Abstract
Objectives
To evaluate the 3-year clinical outcomes of a polymer-free sirolimus-eluting, Nano plus stent for the treatment of coronary artery disease in the NANO multicenter Registry.
Background
The long-term clinical data evaluating the safety and efficacy of the novel polymer-free sirolimus-eluting Nano plus stent (Lepu Medical, Beijing, China) is limited.
Methods
The NANO all-comers Registry trial was a prospective, multicenter clinical registry conducted in 26 centers in China between August 2016 and January 2017. A total of 2481 consecutive patients were exclusively treated with the Nano plus stent. The primary clinical endpoint, target lesion failure (TLF, defined as cardiac death, target vessel nonfatal myocardial infarction, and clinically driven target lesion revascularization [CD-TLR]), was analyzed at 3 years.
Results
At 3 years, 2295 patients (92.5%) were followed. The incidence of TLF was 6.8% (168/2481). The rate of cardiac death was 3.8% (94/2481), target vessel nonfatal myocardial infarction 0.7% (18/2481), and CD-TLR 2.9% (68/2481). The rate of definite/probable stent thrombosis was 0.5% (13/2481). The risk factors of diabetes mellitus, acute myocardial infarction, age, chronic renal failure, in-stent restenosis, chronic total occlusion, and left ventricular ejection fraction < 40% were the independent predictors of 3-year TLF.
Conclusions
At three years, the rate of TLF was relatively low in patients treated with the polymer-free Nano plus stent. The polymer-free Nano plus stent showed a favorable safety and efficacy profile in real-world patients.
Clinical trial registration URL: https://www.clinicaltrials.gov/. Unique identifier: NCT02929030.
Similar content being viewed by others
Introduction
Percutaneous coronary interventions (PCI) with drug-eluting stents (DES) are currently the most common revascularization treatment strategy for coronary artery disease worldwide. DES has dramatically improved clinical outcomes compared to the bare metal stent (BMS) [1]; however, current DES systems always need relatively long (> 6 months) dual antiplatelet therapy (DAPT) [2], which confined their usage on a significant proportion of patients with adherence restraints, such as those at high bleeding risk [3].
The durable polymer has been demonstrated to be associated with vessel wall inflammation and contributes to delay arterial healing, which could lead to late thrombotic risk [4, 5]. Polymer-free coating technologies have then emerged. Polymer-free DESs aim to prevent adverse events caused by hypersensitivity reactions and chronic inflammation to polymer [6, 7]. In patients at high bleeding risk, polymer-free DES was found to be superior to BMS when used with a 1-month course of DAPT [8].
The Nano plus stent is a novel polymer-free stent with nano-sized pores as drug carriers that contain the antiproliferative drug sirolimus and is one of the most widely used DESs in China. Nano plus stent has an improved uniform distribution on the adluminal stent surface than microporous or textured rough surface stents. Nano plus stent has been demonstrated to have comparable safety and efficacy to durable polymer DES for treating de novo coronary artery lesions in a selected randomized controlled trial population [9]. However, the efficacy and safety of the Nano plus stent in real-world practice remained scarce. Previously, we reported the 1-year results of the NANO Registry, showing that the clinical outcome of the Nano plus stent was associated with a low rate of TLF [10]. The current study reports the 3-year outcomes of the NANO all-comers Registry.
Methods
Study design and population
The NANO all-comers Registry trial (NCT02929030) was a prospective, multicenter trial conducted in 26 centers across China between August 2016 and January 2017 with a single arm design. A total of 2481 consecutive patients with symptomatic coronary artery disease scheduled for PCI were enrolled, with no specific inclusion or exclusion criteria [10]. The NANO Registry planned to follow the patients up to 5 years. Patients were contacted at 30 days, 180 days, and 1 year by telephone or scheduled outpatient clinic visit. After 1 year, telephone contact was conducted annually to assess the clinical status and adverse events. In the NANO Registry, each patient provided at least two telephone numbers when he/she participated in the study. If investigators could not reach patients at follow-up, the protocol mandated all possible efforts to be made to trace the patients. Family members or referring cardiologists were contacted if necessary.
