Abstract
Background
Determining the risk factors for brachial-ankle pulse wave velocity (baPWV) may help to identify people susceptible to diabetic atherosclerosis and could prevent diabetic macrovascular complications in the early stages. We aim to comprehensively investigate risk factors contributing to arterial stiffness in patients with and without diabetes.
Methods
BaPWV was measured in 5651 individuals who attended health check-ups at baseline and follow-up. Lasso regression was used to screen for risk factors. Mixed models and multiple linear regressions were subsequently established to evaluate the effect size of the potential risk factors on baPWV and PWV change rates. All analyses were stratified by diabetes. Mediation analysis was also conducted to demonstrate the mechanisms of arterial stiffness in patients with diabetes.
Results
In lasso regression, postprandial 2-h glucose (P2hG), systolic blood pressure (SBP) and age were associated with baPWV regardless of diabetes. Platelet counts (PLT), mean corpuscular volume (MCV) and coronary heart disease (CHD) were associated with baPWV in patients with diabetes. In the mixed models, PLT were positively associated with baPWV in patients with diabetes (βplatelet, perSD = 25.80; 18.26–33.33). Elevated PLTs could also significantly increase the PWV change rate in patients with diabetes (βplatelet, perSD = 54.05; 10.00–107.10). In mediation analysis, diabetes had a significant average direct effect on baPWV. The average causal mediation effect (ACME) of PLTs was 1.76, with a range of 0.17 to 3.70.
Conclusions
Elevated PLT counts can increase baPWV in diabetes and are a potential mediator between diabetes and atherosclerosis.
Similar content being viewed by others
Background
Arterial stiffness is an important risk factor for cardiovascular disease (CVD) and is a key element in the pathogenesis of CVD [1]. Growing evidence shows that increased arterial stiffness can increase both cardiovascular mortality and all-cause mortality [2, 3]. The most common noninvasive index used to quantify these estimates of arterial stiffness is brachial-ankle pulse wave velocity (baPWV) [4]. BaPWV can reflect both central and peripheral arterial stiffness [5, 6]. An increase in baPWV has been related to the severity of peripheral vascular disease and higher cardiovascular risk [7].
Studies have demonstrated various risk factors for arterial stiffness. Elevated blood pressure (BP) [8] and long-term systolic blood pressure (SBP) variability [9] were shown to increase arterial stiffness. Type 2 diabetes (T2DM) has also been confirmed to increase arterial stiffness in several large cohort studies [10, 11] and has been found to have causal association with arterial stiffness in a Chinese population [12]. However, the mechanisms of arterial stiffening in T2DM patients are still not clear. Moreover, the risk factors for arterial stiffness may be different between patients with diabetes and patients without diabetes. Misako Hamamura et al. [13] demonstrated several risk factors for arterial stiffness in T2DM patients, including age, systolic blood pressure, serum uric acid (UA), urinary albumin excretion and lower body mass index (BMI). A cross-sectional study carried out by Bo Youn Won et al. [14] showed that age, mean arterial pressure (MAP), pulse rate, waist circumference and gamma-glutamyl transferase (GGT) could increase baPWV in the elderly population without hypertension, diabetes and statin use. Finding the difference in arterial stiffness risk profiles between patients with and without diabetes would be important for both the etiological study of diabetic macrovascular complications and the prevention of arterial stiffness. However, the difference in arterial stiffness risk profiles between non-T2DM and T2DM patients is still not clear and needs to be further studied. Moreover, whether serum lipids, routine blood indexes or biochemical indexes of liver and kidney function contribute to arterial stiffening has seldom been discussed in previous studies. Determining the risk factors related to baPWV in patients with diabetes may help to identify people susceptible to diabetic atherosclerosis and will aid in the prevention of diabetic macrovascular complications in its early stage.
In a longitudinal study using data from health check-ups in China, we attempted to determine whether traditional risk factors together with routine blood indexes and biochemical indexes contributed to the development of arterial stiffness. Lasso regression was used to screen risk factors, and a mixed model and multiple linear regressions were used to evaluate the effect size of potential risk factors. All analyses were stratified by diabetes, and mediation analysis was also conducted to explore the mechanisms of arterial stiffness in patients with diabetes.
