Abstract
Background
The left atrium (LA) is a key player in the pathophysiology of systolic and diastolic heart failure (HF). Speckle tracking derived LA reservoir strain (LASr) can be used as a prognostic surrogate for elevated left ventricular filling pressure similar to NT-proBNP. The aim of the study is to investigate the correlation between LASr and NT-proBNP and its prognostic value with regards to the composite endpoint of HF hospitalization and all-cause mortality within 1 year.
Methods
Outpatients, sent to the echocardiography core lab because of HF, were enrolled into this study. Patients underwent a transthoracic echocardiographic examination, commercially available software was used to measure LASr. Blood samples were collected directly after the echocardiographic examination to determine NT-proBNP.
Results
We included 174 HF patients, 43% with reduced, 36% with mildly reduced, and 21% with preserved ejection fraction. The study population showed a strong inverse correlation between LASr and log-transformed NT-proBNP (r = − 0.75, p < 0.01). Compared to NT-proBNP, LASr predicts the endpoint with a comparable specificity (83% vs. 84%), however with a lower sensitivity (70% vs. 61%).
Conclusion
LASr is inversely correlated with NT-proBNP and a good echocardiographic predictor for the composite endpoint of hospitalization and all-cause mortality in patients with HF.
Trial registration : https://www.trialregister.nl/trial/7268
Similar content being viewed by others
Background
Heart failure (HF) is among the leading causes of morbidity and mortality worldwide [1]. Early recognition and prompt treatment of heart failure are crucial for the prognosis. Although HF is primarily a clinical diagnosis, N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a valuable diagnostic marker of HF given the fact that symptoms can be aspecific [2]. Patients with normal NT-proBNP levels are unlikely to have HF, contrary to patients with elevated levels who need further cardiac evaluation.
Echocardiography is the modality of choice to establish the diagnosis of HF. The classification is based primarily on measurement of left ventricular ejection fraction (LVEF) into HF with reduced (≤ 40%), mildly reduced (41–49%), or preserved (≥ 50%) ejection fraction [3]. Currently, the evaluation of HF is mainly focused on the left ventricle (LV). This is remarkable since left atrial (LA) volume and function has a pathophysiological significance in different types of HF [4, 5]. Accumulating evidence suggests an added value of measuring the left atrial reservoir strain (LASr) by speckle-tracking echocardiography for both diagnosis and prognosis of HF [6, 7]. Previous studies showed that LASr is around 40% in healthy controls [8] and that it is impaired in HF patients [6, 7]. Additionally, LASr can be used as a prognostic marker similar to NT-proBNP [9].
The aim of the study is to investigate the correlation between LASr and NT-proBNP and its prognostic value in an outpatient population with HF with regards to the composite endpoint of HF hospitalization and all-cause mortality within 1 year.
Methods
Study design
The present study was performed as part of the Heart Failure Classification (HaFaC) project (https://www.trialregister.nl/trial/7268). This prospective, non-randomized, observational, single-center study was designed to develop a HF classification based on objective measurement data. The local ethics committee and the Institutional Review Board approved the study (Medical Research Ethics Committees United study number NL60579.100.17) and all subjects gave written informed consent. The primary outcome was a composite of all-cause mortality or hospitalization for heart failure.
Population
From December 2017 to September 2019, patients referred to the Echocardiography Lab with HF based on the ESC guidelines [10] were prospectively included in the study. To be included, patients needed to be ≥ 18 years old and able to provide written informed consent. Exclusion criteria were: recent cardiothoracic surgery (≤ 90 days) or pregnancy. Patients were also excluded for further analysis in case of inadequate acoustic LA window on echocardiography (> 2 non-visible LA segments), and severe renal failure (glomerular filtration rate ≤ 30 mL/min, calculated by the CKD-EPI formula). Also patients with atrial fibrillation were excluded, because atrial fibrillation on its own induces LA remodeling and influences LASr. All patient data was entered into a prospective database, including demographical, clinical and echocardiographic variables, medications and laboratory biomarkers.
