Abstract
Background
To investigate the impact of hyperoxia that developed immediately after extracorporeal membrane oxygenation (ECMO)-assisted cardiopulmonary resuscitation (ECPR) on patients’ short-term neurological outcomes after out-of-hospital cardiac arrest (OHCA).
Methods
This study retrospectively analyzed data from the Japanese OHCA registry from June 2014 to December 2017. We analyzed adult patients (≥ 18 years) who had undergone ECPR. Eligible patients were divided into the following three groups based on their initial partial pressure of oxygen in arterial blood (PaO2) levels after ECMO pump-on: normoxia group, PaO2 ≤ 200 mm Hg; moderate hyperoxia group, 200 mm Hg < PaO2 ≤ 400 mm Hg; and extreme hyperoxia group, PaO2 > 400 mm Hg. The primary and secondary outcomes were 30-day favorable neurological outcomes. Logistic regression statistical analysis model of 30-day favorable neurological outcomes was performed after adjusting for multiple propensity scores calculated using pre-ECPR covariates and for confounding factors post-ECPR.
Results
Of the 34,754 patients with OHCA enrolled in the registry, 847 were included. The median PaO2 level was 300 mm Hg (interquartile range: 148–427 mm Hg). Among the eligible patients, 277, 313, and 257 were categorized as normoxic, moderately hyperoxic, and extremely hyperoxic, respectively. Moderate hyperoxia was not significantly associated with 30-day neurologically favorable outcomes compared with normoxia as a reference (adjusted odds ratio, 0.86; 95% confidence interval: 0.55–1.35; p = 0.51). However, extreme hyperoxia was associated with less 30-day neurologically favorable outcomes when compared with normoxia (adjusted odds ratio, 0.48; 95% confidence interval: 0.29–0.82; p = 0.007).
Conclusions
For patients with OHCA who received ECPR, extreme hyperoxia (PaO2 > 400 mm Hg) was associated with 30-day poor neurological outcomes. Avoidance of extreme hyperoxia may improve neurological outcomes in patients with OHCA treated with ECPR.
Similar content being viewed by others
Background
Out-of-hospital cardiac arrest (OHCA) is characterized by the loss of cardiac function and the absence of systemic circulation. The American Heart Association reports that the survival rate of patients with OHCA at hospital discharge is approximately 10%, which remains low despite advances in cardiopulmonary resuscitation and post-cardiac arrest syndrome management [1].
Extracorporeal membrane oxygenation (ECMO)-assisted cardiopulmonary resuscitation (ECPR) is the application of venoarterial extracorporeal membrane oxygenation in patients whose cardiac arrest is refractory to conventional cardiopulmonary resuscitation [2]. ECPR has been shown to improve clinical outcomes for patients with OHCA [3,4,5]. The main purpose of ECPR is to restore blood circulation and gas exchange. ECMO provides time for cardiopulmonary interventions that are necessary to obtain adequate spontaneous circulation, including percutaneous coronary intervention, pulmonary thrombectomy, and rewarming.
Existing literature on the potential use of ECMO reports that hyperoxia contributes to the deterioration of patients with post-cardiac arrest syndrome (PCAS) [6]. Therefore, the latest guidelines recommend avoiding hyperoxia after the return of spontaneous circulation [7, 8]. In ECPR, supraphysiological levels of oxygenation are created by the fraction of oxygen in the sweep gas (FDO2), which can lead to the exacerbation of PCAS. However, clinical studies evaluating hyperoxia associated with ECPR are limited [9,10,11]. Therefore, the influence of hyperoxia on neurological outcomes and mortality in patients with OHCA undergoing ECPR remains unclear.
This study aimed to investigate the relationship between hyperoxia and short-term neurological outcomes in adult patients who underwent ECPR. We focused on the partial pressure of oxygen in arterial blood (PaO2) levels in the early phases after ECMO pump-on.