Methods
Study participants
This longitudinal cohort participated in routine health check-ups in Beijing Xiaotangshan Hospital from 2007 to 2015. All participants had check-ups once a year, and those participants who had at least two check-ups with baPWV measurements were included in the current study. The baPWV and other physical indicators were measured at baseline and each check-up. This study was approved by the Ethics Committee of Capital Medical University (No: 2016SY24). Anthropometric and laboratory measurements were performed at each health check-up, and all participants provided consent.
Measurements
The participants’ medical history of chronic disease and medication history were collected by physicians during routine physical examinations. Information on lifestyle risk factors (cigarette smoking, alcohol consumption, etc.) was collected from participants through a face-to-face interview by trained interviewers using uniform questionnaires.
A mercury sphygmomanometer was used to measure blood pressure. Blood pressure measurements were obtained at least 30 min after participants had smoked or had caffeinated products. Blood pressure was measured three times at the right brachial artery while the participant was in a seated position. The average of the last two measurements was used for data analysis.
The baPWV was measured using an automatic waveform analyzer (BP-203RPE II; OMRON, Japan). Participants were asked to rest 5 min in the supine position before measurements were performed. Occlusion and monitoring cuffs were wrapped around the subjects’ arms and ankles. Pulse volume waveforms of the bilateral brachial and posterior tibial arteries were recorded simultaneously to determine the time interval between brachial and tibial waveforms. The height of the participants was used to calculate the transmission distance from the brachium to the ankle. The mean of the right-side and left-side baPWV values was used for analysis. Measurements were conducted by well-trained doctors who were blinded to the clinical information. All physical examinations were performed in the morning after fasting.
After overnight fasting, venous blood samples were collected from all participants for fasting blood analysis and again for an OGTT. Venous blood samples were obtained and stored in a 4 °C refrigerator. All hematological analyses were performed within 8 h. Serum glucose and biochemical parameters were measured by an enzymatic method using a chemistry analyzer (Beckman LX20, Beckman, Brea, CA, USA) in the central laboratory of the hospital. Platelet counts in blood samples collected in EDTA tubes were determined based on the Coulter principle using an automated differential cell counter (Beckman Coulter Act5Diff, Beckman Coulter®).
Definition of atherosclerotic risk factors
Smoking status was determined through three alternatives: “current smoking”, “past smoking”, or “rare/never smoking”. Current smoking was defined as at least 1 cigarette per day over a period of more than 1 year. Not smoking for at least 3 months was defined as past smoking. Alcohol consumption was determined by three alternatives: “current drinking”, “past drinking”, or “rare/never drinking”. Current drinking was defined as drinking at least once per week for the previous month. Drinking at least once per week but not within the last month was defined as past drinking. Rare/never drinking was defined as never drinking or drinking less than once a week.
T2DM was defined according to the 1999 World Health Organization diagnostic criteria as fasting plasma glucose (FPG) ≥7.0 mmol/L or self-reported physician-diagnosed diabetes and/or taking antidiabetic medication. Hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg and/or currently taking antihypertensive medication. Coronary heart disease (CHD) was defined as nonfatal ischemic heart disease, including acute myocardial infarction and angina pectoris.
Statistical analysis
SAS (version 9.4; SAS Institute, Chicago, IL, USA) and R (version 3.6.0) were used for database management and statistical analysis. Continuous variables with normal distribution are expressed as the means ± standard deviations (SDs), while continuous variables without normal distribution are expressed as medians ± interquartile range. Categorical variables are expressed as percentages (%). To compare the difference in each continuous variable between patients with diabetes and patients without diabetes, Student’s t test or Wilcoxon rank sum test were used.
