Abstract
Background
The lack of dystrophin in cardiomyocytes in Duchenne muscular dystrophy (DMD) is associated with progressive decline in cardiac function eventually leading to death by 20–40 years of age. The aim of this prospective study was to determine rate of progressive decline in left ventricular (LV) function in Duchenne muscular dystrophy (DMD) over 5 years.
Methods
Short axis cine and grid tagged images of the LV were acquired in individuals with DMD (n = 59; age = 5.3–18.0 years) yearly, and healthy controls at baseline (n = 16, age = 6.0–18.3 years) on a 3 T MRI scanner. Grid-tagged images were analyzed for composite circumferential strain (ℇcc%) and ℇcc% in six mid LV segments. Cine images were analyzed for left ventricular ejection fraction (LVEF), LV mass (LVM), end-diastolic volume (EDV), end-systolic volume (ESV), LV atrioventricular plane displacement (LVAPD), and circumferential uniformity ratio estimate (CURE). LVM, EDV, and ESV were normalized to body surface area for a normalized index of LVM (LVMI), EDV (EDVI) and ESV (ESVI).
Results
At baseline, LV ℇcc% was significantly worse in DMD compared to controls and five of the six mid LV segments demonstrated abnormal strain in DMD. Longitudinal measurements revealed that ℇcc% consistently declined in individuals with DMD with the inferior segments being more affected. LVEF progressively declined between 3 to 5 years post baseline visit. In a multivariate analysis, the use of cardioprotective drugs trended towards positively impacting cardiac measures while loss of ambulation and baseline age were associated with negative impact. Eight out of 17 cardiac parameters reached a minimal clinically important difference with a threshold of 1/3 standard deviation.
Conclusion
The study shows a worsening of circumferential strain in dystrophic myocardium. The findings emphasize the significance of early and longitudinal assessment of cardiac function in DMD and identify early biomarkers of cardiac dysfunction to help design clinical trials to mitigate cardiac pathology. This study provides valuable non-invasive and non-contrast based natural history data of cardiac changes which can be used to design clinical trials or interpret the results of current trials aimed at mitigating the effects of decreased cardiac function in DMD.
Similar content being viewed by others
Background
Mutations in the dystrophin gene lead to complete absence or partial production of dystrophin protein, giving rise to Duchenne (DMD) and Becker (BMD) muscular dystrophy, respectively. Respiratory and cardiac failure are the leading causes of demise in the DMD patient population [1,2,3], and the number of patients presenting with end-stage heart failure has increased significantly over the last decade due to advancements in respiratory care [2,3,4,5,6].
Based on clinical observation, cardiac involvement in DMD is usually asymptomatic at early ages, and clinically appreciative manifestations present around the age of 10 years with ECG abnormalities and sinus tachycardia [2, 5, 7]. As the disease progresses, individuals develop symptoms of diastolic dysfunction which eventually gives way to systolic dysfunction and dilation of the chambers, thinning of the heart walls, and myocardial fibrosis [5, 7]. Myocardial damage at a cellular level precedes clinically significant cardiac dysfunction in DMD [2, 8, 9].
At present, there is no therapy available to reverse the course of cardiomyopathy in DMD, but various cell and viral vector-based therapies targeted towards cardiomyopathy in DMD are in clinical and preclinical trials [10,11,12,13]. While promising therapies are in the pipeline, there is still a scarcity of natural history data related to development and progression of cardiomyopathy in DMD in the presence of contemporary preventive care standards [14,15,16].
Traditionally, transthoracic echocardiography (TTE), ECG, and MRI have all been used for cardiac assessment in this patient population [17, 18]. Though these non-invasive modalities have identified the effect of disease on global volumetric cardiac function, they each have inherent limitations. TTE is sensitive to changes in cardiac function at a younger age, but with an increase in disease severity, there is a decrease in the acoustic window due to fat deposition and development of scoliosis, limiting its sensitivity [3, 19]. On the other hand, MRI with late gadolinium enhancement (LGE) is widely used to detect focal fibrosis in myocardial tissue but is unreliable in identifying diffuse myocardial fibrosis [4, 20, 21]. In addition, the gadolinium from the contrast agent has been reported to accumulate in organs other than the heart after repeated exposure, leading to potential toxicity [22,23,24]. Based on these limitations, there is a need to develop cardiac assessment tools that are non-invasive, safe, and sensitive to detect cardiac dysfunction at an early stage [2, 3, 19, 25, 26]. Various non-invasive techniques such as circumferential strain using tagged and feature tracking techniques, T1 mapping, and change in volumetric functions have been used to quantify disease progression in DMD [26,27,28,29].
Cardiac magnetic resonance imaging (CMR) using a myocardium tagging approach to calculate peak strain has been shown to be a sensitive measure to detect abnormalities in cardiac function and contractility in DMD. Peak strain can be defined as a measure of biomechanical distortion in tissue length with respect to resting length in the heart wall as it contracts [30, 31]. Studies in DMD have reported circumferential peak strain (ℇcc%) at the mid-ventricular level is a sensitive marker to examine cardiac involvement and the effect of therapeutic interventions on cardiac muscle [3, 27, 28, 32,33,34]. A major advantage of ℇcc% is its ability to detect changes at both global and regional levels of myocardium without use of any contrast [3, 28, 32].
The aim of this study was to evaluate the amount of change in left ventricular (LV) cardiac function and structure over a period of five years in ambulatory and non-ambulatory boys and young men with DMD without the use of a contrast agents. Additionally, the study investigated the value of measuring ℇcc% cross sectionally and longitudinally, in comparison to more traditional LV functional CMR outcomes. We hypothesized that ℇcc%, would be a more sensitive longitudinal marker of myocardial remodeling in people with DMD at both the global and segmental level than the more traditionally used LV functional measures. A secondary aim of this study was to determine if change in cardiac functional parameters in DMD is related to loss of ambulation, age, or the use of cardioprotective drugs.