Methods
Study design and setting
This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology guideline (Additional file 1). The current study was a retrospective analysis of data from the Japanese Association for Acute Medicine Out-of-Hospital Cardiac Arrest (JAAM-OHCA) registry that were collected between June 2014 and December 2017. This registry provides for the nationwide, multicenter, prospectively focused collection of pre-hospital and in-hospital data from patients with OHCA in Japan [12]. The registry included all OHCA patients who were transported to participating institutions. Pre-hospital data were obtained from the All-Japan Utstein Registry of the Fire and Disaster Management Agency, as previously reported [13]. In-hospital data were collected via an internet-based system by physicians or medical staff at each institution. The JAAM-OHCA registry committee integrated pre- and in-hospital data, as previously described [14].
Participants
In this study, we included adult patients (age ≥ 18 years) in the registry who were introduced to ECMO during cardiac arrest in the emergency room. Patients for whom no PaO2 data were available after ECMO initiation were excluded. In addition, we excluded patients who experienced hypoxia (initial measurement of PaO2 < 60 mm Hg) after the start of ECMO.
Data collection
Patient demographics and pre-hospital factors were extracted from the JAAM-OHCA registry. The data was segmented as follows: age, sex, witness status (emergency medical service personnel or others), presence of a bystander who performed cardiopulmonary resuscitation, etiology of cardiac arrest (cardiac or non-cardiac), initial cardiac rhythm, pre-hospital adrenaline administration, pre-hospital airway management, pre-hospital shock delivery, and response time (time from call to scene arrival, time from scene to hospital arrival). In addition, in-hospital factors and outcomes were extracted as follows: cardiac rhythm on arrival, in-hospital shock delivery, in-hospital adrenaline administration, antiarrhythmic drug administration, transient return of spontaneous circulation before ECMO pump-on, time from hospital arrival to ECMO pump-on, time from hospital arrival to initial blood gas analysis after ECMO pump-on, initial blood gas analysis data (pH, PaO2, partial pressure of arterial carbon dioxide [PaCO2], bicarbonate ion concentration, lactate level) after ECMO pump-on, intra-aortic balloon pump use, percutaneous coronary intervention, targeted temperature management, and cerebral performance category 30 days after cardiac arrest.
Exposure and definition
We divided the eligible patients into three groups according to their initial PaO2 levels after ECMO pump-on. The three groups were as follows: normoxia group, 60 mm Hg ≤ PaO2 ≤ 200 mm Hg; moderate hyperoxia group, 200 mm Hg < PaO2 ≤ 400 mm Hg; and extreme hyperoxia group, PaO2 > 400 mm Hg.
Outcome measures
Outcomes were assessed by emergency physicians at participating hospitals 30 days after cardiac arrest. The primary outcome was a 30-day neurologically favorable outcome after cardiac arrest. A neurologically favorable outcome was defined as a cerebral performance category of 1 or 2. The cerebral performance categories included the following five outcomes: (1) good cerebral recovery, (2) moderate cerebral disability, (3) severe cerebral disability, (4) coma or vegetative state, and (5) death or brain death [15]. The secondary outcome was 30-day survival after cardiac arrest.
Statistical analyses
Descriptive statistics were calculated for all variables of interest. Continuous variables are reported as medians and interquartile ranges (IQRs), while categorical variables are summarized using counts and percentages. Categorical variables in the three groups were analyzed using the Chi-square test, and continuous variables were analyzed using the Kruskal–Wallis test. Cubic splines were used to examine the potential nonlinear effects of PaO2 levels on 30-day neurologically favorable outcomes.
We used multiple imputations to compensate for missing data, and ten imputed datasets were generated [16]. Univariate logistic regression analysis was performed to calculate the crude odds ratio (OR) of the PaO2 level group for 30-day favorable neurological outcomes or 30-day survival after cardiac arrest.