Lasso regressions were performed for the participants with questionnaires to screen risk factors for baPWV using the R software glmnet package. The included factors were demographic characteristics: age and sex; lifestyle risk factors: smoking and drinking; disease history: coronary heart disease, hypertension, and type 2 diabetes; medications: antidiabetic drugs, antihypertensive drugs, and lipid-lowering drugs; anthropometric measurements; systolic blood pressure (SBP); diastolic blood pressure (DBP); body mass index (BMI); blood lipid measurements: high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and triglyceride (TG) levels; blood glucose measurements: fasting plasma glucose (FPG) and postprandial 2-h glucose (P2hG); routine blood measurements: red blood cell count (RBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), white blood cell (WBC) count, lymphocyte (LYM) count, lymphocyte percentage (LYM%), monocyte (MONO) count, monocyte percentage (MONO%), eosinophil (EO) count, eosinophil percentage (EO%), basophil (BASO) count, basophil percentage (BASO%), neutrophil (NEUT) count, percentage of neutrophil (NEUT%), hemoglobin (HGB), platelet (PLT) counts, mean platelet volume (MPV), platelet volume distribution width (PDW), plateletcrit (PCT), and erythrocyte sedimentation rate (ESR); and blood biochemical indexes including ① liver function indicators: total bilirubin (TBIL), glutamyl transpeptidase (GGT), albumin (ALB), globulin (GLB), albumin-to-globulin ratio (AG), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) and ② renal function indicators: creatinine (CR), urea nitrogen (BUN), and serum uric acid (UA).
When lasso regressions were conducted for the DM group and non-DM group, “DM” was excluded from the variables mentioned above. Linear mixed-effects models and multiple linear regressions were subsequently conducted in all participants using the SAS mixed procedure to evaluate the effect size of potential risk factors determined by lasso regressions.
Mediation analysis to uncover causal pathways linking diabetes to baPWV was performed by R software mediation packages. The main outputs from the mediation function were total effect (TE), average causal mediation effect (ACME), and average direct effect (ADE). A two-sided P < 0.05 was considered statistically significant.
Results
A total of 5651 participants underwent baPWV measurement in the longitudinal study with a mean follow-up of 5.0 years. The mean age of the participants was 58.0. The age of patients without diabetes (66.80 ± 12.11) was significantly higher than that of patients with diabetes (56.97 ± 13.56). BMI and SBP were significantly higher in patients without diabetes, while DBP was significantly higher in patients with diabetes. The percentage of males was significantly higher in patients with diabetes (87.9% vs. 73.5%, P < 0.001). Most of the routine blood indexes and biochemical indexes, except for TG, UA, MCH, MPV, TP, TBIL, ALT and PLT, were significantly different between patients with and without diabetes. The proportions of patients who reported smoking and drinking were not significantly different between the patients with DM and the patients without DM. Details are shown in Table 1.
Lasso regression was used to screen for risk factors for baPWV. P2hG, age, and SBP were associated with follow-up baPWV in the whole population, patients with diabetes, and patients without diabetes. Hypertension was associated with follow-up baPWV in the whole population and in patients with diabetes. PLT, MCV and CHD were associated with follow-up baPWV only in patients with diabetes.
Mixed models were subsequently used to determine the effect size of potential risk factors selected by lasso regression. All risk factors associated with baPWV in lasso regression were included in the mixed models. Risk factors associated with baPWV are listed in Table 2. P2hG, age and SBP were significantly associated with baPWV regardless of whether the results were stratified by diabetes (P < 0.001). Hypertension and higher PLT can significantly increase baPWV in patients with diabetes. The interaction between DM and PLT was statistically significant in all participants (P = 0.0016).
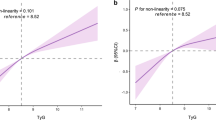
Multiple linear regressions were also used to explore the effect size of risk factors on the PWV change rate. All risk factors associated with baPWV in lasso regression were included in the regression models. Elevated PLT and P2hG increased the PWV change rate in diabetes (βPLT, per SD = 54.05, 10.00–107.10, PPLT = 0.046; βP2hG = 13.85, 2.50–25.21, PP2hG = 0.017, respectively). Age, P2hG and SBP were significantly associated with the baPWV change rate in both the whole population and patients without diabetes. Details are shown in Table 3. The linear relationship between PLT and baPWV change rate per year in patients with diabetes is shown in Fig. 1.