The trial was performed in accordance with the Declaration of Helsinki and was approved by the ethics committees of the Xijing Hospital. All the patients signed the written informed consent prior to participation in the trial. Clinical outcomes were adjudicated by an independent clinical event committee, and three CEC members reviewed all the available cine films and adjudicated the events for each event.
Outcomes
The primary clinical endpoint was target lesion failure (TLF), defined as cardiac death, target vessel nonfatal myocardial infarction (MI), and clinically driven target lesion revascularization (CD-TLR). The safety endpoint was definite and/or probable stent thrombosis (ST). MI was defined according to the third universal definition [11]. Repeat revascularization was defined as any repeat revascularization by PCI or coronary artery bypass graft. ST was defined according to the Academic Research Consortium criteria [12].
Study device
The Nano plus stent is a novel polymer-free sirolimus-eluting stent (Lepu Medical, Beijing, China) with the nanoporous stent surface technology used to carry drug and control drug release. The Nano plus stent system is based on a 316L stainless steel platform and has a high-pressure delivery system with a semi-compliant rapid exchange balloon catheter. The delivery system presents a crossing profile of 0.9–1.2 mm with two radiopaque markers at the ends of the balloon to facilitate correct stent placement. The two ends of the stent have a sinusoidal curve shape, while the center of the stent is composed of a specialized cyclic structure that aligns into a helix. Nano-sized pores (mean pore diameter: 400 nm, 1/800 of the stent thickness) are uniformly distributed on the abluminal stent surface.
Study procedures and medications
In the NANO Registry, PCI was performed per the standard of practice of each participating center [13,14,15]. We recommend that all patients were pretreated with aspirin and a P2Y12 inhibitor (clopidogrel or ticagrelor) according to the standard of care and provided DAPT for ≥ 6 months (stable patients) or ≥ 12 months (acute coronary syndrome) according to the guidelines [13,14,15]. The continuation of DAPT beyond the duration of the recommended guidelines was performed at the physician's discretion. Additional medications for secondary prevention, including statins, beta-blockers, and angiotensin-converting enzyme inhibitors, were prescribed according to the guidelines [13,14,15].
Statistical analysis
Continuous variables are presented as mean ± standard deviation. Categorical data are expressed as percentages. Cumulative event curves were generated using the Kaplan–Meier method. A multivariable Cox proportional hazards model was used to identify the independent predictors of the 3-year TLF. Baseline clinical and procedural variables that were considered clinically relevant or that showed a univariate relationship with TLF (p < 0.10) were entered into the multivariate Cox proportional hazards model. All statistical analyses were performed using the SAS 9.1 software package (SAS Institute, Cary, NC). All tests were 2-sided, and a p value < 0.05 was considered statistically significant.
Results
Baseline demographics and clinical characteristics
Patient baseline and lesion characteristics are reported previously and also in Tables 1 and 2 [10]. The mean age of the patients was 62.8 ± 10.1 years. 40.2% of patients presented acute myocardial infarction (AMI), and 22.8% of patients had diabetes mellitus. 11.6% of patients had multiple vessel PCI, and 63.9% of lesions were American College of Cardiology/American Heart Association (ACC/AHA) type B2 or C lesions, including 17.0% ultra-long lesions (lesion length ≥ 40 mm), 14.5% chronic total occlusions (CTO), 11.7% bifurcations, 5.8% severe calcifications, 2.7% severe tortuosity, and 4.1% referenced vessel diameter < 2.5 mm.