Echocardiographic evaluation
All patients underwent a comprehensive transthoracic echocardiographic examination using commercially available equipment (Philips iE33 or Philips EPIQ, Andover, MA, USA). Examinations were performed by 2 experienced and EACVI certified cardiac sonographers (SB or PH), blinded to other research data. Echocardiogram was stored as Digital Imaging and Communications in Medicine (DICOM) file on a secured server and analysed off-line using commercially available software (QLAB 13, Philips Healthcare, Eindhoven, the Netherlands). Standard 2D- and Doppler-echocardiographic measurements were performed following ASE/EACVI guidelines [11]. LVEF was calculated using the modified biplane Simpson’s rule and maximum LA volume was calculated by the biplane method of disks at end-systole and indexed to body surface area (LAVI). The following parameters were used to determine diastolic dysfunction; average E/e’ > 14, septal e’ velocity < 7 cm/s or lateral e’ velocity < 10 cm/s, tricuspid regurgitation velocity > 2.8 m/s, LAVI > 34 ml/m2, pulmonary vein S/D ratio < 1, mitral inflow velocities and ratio according to the published guidelines [11].
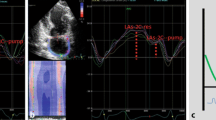
Speckle tracking echocardiography of the LA is a relatively new echocardiographic method. With dedicated software an unique pattern of speckles is identified within the LA wall and these speckles are tracked on frame-by-frame base throughout the cardiac cycle. The measured change in distance between the different speckles is used to calculate LA deformation. LA reservoir strain is a prognostic biomarker, which has been evaluated for patients with HF. Commercially available software (QLAB 13, Philips Healthcare, Eindhoven, the Netherlands) was used to measure LASr on non-foreshortened apical four- and two-chamber views of the LA with a frame rate of 60–80 frames per seconds. The LA endocardial border was automatically drawn followed by manual adjustment if required. The reference point for LA strain analysis was taken at the onset of the QRS-complex (R-R gating) (Fig. 1) [12].
Biomaker analysis
Blood samples were collected and analyzed directly after the echocardiographic examination while the patient was still in a supine position. Levels of NT-proBNP were determined at the department of Clinical Chemistry (Elecsys pro BNP II assay, Roche Diagnostics, Mannheim, DE) [13].
Follow-up
Patients were followed up at our outpatient clinic on a regular base by both clinical visits and telephone calls. All-cause mortality was recorded by consulting the Dutch civil registry. Information on HF hospitalization during the 1-year follow-up period was obtained from a systematic review of all hospital admissions performed by an independent reviewer unaware of clinical and echocardiographic data.
Statistical analysis
Three groups were defined: HF with preserved ejection fraction (HFpEF), HF with mildly reduced ejection fraction (HFmrEF), and HF with reduced ejection fraction (HFrEF) [10]. For continuous variables, normality of distribution was assessed with the Shapiro–Wilk’s test. Normal and skewed continuous variables are presented as means with standard deviation (SD) and medians with interquartile range [IQR], respectively. Statistical comparisons of the three HF subgroups were made using one way ANOVA for normally distributed data or an Kruskal Wallis test for non-normally distributed data. Categorical variables were expressed as proportions and compared using a chi-squared test, or Fishers exact test when the number of positive cases in at least one of the heart failure categories is less than five. Multiple pairwise-comparison between subgroups was performed using Tukey Honest Significant Differences method for normally distributed continuous variables, p values of the other multiple comparisons were corrected using Benjamini–Hochberg correction. A p value of less than 0.05 was considered to indicate statistical significance. The correlation between LASr and NT-proBNP were examined by Pearson’s correlation analysis. Prognostic value of the different parameters was assessed by a receiver-operator curve (ROC-curve), the optimal cut-off point was determined by maximizing the Youden Index. Kaplan–Meier curves are shown for the time-to-event distribution. All analyses were performed using R version 4.0.5 and Rstudio 1.2.1335 (R foundation for Statistical Computing, Vienna, Austria; RStudio InC, Boston, MA).
Results
Patient selection
Two hundred sixty-one outpatients were sent to the echocardiography Core Lab because of HF. Eighty-seven patients were excluded because of atrial fibrillation (n = 63), severe renal failure (n = 4) and insufficient imaging quality for LASr analysis (n = 20). The remaining 174 patients were enrolled into the study; there were 37 patients with HFpEF (21%), 62 with HFmrEF (36%), and 75 with HFrEF (43%) (Fig. 2).
Baseline characteristics
Table 1 shows general characteristics of the total study population and HF subgroups. Patients were predominantly male (69%) with a median age of 68 years. The majority of patients were treated with beta blockers (79%), renin-angiotensin system antagonists (79%), and to a lesser extent with mineralocorticoid antagonists (31%) and loop diuretics (41%).