Anthropometry and biomarkers significantly associated with baPWV in the diabetes group were subsequently examined for their mediation effects between diabetes and baPWV. The results of the mediation analysis are shown in Table 4. Diabetes had a significant average direct effect (ADE) on baPWV in each model (P < 0.05). The average causal mediation effect (ACME) of DBP is 3.219, with a range of 1.327 to 5.860 (P < 0.001). PLT also had significant ACME with a range of 0.17 to 3.70 (ACME = 1.761, P = 0.021).
Discussion
In the current longitudinal study, we found that higher blood pressure can increase the risk of arterial stiffness in both patients with diabetes and patients without diabetes. DBP and PLT may be causally associated with baPWV in patients with diabetes. The most important strengths of the current longitudinal study were a relatively large sample size with a median follow-up of 5 years. Moreover, traditional risk factors together with routine blood indexes and biochemical indexes were included to screen for potential risk factors for baPWV.
It has long been believed that BP is related to arterial stiffness because elastic arterial walls will change in response to chronically elevated blood pressures [15]. The relationship between DBP and arterial stiffness is not consistent. In the Georgia Cardiovascular Twin Study, DBP showed strong positive associations with PWV in American youth and young adults [16]. In a population with a mean age of 68, both systolic hypertension alone and combined with diastolic hypertension were found to be significantly associated with PWV [17]. However, Franklin SS et al. found that a fall in DBP after age 60 was associated with a continual rise in SBP, which was consistent with increased large artery stiffness [18]. Blood pressure (BP) will fluctuate from moment to moment depending on patient- and observer-related factors; thus, the measurement of BP is complicated. In the current study, we did not retake BP measurements for the participants who had abnormal BP. Thus, it was likely that elevated DBP found in a routine health examination was only a transient abnormality. Wingfield D et al. [19] demonstrated that transient high DBP could increase both cardiovascular (CVD) mortality and the incidence of diabetes. Given that arterial stiffness is independently predictive of CVD death [20], transient high DBP may also be associated with arterial stiffness. Moreover, in the current study, we found that DBP is a significant mediator between DM and arterial stiffness. In insulin-resistant patients, plasma asymmetric dimethylarginine (ADMA), which antagonizes endothelium-dependent vasodilatation, was increased independent of hypertension [21]. Moreover, vasodilation in response to insulin concentrations was reduced in insulin-resistant patients [22]. Endothelium-dependent vasodilation dysfunction was related to higher DBP [23]. Thus, patients with diabetes with insulin resistance may have a higher DBP because of endothelial dysfunction. Brachial DBP is a useful marker of central BP [24], and DBP was previously found to be associated with central arterial stiffness, which was measured by carotid-femoral pulse wave velocity (cfPWV) [25]. Thus, a positive association between DBP and baPWV may indicate a role of endothelial dysfunction in central arterial stiffness.