Clinical outcomes up to 3 years follow-up
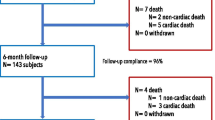
A total of 2295 patients (92.5%) were followed up for 3 years, and 186 patients were lost to follow-up (Fig. 1). The cumulative rate of TLF at 3 years was 6.8% (n = 168) among all patients (Fig. 2), with cardiac death occurred in 3.8% (n = 94) (Fig. 3a), target vessel nonfatal MI in 0.7% (n = 18) (Fig. 3b), and CD-TLR in 2.9% (n = 68) (Fig. 3c) of patients (Table 3). At 3 years, the rate of definite or probable ST was 0.5% (n = 13) (Fig. 3d, Table 3). The rates of clinical outcomes within 1 year, 1–2 years, and 2–3 years are shown in Table 3.
Predictors of TLF at 3 years follow-up
Multivariate analyses of TLF at 3 years using the Cox proportional hazard model showed that the independent predictors of 3-year TLF included diabetes mellitus, AMI, age, chronic renal failure, in-stent restenosis, CTO, and left ventricular ejection fraction < 40% (Table 4).
Discussion
Results of the 3 years analysis of the NANO all-comers Registry show that patients treated with polymer-free Nano plus stent had a relatively low rate of TLF and definite or probable ST, suggesting the polymer-free Nano plus stent was safe and effective in a real-world population.
Stent design, antiproliferative drug, and the presence and type of polymer are the key factors of a DES platform relate to its clinical efficacy. The polymer of DES, which can facilitate loading and controlling the release of antiproliferative drugs, was suspected to be related to inflammatory responses and delayed arterial healing [4]. Polymer-free DESs were initially designed out of hopes that, without the polymer, the risk of polymer-related inflammation and late thrombotic events would be decreased.
However, the safety and efficacy of the polymer-free DES, as compared to durable polymer DES or bioresorbable polymer DES, is still under debate. The SORT OUT IX found that the polymer-free BioFreedom stent did not meet the criteria of non-inferiority regarding MACE (major adverse cardiac events) at 12 months when compared with the ultrathin strut BP sirolimus-eluting Orsiro stent, and the BioFreedom stent had a higher incidence of TLR [16]. In contrast to these findings, most studies demonstrated that compared to durable polymer DES, polymer-free DES has a non-inferior efficacy profile either in a selected population [17, 18], or in a real-world clinical setting [19, 20]-even follow up to 10 years [21].
Several features of the BioFreedom stent should be considered. It has been demonstrated that DES efficacy is closely associated with the release kinetics of the antiproliferative drug in the first 30 days; however, around 90% of biolimus A9 is released from the BioFreedom stent within 48 h of implantation, and the relatively fast drug release may contribute to less efficacy on inhibiting neointimal hyperplasia [16]. Strut thickness is associated with in-stent restenosis [22]. The BioFreedom stent is with a strut thickness of 112 µm, which is thicker than most other newer-generation DES (60–90 µm).
We previously reported that the polymer-free Nano plus stent showed similar safety and efficacy compared with the durable polymer sirolimus-eluting stent in terms of angiographic outcomes at 9 months and clinical outcomes at 2 years [9]. In the current analysis, we showed that using polymer-free Nano plus stent for the treatment of relatively complex lesions in the unselected population is associated with a low and acceptable rate of 3-year TLF. Compared to the BioFreedom stent, the Nano plus stent has a relatively thin strut (91 μm), and 85% of the sirolimus is released within 30 days [9]. However, since there is no data comparing BioFreedom and Nano plus stent, the above-mentioned potential advantage of Nano plus stent is only theoretical and hypothetical.
Compared to durable polymer DES, another benefit of polymer-free DES is its ability to allow a shorter DAPT course after stent implantation. The LEADERS FREE trial showed that polymer-free DES (BioFreedom) has an efficacy and safety advantage over BMS at 1 year in patients at high bleeding risk treated with 1-month DAPT [8]. The benefit of polymer-free DES over BMS was maintained for up to 2 years [23]. The LEADERS FREE II trial reproduced the results of LEADERS FREE in an independent, predominantly North American cohort of high bleeding risk patients [24]. Notwithstanding, this advantage seems to be recently challenged. The ONYX ONE trial observed that among patients at high bleeding risk who received 1 month of DAPT after PCI, durable polymer DES (Resolute Onyx) was non-inferior to polymer-free DES (BioFreedom) in terms of safety and effectiveness composite outcomes [25]. The efficacy of Resolute Onyx and Nano plus stent warrant to be compared in future studies enrolling high bleeding risk population.