Methods
Individuals with a confirmed diagnosis of DMD (based on the genetic report and/or muscle biopsy) and unaffected controls were recruited for participation in a prospective longitudinal cardiac MRI study at the University of Florida (UF) and a retrospective study (pretreatment baseline as a part of interventional clinical trial (NCT02964377)) at the University of California Davis (UCD). At UF. participants underwent a 30–45 min cardiac MRI exam at baseline on a Phllips 3T system, and they returned annually for follow-up cardiac MRI exams for up to 9 years. A separate set of retrospective data was used from data acquired at the UCD, and this cohort underwent a similar cardiac MRI protocol as participants at UF at baseline on a Siemens 3 T system. There were no follow-up visits for this cohort. Participants were defined as non-ambulatory if they were unable to complete the 10 m walk/run test independently within 45 s. The institutional review boards at the University of Florida and the University of California Davis approved the respective studies. Prior to participation, parents of each participant (if participant was younger than 18 years) provided written informed consent, and participants themselves gave written assent. For older participants informed consent was obtained from participants themselves.
MR acquisition
All cardiac MR images at UF were acquired using a 3.0 T whole-body scanner (Philips Achieva Quasar Dual 3T, Philips, Amsterdam, the Netherlands) with a 32-channel torso anterior–posterior cardiac coil. MR images were acquired using a segmented steady-state free precession (SSFP) technique with ECG gating and free breathing. Cinegraphic (cine) long axis two-chamber and four-chamber MR images were acquired from survey scans. Short-axis cine MR images were acquired from the four-chamber view for the left ventricle from base to apex (FOV = 250 × 250 × 91 mm3, TR/TE = 3.6/1.8 ms, slice thickness = 7 mm, minimum number of slices = 11, phases/slice = 25–40). An ECG-triggered fast field echo MR imaging sequence was used to acquire myocardial short-axis tagged images (tag spacing = 6–8 mm, FOV = 260 × 260 mm2, slice thickness = 7 mm, TR/TE = 5.5/3.3 ms, phases/segment = 14–18) at the left mid-ventricular level. The length of the ventricle (tip of the apex to mitral valve) was bisected to identify the mid-ventricular region (typically at the level of papillary muscles).
All cardiac MR images at UC Davis were acquired on a 3 Tesla Siemens scanner (TIM Trio, Siemens Healthineers, Erlangen, Germany). Cardiac functional imaging was performed using retrospective ECG gating with a segmented SSFP technique after localized shimming and/or frequency adjusting. Subjects performed breath holds after expiration. Following localization of two and four-chamber views, short-axis and four-chamber cine SSFP images were acquired for the left ventricle from cardiac base to apex (FOV = 256 × 192 mm2, slice thickness = 6–8 mm, gap = 20%, AVE = 1, TR/TE = 38/1.4 ms). A minimum of 12 slices were acquired with 30 phases/slice. ECG-triggered short axis grid tagged MR images were acquired at the mid-ventricle level. Grid tag spacing was 8 mm. The scan parameters used were: FOV = 192 × 154 mm2, slice thickness = 8 mm, flip angle = 10°, TR/TE = 61/2.4 ms, views per segment = 12.
MRI analysis
All image analyses were performed at UF by two analyzers using FDA-approved software packages to measure cardiac mass and function (Segment;Medviso; Sweden version 2.0 R5585) and Eulerian circumferential strain (Harmonic Phase; Virtue; version 5.4, Myocardial Solutions, Morrisville, NC). There was strong inter and intra-rater reliability for calculation of both strain (inter [r = 0.92], intra [r = 0.96]) and volumetric analysis (inter [r = 0.83], intra [r = 0.94]). Previous studies by Hor et al. [3] and Heiberg et al. [35] have shown the validation and reliability of both these software packages in calculating strain and volumetric functions, respectively. Of all the scans, one tagged image scan, six short-axis scans and eleven four-chambers could not be used because of poor image quality. The number of subjects reported in the tables represents the final subject numbers used for the analysis. Using the Segment software, the short-axis images with the greatest dilation and contraction of the LV cavity were defined as end diastole and systole, respectively. The endocardium and epicardium throughout diastole and systole were automatically segmented for all slices in which myocardium was visible during the cardiac cycle. Following automatic segmentation, myocardium outlines were manually adjusted to accurately delineate the endo and epicardium prior to final measure calculations. Measures obtained from this analysis include left ventricular mass (LVM), left ventricle ejection fraction (LVEF), left ventricle end systolic volume (LVESV), and left ventricle end diastolic volume (LVEDV). To account for short stature in DMD, volumetric functions and masses were normalized to body surface area (BSA) to calculate left ventricular mass index (LVMI), left ventricular end systolic volume index (LVESVI), and left ventricular end diastolic volume index (LVEDVI). BSA was calculated as described previously described [36, 37]. In a separate analysis, left ventricle atrioventricular plane displacement (LVAPD) was derived from the long axis two chamber and four chamber images (averaged together). A decline in LVAPD distance is associated with reduced LV function [38]. Defined anatomical landmarks (interventricular septum, LV lateral point, and apical point) were identified in each set of images, and the displacement of these points was recorded from diastole to systole to calculate LVAPD.
Left mid-ventricular peak and global Eulerian circumferential strain (εcc%) were calculated from the short-axis grid tagged images using the Harmonic Phase software (Osman et al. 1999; Virtue; version 5.4, Myocardial Solutions, Morrisville, NC) commonly used and reported in previous DMD studies [3, 39] The myocardial mesh, representing epicardium and endocardium, was manually drawn on top of tags at end diastole or near end diastole. The software automatically generated the myocardial mesh across frames, from diastole to systole, and manual adjustments were made as needed. Maximum displacement of tags as detected by mesh from diastole to systole for each of the six mid ventricular segments gave rise to peak circumferential strain for each segment. Mean peak composite ℇcc%, for the LV was defined as the average of the maximum ℇcc% (more negative) produced by six segments. Global ℇcc% was calculated by averaging ℇcc% produced by the six midventricular segments at systole.