In this study, we found that elevated PLT can increase baPWV in diabetes, and it may be causally associated with arterial stiffness in diabetes. Type 2 diabetes is characterized by insulin resistance and β-cell dysfunction. Insulin resistance is typically associated with hyperinsulinemia, which results from the feedback response to the loss of the intracellular signaling pathway on glucose transportation. An epidemiological study demonstrated that increased plasminogen activator inhibitor type 1 (PAI-1) levels were associated with type 2 diabetes mellitus and may be a component of insulin-resistance syndrome [26]. Other studies aimed at mimicking the metabolic pattern of type 2 diabetes showed that hyperinsulinemia could cause a sustained increase in the concentration of PAI-1 in the blood in normal subjects [27, 28]. Levels of PAI-1 have been shown to be positively associated with ischemic heart disease, and overexpression of PAI-1 mRNA has been observed in atherosclerotic human arteries, suggesting that PAI-1 is involved in the development and progression of atherosclerosis [29,30,31]. Several studies have shown that platelet (PLT) counts were significantly correlated with PAI-1 (r = 0.44) and platelet aggregation (r = 0.11) [32]. Thus, patients with diabetes with hyperinsulinemia experience PAI-1 elevation and platelet aggregation, which are involved in the pathophysiology of atherosclerosis. Given that PLT is positively correlated with PAI-1 and platelet aggregation, the identification of PLT as a mediator in the current study might indicate the underlying mechanisms of diabetic atherosclerosis. Moreover, Robert H. Lee et al. [33] demonstrated another possible mechanism by which platelet numbers contribute to the pathology of diabetic atherosclerosis. Elevated blood glucose levels can trigger S100 calcium-binding protein A8/A9 (S100A8/A9) and could ultimately lead to increased thrombopoietin (TPO) production by binding to a receptor of advanced glycation end-products (RAGE). TPO causes megakaryocyte proliferation and increased platelet production. Dysregulation of the number and function of platelets might contribute to the development of atherosclerosis.
Many endothelium-derived factors involved in the regulation of blood coagulation may be altered in diabetes, leading to initial platelet adhesion and subsequent platelet aggregate formation. The interaction of activated platelets with endothelial cells and leukocytes, together with the coagulation system, may contribute to the process of atherosclerosis [34]. Mean platelet volume (MPV) is a marker of platelet size and activity. Platelets from diabetic patients are characterized by dysregulation of several signaling pathways [35]. Diabetes can enhance the inflammatory response to bacteria both systemically and at the site of infection [36]. Smaller platelets are considered inflammatory markers in many infectious diseases and are associated with chronic inflammatory processes [37, 38]. In addition, inflammation is also important in the pathogenesis of arterial stiffness and atherosclerosis [39, 40]. A population-based study found that MPV is positively associated with vascular stiffness, and larger platelets are generally considered a strong indicator of atherosclerosis [41].. Thus, the effect of MPV on arterial stiffness in patients with diabetes may be a more complex situation compared with that in the general population. However, few studies have focused on the effect of MPV on arterial stiffness in diabetes. The current study found that only platelet counts could increase baPWV in diabetes but did not find a significant association between MPV and the risk of arterial stiffness. Further studies are still needed in the future to explore the role of platelets and their activity on arterial stiffness in patients with diabetes.
There are some limitations of the current study. First, the patients’ history of disease and medication were self-reported. To minimize bias, the above information was acquired by professional physicians. Second, not all participants completed questionnaires, so it was difficult to evaluate the impact of lifestyle risk factors on arterial stiffness in all participants. We performed lasso regression only in the participants with complete questionnaires. Neither smoking nor drinking was found to be a risk factor for arterial stiffness in lasso regression; thus, the following analyses were performed in all participants without considering their lifestyle risk factors. Third, the current study is a physical examination-based cohort instead of a community-based cohort study. Due to the different possibility for people to choose baPWV measurement in their health examination, the current study may have selection bias to a certain extent. Only participants with more than two baPWV measurements were included in the study, which may also induce selection bias. Older patients and those with metabolic cardiovascular disease might preferentially choose baPWV measurement in their health check-ups several times. To cover this shortage, we adjusted for age, coronary heart disease, blood lipids and medication in the lasso regression model. In addition, people who chose to undergo repeated baPWV measurements might have characteristics that are difficult to measure quantitatively, for example, their health literacy or socioeconomic status. Thus, the results need to be further verified in community-based cohorts. In addition, blood indicators included in the current study are routine items for health check-ups. Because HbA1c was not a routine item and participants seldom chose to test for it in their check-ups, the effect of long-term blood glucose control on atherosclerosis could not be fully evaluated. Moreover, in this research, we cannot determine the mechanism behind the mediation analysis from DM patients relating platelet count to PWV. More research on the potential mechanisms by which platelets increase the risk of diabetic atherosclerosis is needed in the future.
