Abstract
Background
Long term β-blocker therapy after myocardial infarction (MI) reduces mortality and recurrent MI but evidence for this treatment predates contemporary acute coronary care. β-blocker treatment is a key quality of care indicator in the Swedish national quality register for acute coronary care, Riks-HIA. Between 2011 and 2015 a declining number of MI-patients discharged with a β-blocker from the coronary care unit (CCU) at Helsingborg and other hospitals was reported. This retrospective observational study aimed to investigate the causes for discharge without a β-blocker and relate it to outcome, compared to patients discharged with a β-blocker.
Methods
MI-patients registered in Riks-HIA discharged without β-blocker during 2011–2015 (no-β-group) and a control group (β-group) comprised of patients discharged with β-blocker treatment between January 1 to December 31, 2013, were matched by RIKS-HIA criteria for β-blocker use. Clinical characteristics, date of death, readmission for MI, other cardiovascular events were collected from Riks-HIA and medical records.
Results
The no-β-group included 141 patients, where 65.2% had a justified reason for non-β-blocker use. The β-group included 206 patients. There was no difference in cardiovascular risk factor profile. There were a trend towards a higher number of readmissions for MI in the no-β-group was (n = 8 (5.7%) vs n = 2 (1.0%), p = 0.02), but not mortality (6 (4.3%) vs 2 (1.0%), p = 0.07) and combined readmission for angina pectoris, heart failure, arrhythmias or stroke/TIA (n = 23 (16.3%) vs n = 25 (12.1%), p = 0.27).
Conclusion
A majority of the patients in the no-β-group had a justified absence of a β-blocker. β-blocker treatment post-MI showed a trend towards fewer readmissions for MI. But important quality information is lacking to make a firm conclusion of the effect on outcome.
Similar content being viewed by others
Background
In the early 1980s, the importance of β-blockers as long-term treatment after Myocardial infarction (MI) was documented in randomized trials [1]. No randomized controlled trials (RCTs) of β-blockers post-MI have however been conducted in the setting of modern coronary care, i.e. after the introduction of statin treatment, wider use of percutaneous intervention (PCI), and the more efficient antiplatelet drugs such as ADP-receptor blockers [2,3,4]. Observational studies examining the effect of post-MI β-blockers in the setting of a revascularized myocardium have however been conducted, with contradictory results [5,6,7]. A recent meta-analysis of observational studies of β-blocker treatment after acute MI, in patients who had undergone primary PCI, concluded that β-blockers post-MI was associated with lower 1-year all-cause mortality, but not a lower incidence of reinfarction or cardiac death [8].
According to current (2015) guidelines from European Society of Cardiology (ESC) for ST-Elevation Myocardial Infarction (STEMI) patients, long-term treatment with β-blockers is recommended to all patients without contraindications. However, this is a class II, level of evidence B recommendation, since contemporary RCTs are lacking [9]. According to American guidelines, continuation of treatment with β-blocker for 3 years is strongly recommended (Class I) for STEMI patients with normal left ventricular function. Continuation after 3 years in this patient group is considered optional (Class IIa or IIb) [10]. Regarding non-ST-Elevation Myocardial Infarction (NSTEMI) patients, the ESC guidelines recommends long-term β-blocker treatment in patients with an ejection fraction of < 40% [11].
In current (2015) treatment recommendation from the Swedish National board of Health and Welfare, long term treatment with a β-blocker post-MI is only recommended to patients with left ventricular systolic dysfunction (LVDF) [12]. However, The Swedish Society of Cardiology recommends β-blockers as long-term treatment in all patients with a history of acute coronary syndrome (ACS) and who are without contraindications [13]. These recommendations thus deviate from those of the ESC and the Swedish National board of Health and Welfare.
Annual reports from the Swedish national registry of cardiac intensive care (Riks-HIA) has shown that between 2011 and 2015, the coronary care unit (CCU) at Helsingborg hospital as many other hospitals experienced an unsatisfying number of patients receiving β-blocker treatment at discharge, taken into account the Riks-HIA quality of care indicator (Table 1) [14].
This study therefore aimed to investigate the reasons why a growing number of patients were discharged without a β-blocker and to examine the clinical outcome of patients discharged from the CCU at Helsingborg’s hospital with or without β-blocker treatment post MI.