To determine if disease progression leads to development of dyssynchrony in the heart, we calculated the circumferential uniformity ratio estimate (CURE) for the mid ventricular segment. CURE is a dyssynchrony index calculated on a scale of 0 to 1 from tagged images [40], with 1 being completely synchronous and 0 being completely dyssynchronous.
Contrast enhancement was not used so there was no attempt made to draw correlations related to myocardial fibrosis.
Statistical analysis
Statistical analyses were performed using Graph Pad Prism 7 (version 7.0d; GraphPad Software Inc., La Jolla, CA) for cross-sectional comparisons and R v3.5.2 (R Core Team 2018 with readxl and R2Jags packages) for longitudinal analyses [41,42,43]. For cross sectional comparison, all 59 subjects’ data was used while 47 individuals’ data acquired at UF was used for longitudinal analysis. Independent t-tests with Welch’s correction were used for all cross-sectional group comparisons. For longitudinal data analysis, a Bayesian linear model with first order autoregressive error was considered:
where
Here \(y_{i,t}\), \({\text{loa}}_{i,t}\) and \({\text{ace}}_{i,t}\) denote the values of a response variable (e.g.,Peak global strain, LVM etc.), indicator of loss of ambulation, and indicator of cardiac drug use, respectively, for the \(i\)-th individual at time \(t\); \({\text{age}}_{i}^{0}\) denotes the baseline age of the \(i\) th individual; \(\eta_{{{\text{loa}}}}\) and \(\eta_{{{\text{ace}}}}\) denote the probability of losing ambulation and probability of administering a cardiac drug respectively; \(\delta\), \(\gamma_{{{\text{loa}}}}\), \(\gamma_{{{\text{ace}}}}\) are the regression parameters associated with baseline age, loss of ambulation and ACE respectively, and \(\alpha_{t} - \alpha_{0}\) [\((\alpha_{t} =\) response at time t); (\(\alpha_{0} = {\text{response at baseline}}\)) measures the average difference between the response at time \(t\) and at the baseline. Independent vague proper priors for the model parameters were considered. More specifically, the prior for the parameters β, γloa, γace, δ, log σ 2 and αt, t ≥ 0 were taken to be independent, normal (0, 1002) distributions, and the prior for the parameters ηloa and ηace were taken to be independent, uniform (0, 1) distributions. Markov chain Monte Carlo (MCMC) samples from the resulting posterior distribution were generated using JAGS [44]. From these posterior samples, the posterior mean and a 95% credible interval (based on quantiles) were computed for each model parameter and for each of the differences αt − α0, t ≥ 1. A parameter or a parameter difference was considered to be significantly different from zero (with 95% probability) if the associated credible interval did not contain zero. A few subjects (n = 8) had data for more than five visits (up to nine visits) (Figs. 1, 4). Some of the follow ups were lost due to disease progression leading to limitation in travel for study visit or due to bad scan quality. For the longitudinal analysis, we used the data from all visits; however, intercept parameters after the fifth visits were not reported, and we focused on the (marginal) posterior distribution of parameters up to the first five visits. Under this analysis, ignorable missingness (missing at random) was assumed to be ignorable.
In this study, we also looked at the minimal clinically important difference (MCID) to determine if the change in cardiac parameters over 1 year are clinically relevant. We used 1/3 standard deviation at baseline as threshold as reported previously [45].
Results
Baseline demographics
Forty-seven individuals with DMD and 16 unaffected controls enrolled in the study at UF, and an additional 12 participants with DMD enrolled in the study at UC Davis. In the UF cohort, six participants were non-ambulatory at baseline, while nine subjects in the UC Davis cohort were non-ambulatory. Fifty-nine percent of participants took angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) at the start of the study (UF n = 28; UC Davis n = 7). Complete baseline demographics are reported in Table 1.
Baseline differences in cardiac function
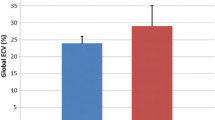
Peak ℇcc% was significantly different in individuals with DMD (− 17.4 ± 2.3%) compared to controls (− 19.6 ± 0.9%; p < 0.0001) (Fig. 2) as was global ℇcc% (DMD = − 16.6 ± 2.5%; controls = − 19.0 ± 1.1%; p < 0.0001) (Additional File 1). When examining strain in each of the six ventricular segments individually, strain was significantly worse in the anterior (p < 0.05), inferior septal (p < 0.05), inferior (p < 0.001), inferior lateral (p < 0.01), and anterior lateral (p < 0.05) segments (Fig. 3). Circumferential uniformity ratio estimate (CURE) was significantly lower in individuals with DMD (0.93 ± 0.03) compared to controls (0.95 ± 0.01; p < 0.001) indicating that the DMD heart is more dyssynchronous (Table 2).
Left ventricular ejection fraction (LVEF) at baseline was significantly lower in DMD compared to unaffected controls (p < 0.001) (Table 2). LVEDV was lower (p < 0.05) in DMD than controls, and there was a trend towards lower LVM (p = 0.07) and ESV (p = 0.06) (Table 2). After normalization to BSA, LVEDVI remained significantly reduced in DMD (p < 0.001). Though these measures were reduced in DMD, all values except for LVEDV and LVEDVI were still in the normal range, as reported in a recent study by van Der Ven et al. [46]. Along with these global cardiac measures of mass and function, we found LVAPD to be significantly reduced in DMD compared to unaffected controls (p < 0.0001) (Table 2). Baseline cardiac measures from just the UF cohort are shown in the supplemental data (Additional Files 4, 5 and 6).
To understand the relationship between of loss of ambulation (LOA) and cardiac function, we compared cardiac function between ambulatory (n = 44) and non-ambulatory (n = 15) DMD subjects at baseline. Of the cardiac parameters measured, strain [global (p = 0.11) and peak (p = 0.15)] were found to show a trend to be deteriorated in non-ambulatory individuals (Table 3) while CURE was significantly deteriorated (p < 0.05). LVM was significantly higher in non-ambulatory participants (p < 0.01), but this significance was lost when mass was normalized to BSA (Table 3). Also, LVEDVI (p < 0.01) and LVESVI (p < 0.05) were significantly lower in non-ambulatory individuals (Table 3).