Subsequently, we performed multiple propensity score analysis in the multivariate analysis in order to adjust and control for multiple independent variables [17]. A multiple propensity score is a conditional probability of patients being categorized into three or more groups given baseline covariates. Multiple propensity score analysis was applied to compare three or more groups [18]. First, we performed a multinomial logistic regression analysis by setting one of the three PaO2 groups as the dependent variable. The following covariates were used to calculate the multiple propensity scores: age, sex, witness, bystander administration of cardiopulmonary resuscitation, initial cardiac rhythm, pre-hospital shock delivery, pre-hospital adrenaline administration, pre-hospital advanced airway management, time from call to scene, time from scene to hospital arrival, etiology, cardiac rhythm on arrival, transient return of spontaneous circulation before ECMO pump-on, time from hospital arrival to ECMO pump-on, and time from hospital arrival to blood gas analysis.
Second, we performed a binomial logistic regression analysis to determine the adjusted ORs of the PaO2 level group for 30-day favorable neurological outcomes or 30-day survival after cardiac arrest, adjusting for multiple propensity scores and in-hospital variables, including PaCO2, bicarbonate ion concentration, lactate level, intra-aortic balloon pump use, percutaneous coronary intervention, and targeted temperature management.
For robustness, we performed the sensitivity analysis (multivariate logistic regression analysis for 1-month neurological favorable outcomes and 1-month survival after cardiac arrest) using data of the cases with all characteristics recorded (complete case analysis).
ORs and 95% confidence intervals (CIs) were calculated. All statistical tests were two-sided, and a p value of < 0.05 was considered significant. All statistical analyses were conducted using R 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS 24.0 for Mac (IBM Corp., Armonk, NY, USA).
Results
Patient enrollment
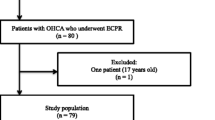
During the study period, 34,754 patients with OHCA were enrolled in the JAAM-OHCA registry. Of these, 1,442 patients underwent ECPR. After excluding 113 patients with PaO2 < 60 mm Hg and 462 for missing blood gas analysis, 847 were finally included in the study (Fig. 1).
Patient characteristics and outcomes
The demographic, pre-hospital, and in-hospital characteristics of patients are shown in Table 1. The median age was 62 years (IQR, 49–69 years), and 79.1% of patients were male. The outcome variables are listed in Table 2. Of the total patients, 280 (33.1%) survived for 30 days after OHCA, and 140 (16.5%) had favorable 30-day neurological outcomes.
PaO2 distribution, grouping, and nonlinear association with neurological outcomes
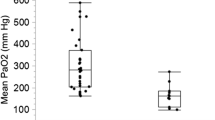
The median PaO2 level was 300 mm Hg (IQR, 148–427 mm Hg), and the distribution of PaO2 levels is shown in Fig. 1. A total of 277, 313, and 257 patients were categorized as normoxic, moderately hyperoxic, and extremely hyperoxic, respectively.
The cubic spline curve demonstrated a nonlinear association between PaO2 levels and log (odds). This means that the odds of a favorable neurological outcome were > 1.0 when log (odds) was > 0 (Fig. 2).
Nonlinear relationship between the logarithm of odds for 30-day favorable neurological outcome according to partial pressure of arterial oxygen levels. The solid line indicates the cubic spline curve of the logarithm of odds. Dotted lines indicate the 95% confidence interval. PaO2, partial pressure of arterial oxygen
Logistic regression analysis
The results of univariate and multivariate logistic regression analyses for neurological outcome at 30 days and survival at 30 days are shown in Table 3. Moderate hyperoxia was not significantly associated with neurological outcome compared with normoxia as a reference (crude OR, 0.92; 95% CI 0.61–1.39; p = 0.69), while extreme hyperoxia was significantly associated with deterioration in neurological outcome compared with normoxia (crude OR, 0.53; 95% CI 0.32–0.86; p = 0.010).