Stent failure remains to occur, which may lead to adverse cardiac events, despite the improvement of the contemporary DES. Identifying the related factors that may predict TLF was of paramount importance. However, predictors of TLF vary in the different postprocedural periods [26]. Previously, we identified that diabetes mellitus, AMI, left ventricular ejection fraction < 40%, and long lesions (> 40 mm) independently predicted 1-year TLF. At 3 years, the independent predictors of TLF included diabetes mellitus, AMI, age, chronic renal failure, in-stent restenosis, CTO, and left ventricular ejection fraction < 40%, the predictors identified in the present analysis are highly consistent with previous studies [26,27,28]. Identifying and intensively managing these predictors may help to reduce the rate of TLF and improve the long-term clinical outcomes.
Limitations
The present study was a single arm, non-randomized study with inherent limitations. However, the current analysis highlights the safety and efficacy of Nano plus stent in an unselected population in a real-world setting. Although the results of the analysis showed that polymer-free Nano plus stent has a favorable safety and efficacy profile, head-to-head comparisons with the newer generation of DES are needed in future studies. In addition, the follow-up rate was relatively low in the present study, which could partially contribute to the low events rate.
Conclusion
At 3 years follow-up, TLF was relatively low in patients treated with polymer-free Nano plus stents in the multicenter NANO Registry trial. The Nano plus stents showed promising safety and efficacy in real-world patients, although longer follow-up is needed for further evaluation.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Stefanini GG, Holmes DR Jr. Drug-eluting coronary-artery stents. N Engl J Med. 2013;368:254–65.
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO and Group ESCSD. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165.
Moussa ID, Colombo A. Antiplatelet therapy discontinuation following drug-eluting stent placement: dangers, reasons, and management recommendations. Catheter Cardiovasc Interv. 2009;74:1047–54.
Hezi-Yamit A, Sullivan C, Wong J, David L, Chen M, Cheng P, Shumaker D, Wilcox JN, Udipi K. Impact of polymer hydrophilicity on biocompatibility: implication for DES polymer design. J Biomed Mater Res A. 2009;90:133–41.
Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202.
Costa RA, Abizaid A, Mehran R, Schofer J, Schuler GC, Hauptmann KE, Magalhaes MA, Parise H, Grube E and BioFreedom FIMCTI. Polymer-free biolimus A9-coated stents in the treatment of de novo coronary lesions: 4- and 12-month angiographic follow-up and final 5-year clinical outcomes of the prospective, multicenter BioFreedom FIM Clinical Trial. JACC Cardiovasc Interv. 2016;9:51–64.
Baquet M, Jochheim D, Mehilli J. Polymer-free drug-eluting stents for coronary artery disease. J Interv Cardiol. 2018;31:330–7.
Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrie D, Naber C, Lipiecki J, Richardt G, Iniguez A, Brunel P, Valdes-Chavarri M, Garot P, Talwar S, Berland J, Abdellaoui M, Eberli F, Oldroyd K, Zambahari R, Gregson J, Greene S, Stoll HP, Morice MC, Investigators LF. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373:2038–47.
Zhang YCF, Muramatsu T, et al. Nine-month angiographic and two-year clinical follow-up of polymer-free sirolimus-eluting stent versus durable-polymer sirolimus-eluting stent for coronary artery disease: the nano randomized trial. Chin Med J (Engl). 2014;127:2153–8.