Longitudinal changes in cardiac function
Both peak (Table 4, Fig. 4) and global ℇcc (%) (Additional Files 2 and 3) significantly worsened over the period of 5 years with an absolute change of 3.0% and 2.7%, respectively. Eighty percent of participants with DMD had global strain values considered hypokinetic (Additional Files 2 and 3) (above − 17%; as defined by HARP straining module), and strain showed worsening with age. Further, on examining the six mid-ventricular segments individually, inferior segments showed significant declines over 5 years with absolute changes of 3.2% in the inferoseptal segment, 4.7% in the inferior segment, and 3.8% in the inferolateral segment (Table 4).
When examining longitudinal changes in volumetric functions and mass, there was no significant change in LVESV, LVEDV, and LVM over 5 years (Table 5). LVEDVI showed a significant decline over 4 years after which it showed a trend towards increase from the 4th to 5th year. LVEF dropped significantly over 5 years with decrease in mean values of of 7.9% (Table 5). Along with these findings, we also found an 18% decline in LVAPD (Table 5). Numerical but nonsignificant decreases were seen for LVMI and CURE.
Effect of age, loss of ambulation, and medication on cardiac function
We examined the effect of covariates including baseline age, ambulatory status, and cardioprotective medication use (ACE inhibitor/ARB) on longitudinal changes in cardiac function. Table 6 reports the effect of the presence of each covariate on the average value of each cardiac parameter at a given point in time. Loss of ambulation (LOA) trended towards worsening of peak εcc(%), and year of age at baseline had a significant worsening effect. Cardioprotective medication showed a trend toward a protective improvement in peak εcc(%) and volumetric and mass parameters, and included significant improvements in LVM, ESV and EDV. An increase in age by 1 year was found to be a significant predictor of change in strain, LV mass, ESV and EDV volume. (Table 6).
Discussion
The main aim of this study was to examine longitudinal changes in cardiac function in DMD. To our knowledge, this is the first prospective cardiac MR study examining cardiac remodeling in a large cohort of individuals with DMD in a longitudinal fashion over a period of five years or more. We found strain to be a sensitive marker to quantify degree of cardiac pathology and its progression over five years in this patient population when most of the global cardiac functions were still relatively preserved and within normal ranges [46]. Circumferential strain was affected earlier than other cardiac parameters when comparing the DMD population to unaffected controls. Evaluating the effect of different covariates, cardioprotective medications in the form of ACE inhibitors/ARBs were found to have a positive impact on cardiac function, while an increase in age and LOA had a negative impact.
Our study provides valuable, longitudinal natural history data of changes in LV function in individuals with DMD in the context of standard of care clinical management. The data provides a valuable reference base for safety measures and evaluation of therapeutic interventions in a progressive disease using non contrast based imaging methods and will help with design and interpretation of clinical trials for DMD cardiomyopathy. Moreover, this study included young DMD boys prior to development of overt cardiomyopathy, which is critical based on the number of preclinical studies that have shown that early interventions may yield a better outcome [19, 47].
Strain and volumetric cardiac function decline in DMD
In our study, we found that volumetric cardiac function, strain, CURE, LVAPD, and LV mass were all different in DMD in comparison to the unaffected control group. We found a significant worsening (less negative) of both peak and global LV strain in DMD. We also found a significant difference in LVEF, LVEDV, and LVEDVI compared to controls at baseline. Though the values for these volumetric and mass parameters were lower than the unaffected population, they were still within the normal range except for LVEDV. Our results agree with previous cross sectional studies [27, 28, 32, 48] which have found composite circumferential strain on tagged and CINE images (using feature tracking) strain to be significantly worse in comparison to healthy controls and to be affected earlier in the disease process prior to structural remodeling [28, 49]. These findings emphasize the importance of measuring strain in this patient population at an early stage of the disease. A study by Khan et al. [50] and a previous study by Lee et al. [51] have reported that individuals with DMD develop cardiac atrophy as evidenced by reduced LVMI. Our findings report a similar trend in the decline in LVM and LVMI. However, these measures were not significantly different from that of controls, which could be attributed to the fact that our participants with DMD were relatively younger with better cardiac functions than those reported in the previous studies. Also, we found a significant decrease in LVEDV and LVEDVI in participants with DMD, and these values were out of normal range. We believe that this change in end diastolic volume can be due to smaller hearts and development of diastolic dysfunction. Further studies are required to understand the pathology and cause of reduced LVEDV out of normal range in this patient population.
To understand changes in the long axis of the heart and circumferential mechanical dyssynchrony, we examined LVAPD (a marker of systolic and diastolic dysfunction) and CURE, respectively. Compared to controls, participants with DMD had lower values for LVAPD, indicating reduced AV plane displacement. Different studies have used AV plane displacement as a marker of adverse cardiac events, and it was found to decline with disease progression and age [38, 52]. The decrease in AV displacement has been reported to be due to a reduction in contractility and relaxation of the heart [38, 52], leading to lower diastolic filling pressure and reduced diastolic volume. In our study, we found that both LVEDV and LVEDVI were significantly lower than controls while there was no significant decline in LV mass and LVESV. This can be due to development of stiffness in heart at an early stage, which in turn leads to less relaxation of heart and less filling pressure, resulting in reduced LVEDV.
In order to determine if all regions of the mid LV were affected to the same extent, we examined regional differences in circumferential strain over time in six mid ventricle segments as defined by the American Heart Association [53]. In comparison to controls, 5 out of 6 mid ventricle segments presented with worsened strain (less negative) in DMD. Our results are in agreement with a previous study by Hor et al. [28], where certain segments produced less negative strain in DMD before any evidence of gadolinium enhancement. The inferior lateral free wall was affected more and earlier than others, which is consistent with autopsy studies describing more fibrotic accumulation in the inferior wall segment [34, 54]. These findings emphasize the importance of measuring composite and regional strain in this patient population and indicate that despite normal LV volumetric function, pathological cardiac remodeling begins at an early age in this patient population supporting cardiac strain as an early biomarker for cardiac dysfunction in DMD.