Even after adjusting for the covariates after ECMO and the multiple propensity scores calculated from factors before ECMO, moderate hyperoxia was not significantly associated with poor neurological outcome compared with normoxia as a reference (adjusted OR, 0.86; 95% CI 0.55–1.35; p = 0.51). Contrariwise, extreme hyperoxia was significantly associated with less neurologically favorable outcomes compared with normoxia (adjusted OR, 0.48; 95% CI 0.29–0.82; p = 0.007).
Univariate and multivariate analyses of 30-day survival also showed that extreme hyperoxia was significantly associated with mortality compared with normoxia (adjusted OR, 0.66; 95% CI: 0.44–1.00; p = 0.048).
Complete case analysis as a sensitivity analysis
In the complete case analysis, a total of 663 cases were analyzed, after 184 cases were excluded due to missing data. Moderate hyperoxia was not significantly associated with neurological outcome compared with normoxia as a reference (adjusted OR, 0.92; 95% CI 0.53–1.58; p = 0.75) (Additional file 2: Table S1). Extreme hyperoxia was also not significantly associated with neurologically favorable outcomes compared with normoxia (adjusted OR, 0.64; 95% CI 0.36–1.13; p = 0.13).
Discussion
Main findings
We investigated the relationship between hyperoxia and short-term neurological outcomes in adult patients who underwent ECPR, focusing on PaO2 levels in the early phases after ECMO. Two-thirds of patients undergoing ECPR were exposed to moderate or greater hyperoxia (PaO2 > 200 mm Hg). There was a nonlinear relationship between PaO2 levels in the early phase after ECMO initiation and neurological outcome 30 days after cardiac arrest in patients with OHCA who underwent ECPR. Extreme hyperoxia (PaO2 > 400 mm Hg) was significantly associated with worse neurological outcomes and higher mortality at 30 days after adjusting for multiple confounders.
Sensitivity analysis showed that hyperoxia tended to be associated with a worse neurological prognosis; however, it could not demonstrate significance. This might have been due to the reduced power, as 22% of the cases were excluded due to missing values.
Effect of hyperoxia in patients with cardiac arrest
Dissolved oxygen that accumulates in the arteries is thought to have adverse effects through a complex combination of mechanisms, including excessive production of reactive oxygen species, pulmonary toxicity, and cardiac and neurological effects [19]. The toxicity of reactive oxygen species is the result of lipid peroxidation, protein oxidation, and DNA damage. When lipid peroxidation affects intracellular or extracellular membranes, it causes enzyme inactivation, thiol oxidation, and inhibition of the mitochondrial respiratory chain [20]. The oxidation of proteins results in resistance to proteolysis by aggregation [21]. The toxic effect of reactive oxygen species on DNA is dominated by cell cycle alterations, apoptosis, and carcinogenesis [22].
Animal studies have shown adverse effects of hyperoxia after cardiac arrest, including increased cell death by apoptosis and cytokine production [23, 24]. Many observational studies and meta-analyses have shown that hyperoxia after cardiac arrest is associated with poor neurological outcomes and increased mortality in post-cardiac arrest patients [25,26,27,28]. However, several controversial studies have reported no association between hyperoxia and clinical outcomes [23, 29, 30]. Two randomized controlled trials are currently ongoing to determine the appropriate oxygenation threshold in post-cardiac arrest patients (NCT03138005 and NCT03141099) [31, 32].
Effect of hyperoxia in patients undergoing ECPR
Oxygenation at supraphysiological levels is common during venoarterial ECMO, including ECPR, which may result in extreme hyperoxia, depending on the FDO2 setting of the sweep gas [11]. Therefore, cardiac arrest patients undergoing ECPR are at higher risk of hyperoxia than those only receiving conventional CPR. Two-thirds of patients undergoing ECPR in this study were exposed to moderate or greater hyperoxia (Fig. 1).
Previous single-center and multicenter studies have reported a relationship between exposure to hyperoxia and poor clinical outcomes (mortality and impaired neurological status) in patients with cardiac arrest undergoing ECPR [9,10,11, 33, 34]. The results of these studies are consistent with those of the present study. However, confounding factors were not adjusted and controlled for. This was due to the small number of patients in the previous studies.