Liu Y, Zhang Y, Li Y, Qi T, Pan D, Wang H, Liu C, Ma D, Fang Z, Zhang R, Mou F, Tao L and Investigators NA-CR. One-year clinical results of the NANO registry: a multicenter, prospective all-comers registry study in patients receiving implantation of a polymer-free sirolimus-eluting stent. Catheter Cardiovasc Interv. 2020;95 Suppl 1:658–64.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESCAAHAWHFTFftUDoMI, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ and Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–35.
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW and Academic Research C. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51.
Kolh P, Windecker S, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol Ç, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Sousa Uva M, Achenbach S, Pepper J, Anyanwu A, Badimon L, Bauersachs J, Baumbach A, Beygui F, Bonaros N, De Carlo M, Deaton C, Dobrev D, Dunning J, Eeckhout E, Gielen S, Hasdai D, Kirchhof P, Luckraz H, Mahrholdt H, Montalescot G, Paparella D, Rastan AJ, Sanmartin M, Sergeant P, Silber S, Tamargo J, ten Berg J, Thiele H, van Geuns RJ, Wagner HO, Wassmann S, Wendler O and Zamorano JL. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2014;46:517–92.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–94.
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Ting HH, O’Gara PT, Kushner FG, Ascheim DD, Brindis RG, Casey DE Jr, Chung MK, de Lemos JA, Diercks DB, Fang JC, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2016;67:1235–50.
Jensen LOEJ, Veien KT, Ahlehof O, Hansen KN, Aziz A, et al. Randomized comparison of the polymer-free biolimus-coated biofreedom stent with the ultrathin strut biodegradable polymer sirolimus-eluting orsiro stent in an all-comers population treated with percutaneous coronary intervention: the SORT out IX Trial. Circulation. 2020;141:2052–63.
Massberg S, Byrne RA, Kastrati A, Schulz S, Pache J, Hausleiter J, Ibrahim T, Fusaro M, Ott I, Schomig A, Laugwitz KL, Mehilli J, Intracoronary S, Angiographic Results: Test Efficacy of S and Probucol-Eluting Versus Zotarolimus- Eluting Stents I. Polymer-free sirolimus- and probucol-eluting versus new generation zotarolimus-eluting stents in coronary artery disease: the Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus- and Probucol-Eluting versus Zotarolimus-eluting Stents (ISAR-TEST 5) trial. Circulation. 2011;124:624–32.
Kufner S, Sorges J, Mehilli J, Cassese S, Repp J, Wiebe J, Lohaus R, Lahmann A, Rheude T, Ibrahim T, Massberg S, Laugwitz KL, Kastrati A and Byrne RA. Randomized trial of polymer-free sirolimus- and probucol-eluting stents versus durable polymer zotarolimus-eluting stents. JACC Cardiovasc Intervent 2016;9:784–792.
Sardella G, Stefanini GG, Briguori C, Tamburino C, Fabbiocchi F, Rotolo F, Tomai F, Paggi A, Lombardi M, Gioffre G, Sclafani R, Rolandi A, Sciahbasi A, Scardaci F, Signore N, Calcagno S, Mancone M, Chiarito M, Giordano A. Safety and efficacy of polymer-free biolimus-eluting stents in all-comer patients: the RUDI-FREE study. EuroIntervention. 2018;14:772–9.
Chiarito M, Sardella G, Colombo A, Briguori C, Testa L, Bedogni F, Fabbiocchi F, Paggi A, Palloshi A, Tamburino C, Margonato A, Pivato CA, Baber U, Calcagno S, Giordano A, Godino C, Stefanini GG. Safety and efficacy of polymer-free drug-eluting stents. Circ Cardiovasc Interv. 2019;12:007311.
Kufner S, Ernst M, Cassese S, Joner M, Mayer K, Colleran R, Koppara T, Xhepa E, Koch T, Wiebe J, Ibrahim T, Fusaro M, Laugwitz K-L, Schunkert H, Kastrati A, Byrne RA. 10-Year Outcomes from a randomized trial of polymer-free versus durable polymer drug-eluting coronary stents. J Am Coll Cardiol. 2020;76:146–58.