Longitudinal changes in cardiac function
In this study we examined changes in cardiac function longitudinally for up to 9 years with statistical analysis over 5 years. To our knowledge, this is the first prospective study examining the changes in cardiac function/remodeling in DMD longitudinally over a period greater than 3 years. In our cohort, we found a consistent decrement in both global and peak composite strain over the 5 years. Along with composite strain, regional strain for inferior segments also showed a significant decline over this period. These findings emphasize that inferior free wall segments of the heart, which had significantly worse strain production in comparison to controls at baseline, are more affected as the disease progresses. This worsening of strain can be due to combination of damage in myocardium leading to cardiac remodeling due to disease pathology [3] and changes in heart hemodynamics which, in turn, can further worsen the disease pathology [55, 56].
Along with strain, we also observed a significant decline in EF over 5 years. The decrease in LVEF can be attributed to various factors, including a loss in heart contractility, dilation of the ventricular cavity, or lower pumping capacity of the heart due to a reduction in LVEDV. We believe this decline in LVEF is dependent on two factors. First, in the early stage, because of a decrease in contractility, there is the development of both systolic and diastolic dysfunction leading to lower LVEDVI (significant decline over 4 years). Second, in the later stage, along with previous abnormal changes, there is the development of dilated cardiomyopathy leading to an increase in LVEDV and, in turn, reduction in LVEF. Of note, although LVEF and other volumetric function show worsening over time, the values still fell within the normal range. On the other hand, strain values (both global and composite) were well below normal at baseline and declined over the 5 years.
This study also looked at the minimal clinically important difference (MCID) on all the cardiac parameters over 1 year. MCID helps us identify whether the deterioration in an outcome measure is clinically relevant for this population. Different studies have reported various methods to calculate MCID using standard error, standard deviation, or anchor-based methods. In this study, we used 1/3 SD at baseline as a threshold [45], and we found that 8 out of 17 cardiac parameters examined showed clinical relevance, including peak strain, global strain, LVAPD, CURE, regional segmental strain (anterior septal strain, inferior septal strain, inferior strain), and ejection fraction. But, when we used ½ SD as a threshold reported previously [57], we found only three parameters CURE, peak strain, and global strain showing the clinically relevant change. Based on these findings, other than strain, CURE was the only parameter which was found to be clinically meaningful at both the thresholds, indicating that this can be a promising tool to understand cardiac pathology along with strain in this patient population. Future studies need be performed to determine its clinical utility in larger cohorts spanning a wider age range.
Heterogeneity in longitudinal disease progression in DMD and the effect of covariates
One of the critical findings of this study was that we found significant heterogeneity among the subjects’ strain values (Fig. 4), with some subjects showing decrements in strain at an early age (less than 7 years of age) and others showing maintenance/improved strain (more negative) even at an older age. Various factors may contribute to this presentation such as presence of genetic modifiers [58, 59], type of mutation in the dystrophin gene [60], skeletal muscle involvement [61], and timing and role of cardioprotective medication [62, 63]. To understand some of these possible contributions, we examined the effect of loss of ambulation, age, and cardioprotective medication as covariates. When comparing ambulatory and non-ambulatory participants both cross sectionally and longitudinally, we found a trend towards worsening of strain values and significant changes in LV mass and volume which was associated with LOA. These findings indicate that non ambulatory subjects present with worse cardiac function and cardiac atrophy as is evident by decreases in LVMI, LVESVI and LVEDVI. Previously, a study conducted by Posner et al. [61] found a significant relationship between skeletal muscle function and cardiac output measures, but there was no cause and effect relationship. One of the confounding issues when comparing ambulatory and non-ambulatory participants across different centers can be ascribed to the difference in definition of LOA [58, 64, 65]. Indeed, if we use an alternative definition of LOA (unable to complete 10 m run/walk under 10 s) as initially defined in UC Davis Cohort (NCT02964377; 11/16/2016) three of the ambulatory participants with the worst cardiac function would be reassigned to the LOA group resulting in significant changes in all cardiac parameters with LOA. This discrepancy emphasizes the need to develop a consistent universal definition of LOA so that data can be interpreted and analyzed across different trials and studies.
In the relatively young cohort of our study, we also found a positive trend for the impact of ACE inhibitors/ARBs on cardiac function. In a study by Raman et al. [33], they also found that a combination of ACE inhibitors/ARBs and eplerenone attenuated decline in cardiac function in DMD. The ACE inhibitor/ARB with eplerenone group reported 1% change in peak strain which was found to be clinically significant; however, this effect might be short-lived as disease pathology eventually negates the positive cardiac remodeling caused by these drugs. A study by Wang et al. [66] and Packer [67] have reported similar findings where they found the effect of ACE inhibitors/ARBs to be transient in a DMD cohort and an adult heart pathology population.
Limitations
There were certain limitations associated with the study. The goal of the study was not to draw correlations to fibrosis, and as such, contrast was not used and no quantitative measurement of native T1 and T2 were obtained in this study. These measurements, in combination with diffusion, could be used to help interpret the strain findings in the context of myocellular and extramyocellular remodeling of the myocardium. It will be important for future studies utilizing quantitative contrast enhancement techniques to delineate the changes in extracellular volume, native T1, T2 , and their relationship to strain. The study did not acquire information about cardiac hemodynamics (including blood pressure) or ECG changes which could potentially help better understanding the relationship between change in strain and volumetric function with disease progression. Also, this study did not acquire any strain data for basal and apical segments which can help interpret changes in rotational mechanics of the heart (68). In this study, we examined the longitudinal effect of covariates including age, LOA, and cardioprotective medications. Unfortunately, we could not compare the trajectories of age-matched patients with cardioprotective medication to patients who are not receiving any drugs and ambulatory to non-ambulatory status because of the limited sample size. While we examined the effect of cardioprotective medication as a covariate on cardiac functions and structure, we were not able to determine the effect of changes in type of medication or drug dosage.