Clinical applications and strength of the present study
In this study, we focused on blood gas levels during the initial period after the start of ECMO. Avoiding early extreme hyperoxia may improve outcomes in patients undergoing ECPR. Therefore, it may be necessary to adjust the FDO2 of the ECMO sweep gas immediately after the ECMO pump is turned on, to maximize the effect of ECPR on improving clinical outcomes.
Our study had several strengths. First, it included a larger sample size than that available in previous studies that assessed hyperoxia in ECPR (9–11, 32). Second, with the benefit of a large sample size and multiple propensity scores, we were able to evaluate the impact of hyperoxia on clinical outcomes after controlling for various confounders. Third, the effect of immortal bias was minimal because we evaluated blood gas data early after the start of ECMO (median time, 1 h after patient presentation).
Study limitations
This study had several limitations. First, the ECPR protocols in each participating institution were standardized. Differences in practices among the facilities may have skewed the results, even after adjusting for various confounders. Second, several clinical outcomes, including ECMO duration and length of hospital and intensive care unit stay, were not registered in the JAAM-OHCA registry. Therefore, the effect of hyperoxia on these outcomes remains unclear. Third, two-thirds of patients who underwent ECPR were excluded because of missing blood gas data in the present study. The missing data may have distorted the results even though our study was strengthened by a large sample size. Fourth, we did not have data regarding cardiac function. During ECPR, peripheral venoarterial ECMO is usually used [35]. A watershed zone known as Harlequin syndrome (North–South syndrome) develops because the retrograde flow from ECMO mixes with the antegrade blood flow from the patient’s own heart [36]. To avoid the influence of the North–South syndrome, we excluded patients with hypoxia. However, PaO2 may be affected by the patient’s cardiac function. Similarly, details of cardiac treatment, such as percutaneous coronary intervention, have not been evaluated. Fifth, we chose PaO2 cutoff values of 200 mm Hg and 400 mm Hg in this study. The cutoff value of 200 mm Hg was chosen because it had been used in previous studies. However, the 400 mm Hg cutoff value was arbitrarily selected before analysis [9, 34] and may not have been appropriate. Furthermore, we have not been able to evaluate the exposure duration of hyperoxia. In view of the limitations of this study, future well-designed studies are required.
Conclusions
Extreme hyperoxia immediately after ECMO was associated with deterioration of short-term neurological outcomes and survival in patients with OHCA who underwent ECPR. Avoidance of extreme hyperoxia may improve neurological outcomes in patients with OHCA treated with ECPR. Further studies are required to determine the optimal PaO2 treatment level.
Availability of data and materials
The use of JAAM-OHCA registry data is limited to members of the Japanese Assciation of Acute Medicine, and permission must be obtained from the society [12].
Abbreviations
- CI:
-
Confidence interval
- ECMO:
-
Extracorporeal membrane oxygenation
- ECPR:
-
Extracorporeal membrane oxygenation-assisted cardiopulmonary resuscitation
- FDO2 :
-
Fraction of oxygen in the sweep gas
- JAAM:
-
Japanese Association for Acute Medicine
- OHCA:
-
Out-of-hospital cardiac arrest
- OR:
-
Odds ratio
- PaO2 :
-
Partial pressure of oxygen in arterial blood
- PCAS:
-
Post-Cardiac Arrest Syndrome
References
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke Statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–492.
Soar J, Maconochie I, Wyckoff MH, Olasveengen TM, Singletary EM, Greif R, et al. 2019 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2019;145:95–150.
Maekawa K, Tanno K, Hase M, Mori K, Asai Y. Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: a propensity-matched study and predictor analysis. Crit Care Med. 2013;41:1186–96.
Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554–61.
Sakamoto T, Morimura N, Nagao K, Asai Y, Yokota H, Nara S, et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85:762–8.
