used as the first-line treatment in hypertension, their wide usage in myocardial infarction, congestive heart failure, cardiac arrhythmias, coronary artery disease, and other conditions may justify its high prevalence in our study.
Our study has several strengths. It is a population-based ongoing cohort conducted in Iran among patients with CVDs, assessing polypharmacy. Our sample size allowed for statistical analysis with sufficient statistical power. Additionally, to reduce recall bias, we asked the patients to bring their medications with them, and a trained nurse checked the list of drugs. However, the study is not free of limitations. First, the cross-sectional design of the study prevents us from establishing a cause and effect relationship between the variables. Second, the temporality between time-dependent variables like obesity and polypharmacy could not be addressed. Also, our study did not discuss the effects of CVDs relapse or untreated CVDs on polypharmacy. Moreover, we asked the patients whether they were diagnosed with CVDs; this might lead to recall and confirmation bias.
Conclusion
The prevalence of polypharmacy was high in patients with CVDs. Higher SES, physical inactivity, tobacco use, existence of several comorbidities, being unhealthy overweight, and abnormal waist-hip ratio were important predictors of polypharmacy in patients with CVDs. Training at physician and patient levels is crucial to inhibit the increasing trend of polypharmacy and subsequent complications.
Availability of data and materials
Data is available upon request by the PCS central board and the corresponding author. (https://persiancohort.com/).
Abbreviations
- NCDs:
-
Non-communicable diseases
- CVDs:
-
Cardiovascular diseases
- DM:
-
Diabetes mellitus
- PCS:
-
Pars cohort study
- LDL:
-
Low density lipoprotein-cholesterol
- HDL:
-
High density lipoprotein-cholesterol
- BMI:
-
Body mass index
- Mets:
-
Metabolic syndrome
- SES:
-
Socioeconomic status
- IPAQ:
-
International Physical Activity Questionnaire
- MET:
-
Metabolic equivalent of task
- GERD:
-
Gastroesophageal reflux disease
- IBS:
-
Irritable bowel syndrome
- SD:
-
Standard deviation
- CI:
-
Confidence interval
- PR:
-
Prevalence ratio
- BB:
-
Beta blocking agents
References
Gyasi RM, Phillips DR. Aging and the rising burden of noncommunicable diseases in sub-Saharan Africa and other low- and middle-income countries: a call for holistic action. Gerontologist. 2019;60(5):806–11.
Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol. 2019;74(20):2529–32.
López-Jaramillo P, González-Gómez S, Zarate-Bernal D, Serrano A, Atuesta L, Clausen C, et al. Polypill: an affordable strategy for cardiovascular disease prevention in low–medium-income countries. Ther Adv Cardiovasc Dis. 2018;12(6):169–74.
von Lueder TG, Atar D. Comorbidities and polypharmacy. Heart Fail Clin. 2014;10(2):367–72.
Sharp CN, Linder MW, Valdes R Jr. Polypharmacy: a healthcare conundrum with a pharmacogenetic solution. Crit Rev Clin Lab Sci. 2019. https://doi.org/10.1080/10408363.2019.1678568.
Taylor LK, Kawasumi Y, Bartlett G, Tamblyn R. Inappropriate prescribing practices: the challenge and opportunity for patient safety. Healthc Q. 2005;8(Spec No):81–5.
Al-Hashar A, Al Sinawi H, Al Mahrizi A, Al-Hatrushi M. Prevalence and covariates of polypharmacy in elderly patients on discharge from a tertiary care hospital in Oman. Oman Med J. 2016;31(6):421.
Al-Amin MM, Zinchenko A, Rana MS, Uddin MMN, Pervin MS. Study on polypharmacy in patients with cardiovascular diseases. J Appl Pharm Sci. 2012;2(12):53.
Tefera YG, Alemayehu M, Mekonnen GB. Prevalence and determinants of polypharmacy in cardiovascular patients attending outpatient clinic in Ethiopia University Hospital. PLoS ONE. 2020;15(6): e0234000.