Conclusion
These findings emphasize the importance of circumferential strain as a sensitive measure to monitor disease progression in DMD as it was able to detect cardiac pathology at an early age with children as young as 5 years of age. We observed that decrements in strain occurred while other measures, such as ejection fraction, showed a decline but were still within the normal range. Decline in strain at both composite and regional levels over 1 year showed minimally clinically important differences with disease progression. The findings also highlight the heterogeneity in cardiac progression in this patient population, which is especially important in designing a clinical trial and understanding the response to therapeutic intervention. Further, these results reveal that cardiac involvement in DMD starts at a relatively early age, and support that the notion that cardiac evaluation and therapeutic intervention should start early.
Availability of data and materials
The datasets used and/or analyzed for this manuscript are available from the corresponding author (Dr. Glenn Walter) on reasonable request.
References
Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9(1):77–93.
McNally EM, Kaltman JR, Benson DW, Canter CE, Cripe LH, Duan D, et al. Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy. Circulation. 2015;131(18):1590–8.
Hor KN, Wansapura J, Markham LW, Mazur W, Cripe LH, Fleck R, et al. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: a cardiac magnetic resonance tagging study. J Am Coll Cardiol. 2009;53(14):1204–10.
Florian A, Ludwig A, Engelen M, Waltenberger J, Rösch S, Sechtem U, et al. Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J Cardiovasc Magn Reson. 2014;16:81.
Finsterer J, Cripe L. Treatment of dystrophin cardiomyopathies. Nat Rev Cardiol. 2014;11(3):168–79.
Ishikawa Y, Miura T, Ishikawa Y, Aoyagi T, Ogata H, Hamada S, et al. Duchenne muscular dystrophy: survival by cardio-respiratory interventions. Neuromuscul Disord. 2011;21(1):47–51.
van Westering TL, Betts CA, Wood MJ. Current understanding of molecular pathology and treatment of cardiomyopathy in duchenne muscular dystrophy. Molecules. 2015;20(5):8823–55.
Jung C, Martins AS, Niggli E, Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signalling and reactive oxygen species-generating pathways. Cardiovasc Res. 2007;77(4):766–73.
Khairallah M, Khairallah R, Young ME, Dyck JR, Petrof BJ, Des RC. Metabolic and signaling alterations in dystrophin-deficient hearts precede overt cardiomyopathy. J Mol Cell Cardiol. 2007;43(2):119–29.
Finanger E, Vandenborne K, Finkel RS, Lee Sweeney H, Tennekoon G, Yum S, et al. Phase 1 Study of Edasalonexent (CAT-1004), an Oral NF-kappaB Inhibitor, in Pediatric Patients with Duchenne Muscular Dystrophy. J Neuromuscul Dis. 2019;6(1):43–54.
Amoasii L, Hildyard JCW, Li H, Sanchez-Ortiz E, Mireault A, Caballero D, et al. Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science. 2018;362(6410):86–91.
Zhu P, Wu F, Mosenson J, Zhang H, He TC, Wu WS. CRISPR/Cas9-mediated genome editing corrects dystrophin mutation in skeletal muscle stem cells in a mouse model of muscle dystrophy. Mol Ther Nucleic Acids. 2017;7:31–41.
Echigoya Y, Nakamura A, Nagata T, Urasawa N, Lim KRQ, Trieu N, et al. Effects of systemic multiexon skipping with peptide-conjugated morpholinos in the heart of a dog model of Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2017;114(16):4213–8.
Quinlivan R, Messer B, Murphy P, Astin R, Mukherjee R, Khan J, et al. Adult north star network (ANSN): consensus guideline for the standard of care of adults with duchenne muscular dystrophy. J Neuromuscul Dis. 2021;8:899–926.
Lee S, Lee M, Hor KN. The role of imaging in characterizing the cardiac natural history of Duchenne muscular dystrophy. Pediatr Pulmonol. 2021;56(4):766–81.
Wittekind SG, Villa CR. Cardiac medication management in Duchenne muscular dystrophy. Pediatr Pulmonol. 2021;56(4):747–52.
Goldberg SJ, Stern LZ, Feldman L, Allen HD, Sahn DJ, Valdes-Cruz LM. Serial two-dimensional echocardiography in Duchenne muscular dystrophy. Neurology. 1982;32(10):1101–5.
de Kermadec JM, Bécane HM, Chénard A, Tertrain F, Weiss Y. Prevalence of left ventricular systolic dysfunction in Duchenne muscular dystrophy: an echocardiographic study. Am Heart J. 1994;127(3):618–23.
Verhaert D, Richards K, Rafael-Fortney JA, Raman SV. Cardiac involvement in patients with muscular dystrophies: magnetic resonance imaging phenotype and genotypic considerations. Circ Cardiovasc Imaging. 2011;4(1):67–76.
Guillaume MD, Phoon CK, Chun AJ, Srichai MB. Delayed enhancement cardiac magnetic resonance imaging in a patient with Duchenne muscular dystrophy. Tex Heart Inst J. 2008;35(3):367–8.
Salerno M. Seeing the unseen fibrosis in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2014;7(10):998–1000.
Perazella MA. Current status of gadolinium toxicity in patients with kidney disease. Clin J Am Soc Nephrol. 2009;4(2):461–9.
Olchowy C, Cebulski K, Łasecki M, Chaber R, Olchowy A, Kałwak K, et al. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity: a systematic review. PLoS ONE. 2017;12(2): e0171704.
Gale EM, Caravan P, Rao AG, McDonald RJ, Winfeld M, Fleck RJ, et al. Gadolinium-based contrast agents in pediatric magnetic resonance imaging. Pediatr Radiol. 2017;47(5):507–21.