Conclusions
In the current study, we have found that platelet (PLT) count is positively associated with baPWV in a diabetic population, and PLT count may be a mediator linking diabetes to arterial stiffness. Our findings indicate that PLT count has the potential to be a predictor of atherosclerosis in patients with diabetes, and the identification of PLT count as a predictor might contribute to the early prevention of diabetic macrovascular complications.
Availability of data and materials
The datasets generated during the current study are not publicly available yet. Data can be made available on reasonable request through contacting the corresponding author.
Abbreviations
- LYM:
-
Absolute value of lymphocyte count
- ALT:
-
Alanine aminotransferase
- ALB:
-
Albumin
- AGR:
-
Albumin and globulin ratio
- AST:
-
Aspartate aminotransferase
- ACME:
-
Average causal mediation effect
- ADE:
-
Average direct effect
- BASO:
-
Basophils count
- BASO%:
-
Basophils percentage
- BP:
-
Blood pressure
- BMI:
-
Body mass index
- BUN:
-
Blood urea nitrogen
- baPWV:
-
Brachial-ankle pulse wave velocity
- CVD:
-
Cardiovascular disease
- CHD:
-
Coronary heart disease
- CR:
-
Creatinine
- DBP:
-
Diastolic blood pressure
- EO:
-
Eosinophils count
- EO%:
-
Eosinophils percentage
- ESR:
-
Erythrocyte sedimentation rate
- FPG:
-
Fasting plasma glucose
- GGT:
-
Gamma-glutamyl transferase
- GLB:
-
Globulin
- GGT:
-
Glutamyl transpeptidase
- HGB:
-
Hemoglobin
- PLT:
-
Platelet counts
- PCT:
-
Platelet crit
- HDLC:
-
High density lipoprotein cholesterol
- LDLC:
-
Low density lipoprotein cholesterol
- MAP:
-
Mean arterial pressure
- MCH:
-
Mean corpuscular hemoglobin
- MCHC:
-
Mean corpuscular hemoglobin concentration
- MCV:
-
Mean corpuscular volume
- MPV:
-
Mean platelet volume
- MONO:
-
Monocyte count
- MONO%:
-
Monocyte percentage
- NEUT:
-
Neutrophil count
- NEUT%:
-
Neutrophil percentage
- LYM%:
-
Lympholeukocyte percentage
- PDW:
-
Platelet volume distribution width
- P2hG:
-
Postprandial 2 h-glucose
- RBC:
-
Red blood cell count
- RDW:
-
Red cell distribution width
- SDs:
-
Standard deviations
- SBP:
-
Systolic blood pressure
- TBIL:
-
Total bilirubin
- TC:
-
Total cholesterol
- TE:
-
Total effect
- TP:
-
Total protein
- TG:
-
Triglyceride
- T2DM:
-
Type 2 diabetes
- UA:
-
Uric acid
- WBC:
-
White blood cell count
References
Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation. 2010;121(4):505–11.
Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam study. Circulation. 2006;113(5):657–63.
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–41.
Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27(10):2022–7.
Tsuchikura S, Shoji T, Kimoto E, Shinohara K, Hatsuda S, Koyama H, Emoto M, Nishizawa Y. Brachial-ankle pulse wave velocity as an index of central arterial stiffness. J Atheroscler Thromb. 2010;17(6):658–65.
Lacroi V, Willemet M, Verhelst R, Beauloye C, Jacquet L, Astarci P, Persu A, Marchandise. E. Central and peripheral pulse wave velocities are associated with ankle–brachial pressure index. Artery Res. 2012;6(1):28–33.
Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, Inoguchi T, Maeda Y, Kohara K, Tabara Y, et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension. 2017;69(6):1045–52.
Zheng M, Xu X, Wang X, Huo Y, Xu X, Qin X, Tang G, Xing H, Fan F, Cui W, et al. Age, arterial stiffness, and components of blood pressure in Chinese adults. Medicine. 2014;93(29):e262.
Tedla YG, Yano Y, Carnethon M, Greenland P. Association between long-term blood pressure variability and 10-year progression in arterial stiffness: the multiethnic study of atherosclerosis. Hypertension. 2017;69(1):118–27.