Young EH, Pan S, Yap AG, Reveles KR, Bhakta K. Polypharmacy prevalence in older adults seen in United States physician offices from 2009 to 2016. PLoS ONE. 2021;16(8): e0255642.
Morin L, Johnell K, Laroche ML, Fastbom J, Wastesson JW. The epidemiology of polypharmacy in older adults: register-based prospective cohort study. Clin Epidemiol. 2018;10:289–98.
Khezrian M, McNeil CJ, Murray AD, Myint PK. An overview of prevalence, determinants and health outcomes of polypharmacy. Ther Adv Drug Saf. 2020;11:2042098620933741.
Abolhassani N, Castioni J, Marques-Vidal P, Vollenweider P, Waeber G. Determinants of change in polypharmacy status in Switzerland: the population-based CoLaus study. Eur J Clin Pharmacol. 2017;73(9):1187–94.
Silva IR, Gonçalves LG, Chor D, Fonseca M, Mengue SS, Acurcio FA, et al. Polypharmacy, socioeconomic indicators and number of diseases: results from ELSA-Brasil. Rev Bras de Epidemiol. 2020;23:e200077.
Wastesson JW, Morin L, Tan ECK, Johnell K. An update on the clinical consequences of polypharmacy in older adults: a narrative review. Expert Opin Drug Saf. 2018;17(12):1185–96.
Koper D, Kamenski G, Flamm M, Böhmdorfer B, Sönnichsen A. Frequency of medication errors in primary care patients with polypharmacy. Fam Pract. 2013;30(3):313–9.
Lum MV, Cheung MYS, Harris DR, Sakakibara BM. A scoping review of polypharmacy interventions in patients with stroke, heart disease and diabetes. Int J Clin Pharm. 2020;42(2):378–92.
Gandomkar A, Poustchi H, Moini M, Moghadami M, Imanieh H, Fattahi MR, et al. Pars cohort study of non-communicable diseases in Iran: protocol and preliminary results. Int J Public Health. 2017;62(3):397–406.
Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230.
WHO. Anatomical Therapeutic Chemical (ATC) Classification [Available from: https://www.who.int/tools/atc-ddd-toolkit/atc-classification.
World Health Organization . Waist circumference and waist-hip ratio: report of a WHO expert consultation. Geneva. 2008;8–11:2011.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–5.
Moghaddam MHB, Aghdam F, Asghari Jafarabadi M, Allahverdipour H, Nikookheslat S, Safarpour S. The Iranian Version of International Physical Activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci J. 2012;18:1073–80.
Moezi P, Salehi A, Molavi H, Poustchi H, Gandomkar A, Imanieh MH, et al. Prevalence of chronic constipation and its associated factors in pars cohort study: a study of 9000 adults in Southern Iran. Middle East J Dig Dis. 2018;10(2):75–83.
Al-Dahshan A, Al-Kubiasi N, Al-Zaidan M, Saeed W, Kehyayan V, Bougmiza I. Prevalence of polypharmacy and the association with non-communicable diseases in Qatari elderly patients attending primary healthcare centers: a cross-sectional study. PLoS ONE. 2020;15(6): e0234386.
Castioni J, Marques-Vidal P, Abolhassani N, Vollenweider P, Waeber G. Prevalence and determinants of polypharmacy in Switzerland: data from the CoLaus study. BMC Health Serv Res. 2017;17(1):840.
Rawle MJ, Richards M, Davis D, Kuh D. The prevalence and determinants of polypharmacy at age 69: a British birth cohort study. BMC Geriatr. 2018;18(1):118.
Aggarwal A, Patel P, Lewison G, Ekzayez A, Coutts A, Fouad FM, et al. The Profile of Non-Communicable Disease (NCD) research in the Middle East and North Africa (MENA) region: analyzing the NCD burden, research outputs and international research collaboration. PLoS ONE. 2020;15(4): e0232077.
Sechana K, Rashmi A. Assessment on prevalence of polypharmacy in geriatric patients with cardiovascular diseases. Int J Innov Sci Res Technol. 2020;5(6).