Olivieri LJ, Kellman P, McCarter RJ, Cross RR, Hansen MS, Spurney CF. Native T1 values identify myocardial changes and stratify disease severity in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson. 2016;18(1):72.
Maforo NG, Magrath P, Moulin K, Shao J, Kim GH, Prosper A, et al. T1-Mapping and extracellular volume estimates in pediatric subjects with Duchenne muscular dystrophy and healthy controls at 3T. J Cardiovasc Magn Reson. 2020;22(1):85.
Siddiqui S, Alsaied T, Henson SE, Gandhi J, Patel P, Khoury P, et al. Left ventricular magnetic resonance imaging strain predicts the onset of Duchenne muscular dystrophy-associated cardiomyopathy. Circ Cardiovasc Imaging. 2020;13(11): e011526.
Hor KN, Kissoon N, Mazur W, Gupta R, Ittenbach RF, Al-Khalidi HR, et al. Regional circumferential strain is a biomarker for disease severity in duchenne muscular dystrophy heart disease: a cross-sectional study. Pediatr Cardiol. 2015;36(1):111–9.
Zhan-Qiu Liu NM, Dual S, Prosper A, Renella P, Halnon N, Wu H, Ennis D. Early non-contrast biomarkers of left ventricular cardiomyopathy in children with Duchenne muscualr dystrophy. Proceedings of International Society of Magnetic Resonance 2021.
Jeung MY, Germain P, Croisille P, Elghannudi S, Roy C, Gangi A. Myocardial tagging with MR imaging: overview of normal and pathologic findings. Radiographics. 2012;32(5):1381–98.
Merlocco A, Cross RR, Kellman P, Xue H, Olivieri L. Validation of cardiac magnetic-resonance-derived left ventricular strain measurements from free-breathing motion-corrected cine imaging. Pediatr Radiol. 2019;49(1):68–75.
Ashford MW, Liu W, Lin SJ, Abraszewski P, Caruthers SD, Connolly AM, et al. Occult cardiac contractile dysfunction in dystrophin-deficient children revealed by cardiac magnetic resonance strain imaging. Circulation. 2005;112(16):2462–7.
Raman SV, Hor KN, Mazur W, Halnon NJ, Kissel JT, He X, et al. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015;14(2):153–61.
Bilchick KC, Salerno M, Plitt D, Dori Y, Crawford TO, Drachman D, et al. Prevalence and distribution of regional scar in dysfunctional myocardial segments in Duchenne muscular dystrophy. J Cardiovasc Magn Reson. 2011;13:20.
Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment–freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10:1.
Batra A, Harrington A, Lott DJ, Willcocks R, Senesac CR, McGehee W, et al. Two-year longitudinal changes in lower limb strength and its relation to loss in function in a large cohort of patients with duchenne muscular dystrophy. Am J Phys Med Rehabil. 2018;97(10):734–40.
Mathur S, Lott DJ, Senesac C, Germain SA, Vohra RS, Sweeney HL, et al. Age-related differences in lower-limb muscle cross-sectional area and torque production in boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 2010;91(7):1051–8.
Alam M. The atrioventricular plane displacement as a means of evaluating left ventricular systolic function in acute myocardial infarction. Clin Cardiol. 1991;14(7):588–94.
Soslow JH, Xu M, Slaughter JC, Stanley M, Crum K, Markham LW, et al. Evaluation of echocardiographic measures of left ventricular function in patients with Duchenne muscular dystrophy: assessment of reproducibility and comparison to cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2016;29(10):983–91.
Delgado V, Bax JJ. Assessment of systolic dyssynchrony for cardiac resynchronization therapy is clinically useful. Circulation. 2011;123(6):640–55.
Team RDC. R: a language and environment for statistical computing. Vienna: R foundation for staitsical computing; 2018.
Wickham H, Jennifer Bryan. readxl:Read Excel Files. 2018.
Yu-Sung Su MY. Using R to Run 'JAGS'. 0.6 ed2015.
Plummer M. JAGS: a program for analysis of bayesian graphical models using gibbs sampling. 3rd International Workshop on Distributed Statistical Computing (DSC 2003); Vienna, Austria. 2003;124.
McDonald CM, Henricson EK, Abresch RT, Florence J, Eagle M, Gappmaier E, et al. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve. 2013;48(3):357–68.
van der Ven JPG, Sadighy Z, Valsangiacomo Buechel ER, Sarikouch S, Robbers-Visser D, Kellenberger CJ, et al. Multicentre reference values for cardiac magnetic resonance imaging derived ventricular size and function for children aged 0–18 years. Eur Heart J Cardiovasc Imaging. 2020;21(1):102–13.
Townsend D, Yasuda S, Chamberlain J, Metzger JM. Cardiac consequences to skeletal muscle-centric therapeutics for duchenne muscular dystrophy. Trends Cardiovasc Med. 2009;19(2):49–54.
Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, et al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC: Cardiovasc Imaging. 2010;3(2):144–51.
Hor KN, Wansapura J, Markham LW, Mazur W, Cripe LH, Fleck R, et al. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy. J Am Coll Cardiol. 2009;53(14):1204–10.
Khan S, Cheeran D, Garg S, Grodin J, Morlend R, Araj F, et al. Cardiac atrophy: a novel mechanism for duchenne muscular dystrophy (dmd)-associated cardiomyopathy. J Am Coll Cardiol. 2017;69(11 Supplement):946.
Lee TH, Eun LY, Choi JY, Kwon HE, Lee YM, Kim HD, et al. Myocardial atrophy in children with mitochondrial disease and Duchenne muscular dystrophy. Korean J Pediatr. 2014;57(5):232–9.
Seemann F, Pahlm U, Steding-Ehrenborg K, Ostenfeld E, Erlinge D, Dubois-Rande JL, et al. Time-resolved tracking of the atrioventricular plane displacement in Cardiovascular Magnetic Resonance (CMR) images. BMC Med Imaging. 2017;17(1):19.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002;18(1):539–42.