Roberts BW, Kilgannon JH, Hunter BR, Puskarich MA, Pierce L, Donnino M, et al. Association between early hyperoxia exposure after resuscitation from cardiac arrest and neurological disability: prospective multicenter protocol-directed cohort study. Circulation. 2018;137:2114–24.
Berg KM, Soar J, Andersen LW, Böttiger BW, Cacciola S, Callaway CW, et al. Adult advanced life support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2020;142:S92–139.
Nolan JP, Sandroni C, Böttiger BW, Cariou A, Cronberg T, Friberg H, et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Resuscitation. 2021;161:220–69.
Chang WT, Wang CH, Lai CH, Yu HY, Chou NK, Wang CH, et al. Optimal arterial blood oxygen tension in the early postresuscitation phase of extracorporeal cardiopulmonary resuscitation: a 15-year retrospective observational study. Crit Care Med. 2019;47:1549–56.
Halter M, Jouffroy R, Saade A, Philippe P, Carli P, Vivien B. Association between hyperoxemia and mortality in patients treated by ECPR after out-of-hospital cardiac arrest. Am J Emerg Med. 2020;38:900–5.
Munshi L, Kiss A, Cypel M, Keshavjee S, Ferguson ND, Fan E. Oxygen thresholds and mortality during extracorporeal life support in adult patients. Crit Care Med. 2017;45:1997–2005.
JAAM-OHCA registry. http://www.jaamohca-web.com/. Accessed 11 Feb 2022.
Kitamura T, Iwami T, Kawamura T, Nagao K, Tanaka H, Hiraide A, et al. Nationwide public-access defibrillation in Japan. N Engl J Med. 2010;362:994–1004.
Kitamura T, Iwami T, Atsumi T, Endo T, Kanna T, Kuroda Y, et al. The profile of Japanese Association for Acute Medicine: out-of-hospital cardiac arrest registry in 2014–2015. Acute Med Surg. 2018;5:249–58.
Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein resuscitation registry templates for out-of-hospital cardiac arrest: a statement for healthcare professionals from a Task Force of the International liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, resuscitation Council of Southern Africa, resuscitation Council of Asia); and the American Heart Association emergency cardiovascular care committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation. 2015;132:1286–300.
Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393.
Feng P, Zhou XH, Zou QM, Fan MY, Li XS. Generalized propensity score for estimating the average treatment effect of multiple treatments. Stat Med. 2012;31:681–97.
Bangalore S, Pencina MJ, Kleiman NS, Cohen DJ. Prognostic implications of procedural vs spontaneous myocardial infarction: results from the Evaluation of Drug Eluting Stents and ischemic Events (EVENT) registry. Am Heart J. 2013;166:1027–34.
Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:599–602.
Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009;47:469–84.
Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–6.
Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. 2013;13:349–61.
Bellomo R, Bailey M, Eastwood GM, Nichol A, Pilcher D, Hart GK, et al. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15:R90.
Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303:2165–71.
Wang HE, Prince DK, Drennan IR, Grunau B, Carlbom DJ, Johnson N, et al. Post-resuscitation arterial oxygen and carbon dioxide and outcomes after out-of-hospital cardiac arrest. Resuscitation. 2017;120:113–8.
Young PJ, Bailey M, Bellomo R, Bernard S, Bray J, Jakkula P, et al. Conservative or liberal oxygen therapy in adults after cardiac arrest: an individual-level patient data meta-analysis of randomised controlled trials. Resuscitation. 2020;157:15–22.
Youn CS, Park KN, Kim SH, Lee BK, Oh SH, Jeung KW, et al. The cumulative partial pressure of arterial oxygen is associated with neurological outcomes after cardiac arrest treated with targeted temperature management. Crit Care Med. 2018;46:e279–85.
Patel JK, Kataya A, Parikh PB. Association between intra- and post-arrest hyperoxia on mortality in adults with cardiac arrest: a systematic review and meta-analysis. Resuscitation. 2018;127:83–8.