Thompson AE, Anisimowicz Y, Miedema B, Hogg W, Wodchis WP, Aubrey-Bassler K. The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Fam Pract. 2016;17(1):38.
Ranjbaran S, Shojaeizadeh D, Dehdari T, Yaseri M, Shakibazadeh E. Determinants of medication adherence among Iranian patients with type 2 diabetes: an application of health action process approach. Heliyon. 2020;6(7): e04442.
Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. The Lancet. 2017;389(10076):1323–35.
Rieckert A, Trampisch US, Klaaßen-Mielke R, Drewelow E, Esmail A, Johansson T, et al. Polypharmacy in older patients with chronic diseases: a cross-sectional analysis of factors associated with excessive polypharmacy. BMC Fam Pract. 2018;19(1):1–9.
Zibaeenezhad MJ, Sayadi M, Karimi-Akhormeh A, Ardekani A, Parsa N, Razeghian-Jahromi I. Potential of four definitions of metabolic syndrome to discriminate individuals with different 10-year cardiovascular disease risk scores: a cross-sectional analysis of an Iranian cohort. BMJ Open. 2022;12(2): e058333.
Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91.
Azadi M, Kamalipour A, Molavi Vardanjani H, Poustchi H, Taherifard E, Sharifi MH, et al. Prevalence, pattern, and correlates of polypharmacy among Iranian Type II diabetic patients: results from pars cohort study. Arch Iran Med. 2021;24(9):657–64.
Woudstra OI, Kuijpers JM, Meijboom FJ, Post MC, Jongbloed MRM, Duijnhouwer AL, et al. High burden of drug therapy in adult congenital heart disease: polypharmacy as marker of morbidity and mortality. Eur Heart J Cardiovasc Pharmacother. 2019;5(4):216–25.
Abolbashari M, Macaulay TE, Whayne TF, Mukherjee D, Saha S. Polypharmacy in cardiovascular medicine: problems and promises! Cardiovasc Hematol Agents Med Chem. 2017;15(1):31–9.
Chris-Olaiya A, Palmer W, Stancampiano F, Lacy B, Heckman M, Chirila R, et al. Medication use and polypharmacy in patients referred to a tertiary gastroenterology practice. Rom J Intern Med. 2020;58(4):228–32.
Borghi C, Rossi F, Force ST, Force SIFT. Role of the renin-angiotensin-aldosterone system and its pharmacological inhibitors in cardiovascular diseases: complex and critical issues. High Blood Press Cardiovasc Prev. 2015;22(4):429–44.
Martínez-Milla J, Raposeiras-Roubín S, Pascual-Figal DA, Ibáñez B. Role of beta-blockers in cardiovascular disease in 2019. Rev Española de Cardiol (English Edition). 2019;72(10):844–52.
Acknowledgements
This paper was a part of the thesis of Pooran Mohsenzadeh for obtaining the Master of Public Health degree.
Funding
The Pars Cohort Study received financial support from the Iranian Ministry of Health and Medical Education (grant no. 700/107). Also, Shiraz University of Medical Sciences supported this project (grant no. 910210).
Author information
Authors and Affiliations
Contributions
Conceptualization and design: PM, HP, ZM, HBD, and HMV. Supervision of data gathering: HP, ZM, HBD, and HMV. Data analysis: PM, AA, SRAM, ZR, ET, AN, AK, FM, and HMV. Writing and reviewing the manuscript: PM, AA, HP, ZM, SRAM, HBD, ZR, ET, AN, AK, BM, FM, and HMV. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was reviewed and accepted by the Ethics Committees of Shiraz University of Medical Sciences (IR.SUMS.MED.REC.1401.263). Informed consent was obtained from all subjects or their legal guardian(s). All methods were carried out in accordance with relevant guidelines and regulations or declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mohsenzadeh, P., Ardekani, A., Poustchi, H. et al. Population-based pattern of medication use and prevalence of polypharmacy among patients with cardiovascular diseases: results of the Pars cohort study from Iran. BMC Cardiovasc Disord 22, 435 (2022). https://doi.org/10.1186/s12872-022-02872-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02872-7