Ryan TD, Taylor MD, Mazur W, Cripe LH, Pratt J, King EC, et al. Abnormal circumferential strain is present in young Duchenne muscular dystrophy patients. Pediatr Cardiol. 2013;34(5):1159–65.
Liu H, Wang J, Pan Y, Ge Y, Guo Z, Zhao S. Early and quantitative assessment of myocardial deformation in essential hypertension patients by using cardiovascular magnetic resonance feature tracking. Sci Rep. 2020;10(1):3582.
Tadic M, Sala C, Carugo S, Mancia G, Grassi G, Cuspidi C. Myocardial strain in hypertension: a meta-analysis of two-dimensional speckle tracking echocardiographic studies. J Hypertens. 2021;39(10):2103–12.
Katz P, Morris A, Trupin L, Yazdany J, Yelin E. Disability in valued life activities among individuals with systemic lupus erythematosus. Arthritis Rheum. 2008;59(4):465–73.
Flanigan KM, Ceco E, Lamar KM, Kaminoh Y, Dunn DM, Mendell JR, et al. LTBP4 genotype predicts age of ambulatory loss in Duchenne muscular dystrophy. Ann Neurol. 2013;73(4):481–8.
Barp A, Bello L, Politano L, Melacini P, Calore C, Polo A, et al. Genetic modifiers of duchenne muscular dystrophy and dilated cardiomyopathy. PLoS ONE. 2015;10(10): e0141240.
Tandon A, Jefferies JL, Villa CR, Hor KN, Wong BL, Ware SM, et al. Dystrophin genotype-cardiac phenotype correlations in Duchenne and Becker muscular dystrophies using cardiac magnetic resonance imaging. Am J Cardiol. 2015;115(7):967–71.
Posner AD, Soslow JH, Burnette WB, Bian A, Shintani A, Sawyer DB, et al. The correlation of skeletal and cardiac muscle dysfunction in duchenne muscular dystrophy. J Neuromuscul Dis. 2016;3(1):91–9.
Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G, Becane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45(6):855–7.
Raman SV, Hor KN, Mazur W, He X, Kissel JT, Smart S, et al. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: results of a two-year open-label extension trial. Orphanet J Rare Dis. 2017;12(1):39.
Naarding KJ, Reyngoudt H, van Zwet EW, Hooijmans MT, Tian C, Rybalsky I, et al. MRI vastus lateralis fat fraction predicts loss of ambulation in Duchenne muscular dystrophy. Neurology. 2020;94(13):e1386–94.
Bello L, Gordish-Dressman H, Morgenroth LP, Henricson EK, Duong T, Hoffman EP, et al. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study. Neurology. 2015;85(12):1048–55.
Wang M, Birnkrant DJ, Super DM, Jacobs IB, Bahler RC. Progressive left ventricular dysfunction and long-term outcomes in patients with Duchenne muscular dystrophy receiving cardiopulmonary therapies. Open Heart. 2018;5(1): e000783.
Packer M. Love of Angiotensin-converting enzyme inhibitors in the time of cholera. JACC Heart Fail. 2016;4:403–8.
Reyhan ML, Wang Z, Kim HJ, Halnon NJ, Finn JP, Ennis DB. Effect of free-breathing on left ventricular rotational mechanics in healthy subjects and patients with duchenne muscular dystrophy. Magn Reson Med. 2016;77:864–9.
Acknowledgements
We especially thank Dr. Steve Chrzanowski for his help in performing reliability analysis of data. Gerard Sonico, Erica Goude, Alina Nicorici for their help in collecting data at UC Davis site
Funding
The study was funded by Paul D. Wellstone Muscular Dystrophy Cooperative Research Center Grant (NIAMS: U54AR052646,P50AR052646). A. Batra was supported by Paul D. Wellstone Muscular Dystrophy Cooperative Research Center Grant (NIAMS: U54AR052646). AM Barnard was also supported by NIAMS: U54AR052646 during the conduct of this study as well as by T32HL134621 from the National Heart, Lung, and Blood Institute.
Author information
Authors and Affiliations
Contributions
AB: Contributed to the study design, data collection, analysis and interpretation, drafted, edited, revised and approved final version of the manuscript. AlB: Contributed to the study design, data collection, interpretation, drafted, edited, revised and approved final version of the manuscript. DL, RW, SF, EH, JD, CS, WT, JA: Contributed to the study design, data collection, edited, revised and approved final version of the manuscript. SC, MD: Contributed to statistical analysis, edited, revised and approved final version of the manuscript. LS, BB, CM: Contributed to the study design, data collection, interpretation, drafted, edited, revised and approved final version of the manuscript. GW, KV: Conceived and designed research, edited, revised and approved final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the institutional review boards at the University of Florida and the University of California Davis. The study was conducted in accordance with the Helsinki Declaration. Prior to participation, parents of each participant (if participant is below 18 years) provided written informed consent, and participants themselves gave written assent. For older participants informed consent was obtained from participants themselves.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Global mid ventricular strain (εcc %) in unaffected controls (n=15) and individuals with DMD (n=58) at baseline.
Additional file 2:
Longitudinal changes in global strain in DMD. Solid line for global strain was defined based on normal zone cut off of -17% as given by HARP software. Red lines indicates subjects with more than 5 years data, filled triangles represent unaffected controls.
Additional file 3:
Longitudinal change in global strain for mid ventricle of DMD.
Additional file 4:
Peak and global mid ventricular strain (εcc %) in unaffected controls (n=15) and individuals with DMD (n=46) at baseline (UF Cohort).
Additional file 5:
Strain for each LV segment in controls and individuals with DMD (n=46) at baseline (UF cohort).
Additional file 6:
Comparison of cardiac function in control and DMD subjects at baseline (UF Cohort).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Batra, A., Barnard, A.M., Lott, D.J. et al. Longitudinal changes in cardiac function in Duchenne muscular dystrophy population as measured by magnetic resonance imaging. BMC Cardiovasc Disord 22, 260 (2022). https://doi.org/10.1186/s12872-022-02688-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02688-5