Kastrati AMJ, Dirschinger J, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103:2816–21.
Garot P, Morice M-C, Tresukosol D, Pocock SJ, Meredith IT, Abizaid A, Carrié D, Naber C, Iñiguez A, Talwar S, Menown IBA, Christiansen EH, Gregson J, Copt S, Hovasse T, Lurz P, Maillard L, Krackhardt F, Ong P, Byrne J, Redwood S, Windhövel U, Greene S, Stoll H-P, Urban P, Urban P, Morice M-C, Abizaid A, Meredith IT, Pocock SJ, Carrié D, Naber C, Greene S, Stoll H-P. 2-Year outcomes of high bleeding risk patients after polymer-free drug-coated stents. J Am Coll Cardiol. 2017;69:162–71.
Krucoff MW, Urban P, Tanguay J-F, McAndrew T, Zhang Y, Rao SV, Morice M-C, Price MJ, Cohen DJ, Abdel-Wahab M, Mehta SR, Faurie B, McLaurin B, Diaz C, Stoll H-P, Pocock S and Leon MB. Global approach to high bleeding risk patients with polymer-free drug-coated coronary stents. Circul. Cardiovasc. Intervent. 2020;13.
Windecker S, Latib A, Kedhi E, Kirtane AJ, Kandzari DE, Mehran R, Price MJ, Abizaid A, Simon DI, Worthley SG, Zaman A, Hudec M, Poliacikova P, Abdul Ghapar AKB, Selvaraj K, Petrov I, Mylotte D, Pinar E, Moreno R, Fabbiocchi F, Pasupati S, Kim HS, Aminian A, Tie C, Wlodarczak A, Hur SH, Marx SO, Jankovic I, Brar S, Bousquette L, Liu M, Stone GW, Investigators OO. Polymer-based or polymer-free stents in patients at high bleeding risk. N Engl J Med. 2020;382:1208–18.
Konigstein M, Madhavan MV, Ben-Yehuda O, Rahim HM, Srdanovic I, Gkargkoulas F, Mehdipoor G, Shlofmitz E, Maehara A, Redfors B, Gore AK, McAndrew T, Stone GW, Ali ZA. Incidence and predictors of target lesion failure in patients undergoing contemporary DES implantation-Individual patient data pooled analysis from 6 randomized controlled trials. Am Heart J. 2019;213:105–11.
Taniwaki M, Stefanini GG, Silber S, Richardt G, Vranckx P, Serruys PW, Buszman PE, Kelbaek H, Windecker S. 4-Year clinical outcomes and predictors of repeat revascularization in patients treated with new-generation drug-eluting stents. J Am Coll Cardiol. 2014;63:1617–25.
Ananthakrishna R, Kristanto W, Liu L, Chan SP, Loh PH, Tay EL, Chan KH, Chan MY, Lee CH, Low AF, Tan HC, Loh JP. Incidence and predictors of target lesion failure in a multiethnic Asian population receiving the SYNERGY coronary stent: a prospective all-comers registry. Catheter Cardiovasc Interv. 2018;92:1097–103.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YD, RW and FC analyzed and interpreted data, wrote the first draft of the article, and contributed to all revisions. YZ, CG, YC, LT, HH and PY contributed to the conception and design of the study, gathered and interpreted data and contributed to critical revision of the manuscript. YL, RZ, and BZ contributed to the data collection and revised the manuscript critically for important intellectual content. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The trial was performed in accordance with the Declaration of Helsinki and was approved by the ethics committees of the Xijing Hospital. All the patients signed the written informed consent prior to participation in the trial.
Consent for publication
Not applicable.
Competing interests
All the authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dai, Y., Wang, R., Chen, F. et al. Clinical outcomes in 2481 unselected real-world patients treated with a polymer-free sirolimus-eluting stent: 3 years results from the NANO multicenter Registry. BMC Cardiovasc Disord 21, 537 (2021). https://doi.org/10.1186/s12872-021-02356-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-021-02356-0