Methods
The study population was selected from Riks-HIA, which has been used as a national quality registry for cardiac intensive care since 1995 and covers all the 73 CCUs in Sweden. The aim of the registry is to monitor and compare how well the CCUs adhere to the guidelines and implementation of new treatments. It also monitors and compares short- and long-term survival at the different CCUs [13]. Riks-HIA comprises over 100 variables and includes the majority of patients admitted to the CCUs of the participating hospitals. Two patient groups discharged from Helsingborg CCU were identified using the registry. The first patient group (no-β-group) comprised all MI patients who were discharged without a β-blocker, during 1 January 2011 to 1 January 2015. A control group (β-group) comprised all patients discharged with β-blocker treatment during 1st January 2013 to 31st December 2013. Patients eligible for β-blocker treatment was chosen based on Riks-HIA’s criteria for β-blocker treatment, (age < 80, discharged alive, absence of AV-block II or III and discharged with a diagnosis of a type 1 MI) [15].
In order to investigate similarities and differences between the no-β-group and β-group, relevant variables were selected and collected from the registries and from the patient’s medical records. All medical records were reviewed in order to verify the accuracy of the data collected from the registers, and to collect additional information. Regarding the no-β group, if a reason was stated why they did not receive a β-blocker and whether they were prescribed a β-blocker within a year from index event, this information was obtained. In the β-group, information was collected from the medical record regarding side effects attributable to β-blocker and whether treatment was terminated within a year.
Final diagnosis was determined using the WHO definition of type 1 MI [16]. In the medical record, this was identified by main diagnosis at discharge coded I21 in the international classification of disease (ICD) diagnostic tool, and subclass of MI (NSTEMI or STEMI) was obtained from the medical records.
The primary outcome was readmission for MI during 1 year after the index event. Secondary outcomes included all cause death, cardiovascular death or readmission for all cardiovascular events.
Data processing and statistical calculations
IBM SPSS Statistics version 23 was used for all statistical calculations. Comparisons between the two groups were conducted using Fisher’s exact test for categorical variables, and Mann-Whitney U-test for continuous variables. The Bonferroni correction was applied for comparison of characteristics to adjust for multiple testing. Hence, a p-value of < 0.01 was considered significant after correction [17].
Results
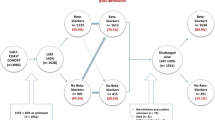
Data from Riks-HIA showed that of 1631 MI patients discharged from the CCU at Helsingborg hospital during the study period 1st of January 2011 to 1st of January 2015, 1155 patients met the criteria for β-blocker treatment. Among these 1155, a total of 171 patients (14.8%) were not prescribed a β-blocker. After reviewing the medical records, 24 of these patients (14.0%) turned out to be incorrectly registered as they did not meet the criteria for β-blocker treatment (n = 14) or were discharged with a β-blocker (n = 10). Consequently, 141 patients were included in the no-β-group (Fig. 1). The β-group included 206 patients. Among the 141 patients in the no-β-group, 92 patients (65.2%) had a reason stated in the medical record why they were discharged without and 49 patients (34.8%) were discharged without a β-blocker with no obvious reason (Fig. 1).
Flow-chart on patients who were admitted with a myocardial infarction to the coronary care unit at Helsingborg’s hospital between 1st January 2011 and 1st January 2015 and discharge without a β-blocker prescription. AV-block = Atrioventricular block, COPD = Chronic obstructive pulmonary disease, Riks-HIA = The Register of Information and Knowledge about Swedish heart intensive care
The two groups were similar to a large extent regarding risk factor profile (Table 2). There was a high prescription rate in both groups of the recommended secondary prevention related medications; statins, ASA and ADP receptor inhibitors. PCI was performed in 78.7% in the no-β-group, and in 82.5% in the β-group (Table 2). The baseline characteristics showed that mean heart rate and systolic blood pressure at admission was lower in the no-β-group (p = < 0.001 and p = 0.01) (Table 2). Ejection fraction < 50% at discharge was more common in the β-group (p = 0.01) (Table 2). Among medications at discharge, ACE inhibitors was to a wider extent prescribed in the β-group (p = < 0.001) (Table 2).