Peluso L, Belloni I, Calabró L, Dell’Anna AM, Nobile L, Creteur J, et al. Oxygen and carbon dioxide levels in patients after cardiac arrest. Resuscitation. 2020;150:1–7.
Jakkula P, Reinikainen M, Hästbacka J, Loisa P, Tiainen M, Pettilä V, et al. Targeting two different levels of both arterial carbon dioxide and arterial oxygen after cardiac arrest and resuscitation: a randomised pilot trial. Intensive Care Med. 2018;44:2112–21.
U.S. National Library of Medicine. Blood pressure and OXygenation targets after OHCA. https://www.clinicaltrials.gov/ct2/show/NCT03141099. Accessed 11 Feb 2022.
U.S. National Library of Medicine. Reduction of oxygen after cardiac arrest. https://clinicaltrials.gov/ct2/show/NCT03138005. Accessed 11 Feb 2022.
Al-Kawaz MN, Canner J, Caturegli G, Kannapadi N, Balucani C, Shelley L, et al. Duration of hyperoxia and neurologic outcomes in patients undergoing extracorporeal membrane oxygenation. Crit Care Med. 2021;49:e968–77.
Mckenzie N, Finn J, Dobb G, Bailey P, Arendts G, Celenza A, et al. Non-linear association between arterial oxygen tension and survival after out-of-hospital cardiac arrest: a multicentre observational study. Resuscitation. 2021;158:130–8.
Swol J, Belohlávek J, Brodie D, Bellezzo J, Weingart SD, Shinar Z, et al. Extracorporeal life support in the emergency department: A narrative review for the emergency physician. Resuscitation. 2018;133:108–17.
Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ Heart Fail. 2018;11:e004905.
Acknowledgements
We would like to thank Editage (www.editage.com) for their writing support.
Funding
No funding was received regarding this study.
Author information
Authors and Affiliations
Contributions
MK contributed to the study conception and design, data acquisition, statistical analysis and interpretation, and drafting of the manuscript. HY, YK, KT, MN, KH, HT, and TM contributed to the conception and design of the study and data interpretation. All authors critically revised the manuscript for important intellectual content, provided intellectual input to the study and manuscript, and read and approved the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The registry protocol was approved by the Institutional Review Board of each participating hospital. The list of participating hospitals and approval numbers for the Institutional Review Board of each institution are provided in Additional file 3. Informed consent was waived because of the observational study design that posed minimal risk to patients and preserved their anonymity. An opportunity to opt out from the registry was provided for patients and their respective families.
Consent for publication
Not applicable. No individual patient data will be reported.
Competing interests
Masahiro Kashiura, Hideto Yasuda, Yuki Kishihara, Keiichiro Tominaga, Masaaki Nishihara, Ken-ichi Hiasa, and Takashi Moriya each declare that they have no conflict of interest. Hiroyuki Tsutsui reports grants from Daiichi Sankyo, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, IQVIA Services Japan, Omron Healthcare, and MEDINET; personal fees from Daiichi Sankyo, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, AstraZeneca, Ono Pharmaceutical, Otsuka Pharmaceutical, Novartis Pharma, Bayer Yakuhin, Pfizer Japan, Bristol-Myers Squibb, Kowa, and Nippon Rinsho outside the submitted work. Hiroyuki Tsutsui is the President of the Japanese Heart Failure Society.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
STROBE statement checklist.
Additional file 2:
Complete case analysis as a sensitivity analysis.
Additional file 3:
The list of participating hospitals and approval numbers for the Institutional Review Board of each institution.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kashiura, M., Yasuda, H., Kishihara, Y. et al. Association between short-term neurological outcomes and extreme hyperoxia in patients with out-of-hospital cardiac arrest who underwent extracorporeal cardiopulmonary resuscitation: a retrospective observational study from a multicenter registry. BMC Cardiovasc Disord 22, 163 (2022). https://doi.org/10.1186/s12872-022-02598-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02598-6