Sung KC, Lim YH, Park S, Kang SM, Park JB, Kim BJ, Shin JH. Arterial stiffness, fatty liver and the presence of coronary artery calcium in a large population cohort. Cardiovasc Diabetol. 2013;12:162.
Muhammad IF, Borne Y. Arterial stiffness and incidence of diabetes: a population-based cohort study. Diabetes Care. 2017;40(12):1739–45.
Xu M, Huang Y, Xie L, Peng K, Ding L, Lin L, Wang P, Hao M, Chen Y, Sun Y, et al. Diabetes and risk of arterial stiffness: a Mendelian randomization analysis. Diabetes. 2016;65(6):1731–40.
Hamamura M, Mita T, Osonoi Y, Osonoi T, Saito M, Tamasawa A, Nakayama S, Someya Y, Ishida H, Gosho M, et al. Relationships among conventional cardiovascular risk factors and lifestyle habits with arterial stiffness in type 2 diabetic patients. J Clin Med Res. 2017;9(4):297–302.
Won BY, Park SG, Lee SH, Kim MJ, Chun H, Hong D, Kim YS. Characteristics of metabolic factors related to arterial stiffness in young and old adults. Clin Exp Hypertens. 2020;42(3):225–32.
O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45(4):652–8.
Ge D, Young TW, Wang X, Kapuku GK, Treiber FA, Snieder H. Heritability of arterial stiffness in black and white American youth and young adults. Am J Hypertens. 2007;20(10):1065–72.
Verwoert GC, Franco OH, Hoeks AP, Reneman RS, Hofman A, CM VD, Sijbrands EJ, Witteman JC, Mattace-Raso FU. Arterial stiffness and hypertension in a large population of untreated individuals: the Rotterdam study. J Hypertens. 2014;32(8):1606–12 discussion 1612.
Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96(1):308–15.
Wingfield D, Grodzicki T, Palmer AJ, Wells F, Bulpitt CJ. Transiently elevated diastolic blood pressure is associated with a gender-dependent effect on cardiovascular risk. J Hum Hypertens. 2005;19(5):347–54.
Shoji T, Maekawa K, Emoto M, Okuno S, Yamakawa T, Ishimura E, Inaba M, Nishizawa Y. Arterial stiffness predicts cardiovascular death independent of arterial thickness in a cohort of hemodialysis patients. Atherosclerosis. 2010;210(1):145–9.
Stuhlinger MC, Abbasi F, Chu JW, Lamendola C, McLaughlin TL, Cooke JP, Reaven GM, Tsao PS. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA. 2002;287(11):1420–6.
Lind L. Endothelium-dependent vasodilation, insulin resistance and the metabolic syndrome in an elderly cohort: the prospective investigation of the vasculature in Uppsala seniors (PIVUS) study. Atherosclerosis. 2008;196(2):795–802.
Lind L. Endothelium-dependent vasodilation in relation to different measurements of blood pressure in the elderly: the prospective investigation of the vasculature in Uppsala seniors study. Blood Press Monit. 2008;13(5):245–50.
Safar M, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, Laurent S, London G, Pannier B, Protogerou A, et al. Interaction between hypertension and arterial stiffness. Hypertension. 2018;72(4):796–805.
Tsai JP, Wang JH, Chen ML, Yang CF, Chen YC, Hsu BG. Association of serum leptin levels with central arterial stiffness in coronary artery disease patients. BMC Cardiovasc Disord. 2016;16:80.
Juhan-Vague I, Alessi MC, Vague P. Increased plasma plasminogen activator inhibitor 1 levels. A possible link between insulin resistance and atherothrombosis. Diabetologia. 1991;34(7):457–62.
Carmassi F, Morale M, Ferrini L, Dell'Omo G, Ferdeghini M, Pedrinelli R, De Negri F. Local insulin infusion stimulates expression of plasminogen activator inhibitor-1 and tissue-type plasminogen activator in normal subjects. Am J Med. 1999;107(4):344–50.