On echocardiography, median LVEF was 44% [34–49], median left ventricular end-diastolic volume (LVEDV) 142 ml [100–195], and median LAVI 37 ml/m2 [28–47] with a median LASr 27% [20–35]. On subgroup analysis, patients with HFrEF had significantly larger LVEDV and prevalence of mitral valve regurgitation was higher compared with HFmrEF (p < 0.01). LA size did not differ significantly between HFrEF and HFpEF patients, however LASr was lower in the HFrEF compared with HFpEF and HFmrEF patients (p = 0.02 and p < 0.01, respectively) (Table 2).
Median NT-proBNP (568 pg/mL [276–1114]) was significantly higher in the HFrEF group (HFmrEF p < 0.01, HFpEF p = 0.02) (Table 2).
Correlation between biomarkers and LAS r
The study population showed a moderate inverse correlation between NT-proBNP and LASr (r = − 0.55 p < 0.01), which improved after 10log-transformation of NT-proBNP (r = − 0.75, p < 0.01) (Fig. 3). For the HF subgroups, no significant differences were found between the degree of correlation between 10log-transformed NT-proBNP and LASr.
Correlation between conventional echocardiographic diastolic parameters and LAS r
Both deceleration time of early mitral inflow (E) and early diastolic mitral annular velocity (e’) had a weak correlation with LASr (r = 0.35 and r = 0.24, respectively). E/A and E/e’ ratio had a moderate inverse correlation with LASr (r = − 0.44 and r = − 0.42, respectively).
Follow-up
Twenty-three patients (13%) reached the composite endpoint of all-cause mortality and heart failure hospitalization (Table 1).
Prognostic value of LAS r and biomarkers
Results of receiver operating characteristic (ROC) analysis for all predictors of the endpoint are shown in Fig. 4 and Table 3. NT-proBNP showed the highest area under the ROC curve (AUC 0.83) to predict the primary endpoint of death or heart failure hospitalization up to 12 months of follow-up. As for echocardiographic parameters, LASr outperformed LVEF with a AUC-value of 0.79. The AUC of LASr differed significantly from LAVI and LVEDV (p < 0.01 and p = 0.03), however not from LVEF and NT-proBNP (p = 0.10 and p = 0.25). Figure 5 shows survival curves by Kaplan Meier analysis for patients stratified by LASr (Panel A) and NT-proBNP (Panel B). Patients with LASr ≤ 17% showed significantly worse survival than patients with LASr > 17%. Patients with NT-proBNP ≥ 1191 pg/mL also showed significantly worse survival.
Discussion
The key findings of the present study are as follows. First, LASr is a strong echocardiographic predictor of the composite endpoint of HF hospitalization and all-cause mortality. Compared to NT-proBNP, LASr predicts the endpoint with a comparable specificity (83% vs. 84%), however with a lower sensitivity (70% vs. 61%). Also, LASr correlates strongly with 10log-transformed NT-proBNP levels.
LASr has enhanced prognostic value beyond conventional echocardiographic measures to discriminate which heart failure patients are at greater risk for hospital admission or death. For HF subgroups, Carluccio et al. [15] and Freed et al. [16] showed that assessment of LASr by speckle-tracking strain echocardiography had powerful prognostication in patients with HFrEF and HFpEF, respectively.
Although limited in number, previous studies that investigated the correlation between NT-proBNP and LASr, are in line with our results. Al Saikhan [7] demonstrated a modest inverse correlation in patients with both HFpEF (r = − 0.57) and HFmrEF (r = − 0.53). Another study showed that LASr had moderate inverse correlation with NT-proBNP (r = − 0.42) [14]. In both studies, the correlation between LASr and a log-transformation of NT-proBNP levels was not reported, although it has been shown that plasma concentrations of NT-proBNP follow a log-normal distribution in patients with HF [13]. Prastaro [5] evaluated the relationship between NT-proBNP and LA function in patient with HFrEF. In their study, LA function was based on measuring fractional active and total emptying from M- and B-mode images and showed a significant correlation between NT-proBNP and LA function [5].
LA reservoir strain and elevated LV filling pressure
Assessment of LV filling pressure has important diagnostic and prognostic implications in patients with HF [10, 11]. Although right-sided cardiac catheterization is the gold standard to determine LV filling pressure, it is unattractive for routine clinical use given its invasiveness. In the continuing search for non-invasive markers to estimate LV filling pressure, NT-proBNP provides a reliable estimation, especially for left ventricular end-diastolic pressure (LVEDP) [17]. The prognostic value of NT-proBNP is well established [9, 10] and the results of our study are in line with previous reports. The LA, on the other hand, is more and more acknowledged as key player in the pathophysiology of systolic and diastolic HF [18]. Indeed, elevated LV filling pressure results in pressure overload that induces LA failure characterized by dilation and decrease in reservoir function. Wakami [19] showed that an increase in LVEDP is associated with a decrease in LASr. Moreover, LASr can accurately categorize patients based on a normal or elevated LV filling pressures [14, 20].