Calles-Escandon J, Mirza SA, Sobel BE, Schneider DJ. Induction of hyperinsulinemia combined with hyperglycemia and hypertriglyceridemia increases plasminogen activator inhibitor 1 in blood in normal human subjects. Diabetes. 1998;47(2):290–3.
Wiman B, Andersson T, Hallqvist J, Reuterwall C, Ahlbom A, deFaire U. Plasma levels of tissue plasminogen activator/plasminogen activator inhibitor-1 complex and von Willebrand factor are significant risk markers for recurrent myocardial infarction in the Stockholm heart epidemiology program (SHEEP) study. Arterioscler Thromb Vasc Biol. 2000;20(8):2019–23.
Hamsten A, de Faire U, Walldius G, Dahlen G, Szamosi A, Landou C, Blomback M, Wiman B. Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. Lancet. 1987;2(8549):3–9.
Schneiderman J, Sawdey MS, Keeton MR, Bordin GM, Bernstein EF, Dilley RB, Loskutoff DJ. Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci U S A. 1992;89(15):6998–7002.
Cancelas JA, Garcia-Avello A, Garcia-Frade LJ. High plasma levels of plasminogen activator inhibitor 1 (PAI-1) in polycythemia vera and essential thrombocythemia are associated with thrombosis. Thromb Res. 1994;75(5):513–20.
Lee RH, Bergmeier W. Sugar makes neutrophils RAGE: linking diabetesassociated hyperglycemia to thrombocytosis and platelet reactivity. J Clin Invest. 2017;127(6):2040–3.
Sobol AB, Watala C. The role of platelets in diabetes-related vascular complications. Diabetes Res Clin Pract. 2000;50(1):1–16.
Shah B, Sha D, Xie D, Mohler ER 3rd, Berger JS. The relationship between diabetes, metabolic syndrome, and platelet activity as measured by mean platelet volume: the National Health and Nutrition Examination Survey, 1999-2004. Diabetes Care. 2012;35(5):1074–8.
Graves DT, Liu R, Oates TW. Diabetes-enhanced inflammation and apoptosis: impact on periodontal pathosis. Periodontol. 2007;45:128–37.
Cakiroglu Y, Vural F, Vural B. The inflammatory markers in polycystic ovary syndrome: association with obesity and IVF outcomes. J Endocrinol Investig. 2016;39(8):899–907.
Akelma AZ, Mete E, Cizmeci MN, Kanburoglu MK, Malli DD, Bozkaya D. The role of mean platelet volume as an inflammatory marker in children with chronic spontaneous urticaria. Allergol Immunopathol. 2015;43(1):10–3.
Schnabel R, Larson MG, Dupuis J, Lunetta KL, Lipinska I, Meigs JB, Yin X, Rong J, Vita JA, Newton-Cheh C, et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 2008;51(6):1651–7.
Tuttolomondo A, Di Raimondo D, Pecoraro R, Serio A, D'Aguanno G, Pinto A, Licata G. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke. Atherosclerosis. 2010;213(1):311–8.
Panova-Noeva M, Arnold N, Hermanns MI, Prochaska JH, Schulz A, Spronk HM, Binder H, Pfeiffer N, Beutel M, Blankenberg S, et al. Mean platelet volume and arterial stiffness - clinical relationship and common genetic variability. Sci Rep. 2017;7:40229.
Acknowledgements
The authors thank all the participants for their continuing participation in this research effort.
Not applicable.
Funding
This study was supported by the Natural Science Foundation of China (81602908, 81530087). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
LK analysed data and wrote the manuscript. XJF and SY contributed to data collection in the study. TLX and YK contributed to the data analysis and data interpretation. GXH contributed to the design and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Capital Medical University (No:2016SY24). All participants enrolled in this study have signed informed consent.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, K., Xu, J., Tao, L. et al. Platelet counts are associated with arterial stiffness in Chinese Han population: a longitudinal study. BMC Cardiovasc Disord 20, 353 (2020). https://doi.org/10.1186/s12872-020-01634-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-020-01634-7