LA reservoir strain and heart failure
In HFpEF a fortiori, elevated filling pressures are the main physiologic consequence of the diastolic dysfunction [21]. Current guidelines [11] use various echocardiographic parameters for determination of diastolic dysfunction. LASr provides potentially clinical relevance in the detection of LV diastolic dysfunction, because LASr detects subtle dysfunction, even before the LA begins to enlarge [22]. LASr decreases in a linear fashion as LV diastolic dysfunction progresses [23]. In line with this, the latest EACVI document [24] encourages the use of LA strain in the assessment of diastolic function and filling pressures in HFpEF, however LASr should not be used in patients with atrial fibrillation [25]. We previously showed a relationship between increased coronary microvascular resistance and reduced LASr, that seemed to precede conventional measures of LV diastolic dysfunction [26]. Moreover, LASr is not only influenced by diastolic, but also by systolic LV function. As LA expansion is also determined by the base-to-apex displacement during LV systolic contraction [27], any condition that influences LV myocardial function is expected to influence LASr. Thus, LASr correlates with both LV filling pressures and systolic performance.
Clinical implications
The LA seems to have a central role in HF. LA function can be easily studied using speckle-tracking strain echocardiography. The LA strain measurements should be included in the standard evaluation of outpatients with heart failure, because it can stratify their risk for hospital admission and death more reliable than LVEF. Further research is need if a closer follow-up of these patients will reduce their morbidity and mortality.
Study limitations
Although the current study is based on a real-world, prospective, observational data of an outpatient HF population referred to the echocardiography lab, the subgroups of different HF types were relatively small. Second, the number of events was too small to perform a multivariate Cox regression analysis. A logistic regression with NT-proBNP and echocardiographic parameters did not result in an improvement in AUC. However, follow-up research with a larger population and more events is needed to draw conclusions on the AUC of combined laboratory and echocardiographic parameters. Third, the length of follow-up in the current study was limited to 1 year, so we were unable to determine the long-term prognostic value of LASr. Fourth, in this prospective study a combined endpoint of HF hospitalization and all-cause mortality has been chosen. Cardiovascular death might have been a stronger endpoint. However, the exact cause of death was not known in all of our HF patients. Finally, in 7% of the study population, LASr could not be analyzed due to poor image quality.
Conclusions
LASr is a strong echocardiographic predictor of the composite endpoint of HF hospitalization and mortality. LASr is inversely correlated with NT-proBNP and predicts the endpoint with a comparable specificity.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HF:
-
Heart failure
- HFmrEf:
-
Heart failure with mildly reduced ejection fraction
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- LA:
-
Left atrial
- LASr :
-
Left atrial reservoir strain
- LAVI:
-
Left atrial volume indexed to body surface are
- LV:
-
Left ventricle
- LVEDP:
-
Left ventricular end-diastolic pressure
- LVEDV:
-
Left ventricular end-diastolic volume
- LVEF:
-
Left ventricular ejection fraction
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
References
Abbafati C, Machado DB, Cislaghi B, Salman OM, Karanikolos M, McKee M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22.
Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–63.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726.
Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2(1):10–5.
Prastaro M, Paolillo S, Savarese G, Dellegrottaglie S, Scala O, Ruggiero D, et al. N-terminal pro-B-type natriuretic peptide and left atrial function in patients with congestive heart failure and severely reduced ejection fraction. Eur J Echocardiogr. 2011;12(7):506–13.
Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Hear Fail. 2015;8(2):295–303.
Al Saikhan L, Hughes AD, Chung WS, Alsharqi M, Nihoyannopoulos P. Left atrial function in heart failure with mid-range ejection fraction differs fromthat of heart failure with preserved ejection fraction: A 2D speckle-tracking echocardiographic study. Eur Heart J Cardiovasc Imaging. 2019;20(3):279–90.
Pathan F, D’Elia N, Nolan MT, Marwick TH, Negishi K. Normal ranges of left atrial strain by speckle-tracking echocardiography: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2017;30(1):59–70.
Stanek B, Frey B, Hülsmann M, Berger R, Sturm B, Strametz-Juranek J, et al. Prognostic evaluation of neurohumoral plasma levels before and during beta-blocker therapy in advanced left ventricular dysfunction. J Am Coll Cardiol. 2001;38(2):436–42.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2016;18(8):891–975.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–60.
Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19(6):591–600.
Schou M, Gustafsson F, Kjaer A, Hildebrandt PR. Long-term clinical variation of NT-proBNP in stable chronic heart failure patients. Eur Heart J. 2007;28(2):177–82.
Kurt M, Tanboga IH, Aksakal E, Kaya A, Isik T, Ekinci M, et al. Relation of left ventricular end-diastolic pressure and N-terminal pro-brain natriuretic peptide level with left atrial deformation parameters. Eur Heart J Cardiovasc Imaging. 2012;13(6):524–30.
Carluccio E, Biagioli P, Mengoni A, Francesca Cerasa M, Lauciello R, Zuchi C, et al. Left atrial reservoir function and outcome in heart failure with reduced ejection fraction. Circ Cardiovasc Imaging. 2018;11(11):e007696.
Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. 2016;9(3)
Bansal M, Marwick TH. Natriuretic peptides and filling pressure at rest and stress. Heart Fail Clin. 2008;4(1):71–86.
Rossi A, Gheorghiade M, Triposkiadis F, Solomon SD, Pieske B, Butler J. Left atrium in heart failure with preserved ejection fraction structure, function, and significance. Circ Hear Fail. 2014;7(6):1042–9.
Wakami K, Ohte N, Asada K, Fukuta H, Goto T, Mukai S, et al. Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr. 2009;22(7):847–51.
Tan TS, Akbulut IM, Demirtola AI, Serifler NT, Ozyuncu N, Esenboga K, et al. LA reservoir strain: a sensitive parameter for estimating LV filling pressure in patients with preserved EF. Int J Cardiovasc Imaging. 2021;37(9):2707–16.
Brutsaert DL, Sys SU, Gillebert TC. Diastolic failure: Pathophysiology and therapeutic implications. J Am Coll Cardiol. 1993;22(1):318–25.
Morris DA, Belyavskiy E, Aravind-Kumar R, Kropf M, Frydas A, Braunauer K, et al. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging. 2018;11(10):1405–15.
Kim J, Yum B, Palumbo MC, Sultana R, Wright N, Das M, et al. Left atrial strain impairment precedes geometric remodeling as a marker of post-myocardial infarction diastolic dysfunction. JACC Cardiovasc Imaging. 2020;13(10):2099–113.
Smiseth OA, Morris DA, Cardim N, Cikes M, Delgado V, Donal E, et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Hear J Cardiovasc Imaging. 2021. https://doi.org/10.1093/ehjci/jeab154.
Inoue K, Khan FH, Remme EW, Ohte N, García-Izquierdo E, Chetrit M, et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur Hear journal Cardiovasc Imaging 23(1):61–70
Keulards DCJ, Bouwmeester S, de Vos AMJ, Dekker LRC, Pijls NHJ, Houthuizen P. High microvascular resistance and reduced left atrial strain in patients with coronary microvascular dysfunction: The micro-strain study. Int J Cardiol. 2021;333:21–8.
Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation. 1999;100(4):427–36.
Acknowledgements
Not applicable.
Funding
Catharina Research Fund, Netherlands Enterprise Agency and part of the reagents for laboratory analysis were provided free of charge by Roche Diagnostics Nederland BV. Funders had no role in the trial design; the collection, management, analysis, or interpretation of the data; or the writing of the manuscript and the decision to submit it for publication.
Author information
Authors and Affiliations
Contributions
S.B and J.S: planning, conducting, patient enrollment, data collection, article writing and submission. S.L: planning, conducting, patient enrollment and data collection. N.R: article writing. A.B: planning, article writing. L.D: patient enrollment, article writing. V.S: planning, data collection and article writing. P.H: planning, conducting, patient enrollment, data collection and article writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The investigation complied with the principles of the Declaration of Helsinki and was approved by the Catharina hospital ethics committee and the Institutional Review Board (Medical Research Ethics Committees United study number NL60579.100.17) and all subjects gave written informed consent.
Consent for publication
Not applicable.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bouwmeester, S., van der Stam, J.A., van Loon, S.L.M. et al. Left atrial reservoir strain as a predictor of cardiac outcome in patients with heart failure: the HaFaC cohort study. BMC Cardiovasc Disord 22, 104 (2022). https://doi.org/10.1186/s12872-022-02545-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02545-5