The non-β-group had a trend towards higher rate of readmissions for MI within 1 year after index event, compared with the β-group (Table 3). However, no statistically significant difference between the groups was found regarding other outcome parameters including all cause death, cardiovascular death, morbidity or readmission for all cardiovascular events (Table 3).
Discussion
We studied the reasons to why β-blockers were not prescribed to all patients post-MI without an obvious contraindication at Helsingborg hospital during 2011–2015, and how this corresponded to clinical outcome. We found that, after reviewing the medical records, the treating cardiologist had motivated the absence of β-blocker in a majority of the patients. These motivations are however not visible in the registry and thus not presented in the annual reports from Riks-HIA.
When studying the correlation between lack of a β-blocker prescription and clinical outcome, our study showed that the patient group discharged without a β-blocker after MI had a significantly higher rate of readmissions for MI during 1 year after index event, compared with the patient group that was discharged with a β-blocker. However, no statistically significant difference between the patient groups was found regarding all cause death, cardiovascular death or readmission for all cardiovascular events, although a trend towards higher mortality without β-blockers was observed.
No RCTs have been conducted on β-blockers post-MI since the introduction of primary PCI and modern secondary prevention treatment. Several registers based observational studies with many included patients have been conducted to address the question if β-blockers as secondary prevention after acute MI still is associated with improved prognosis [5,6,7, 18,19,20,21,22,23,24]. A meta-analysis published in 2015 included 10 observational studies published between 2000 and 2014 [8]. The authors concluded that β-blocker prescription after acute MI was associated with a reduction of all-cause death during 1-year follow-up compared to patients without β-blocker prescription. There was no difference however in cardiac death, readmission for MI or heart failure [8]. Further, the study revealed that patients suffering from a NSTEMI, had LVDF or who were sub optimally treated for secondary prevention had the strongest association with better outcome when treated with a β-blocker [8].
The present study differs in several ways from the studies included in the meta-analysis [8]. The criteria for inclusion in the no-β-group (were patients < 80 years, with a type-1 MI (i.e. STEMI and NSTEMI), discharged alive without a β-blocker and with absence of AV-block II-III, which differs from studies that only included patients with STEMI and who underwent PCI [6, 7, 18, 22,23,24]. A study by Choo and co-workers included patients with preserved systolic function after acute MI, treated with PCI, and a study by Ellis and co-workers included patients with acute MI treated with PCI [19, 21]. A study by Chen and co-workers investigated the effect of β-blockers in elderly patients and thus included only patients > 65 years [5].
Unique to this study was that we collected information on the reason why patients were discharged without β-blocker treatment. All of these differences constitute important reasons why comparison between studies becomes difficult and may explain why the results differ. Results from the present study regarding the clinical outcome of all-cause death was not consistent with results from the meta-analysis, where both statistically unadjusted relative risk, and statistically adjusted hazard ratio for all cause death was lower in the group treated with β-blockers [8]. Regarding the study outcome of readmission for MI, the present study could show a lower incidence with β-blocker treatment, whilst the meta-analysis did not show an association of β-blocker treatment and a lower risk for MI [8]. Another possible explanation might be found among the reasons why patients were not prescribed a β-blocker, instead of the absence of a β-blocker per se, as described in Fig. 1.
In an observational study such as the present, the β- and no-β-group differ in a systematically fashion. Among the known reasons in the present study why patients were not prescribed a β-blocker were peripheral limb ischemia, patient negative to drugs and the presence of multiple comorbidities. This indicates comorbidities not found among the baseline characteristics. On the contrary, in the present study, some patients in the no-β-group were not prescribed a β-blocker since they had normal blood pressure, or a normal EF. This is in line with current treatment recommendation from ESC, that recommends β-blockers mainly to patients with reduced left ventricular function post MI [11, 25]. Since the reasons why patients were not treated with a β-blocker were not reported in the meta-analysis, one might speculate that some of these patients were instead considered too healthy for this treatment, perhaps to a larger extent than in the present study, since the Swedish quality registry provides an incentive that all patients without contraindications should be prescribed a β-blocker [26]. This might be an explanation for the different results regarding readmissions for MI.
There were some significant differences in baseline characteristics between the groups (Table 2). These included a significantly lower prevalence of EF < 50%, and a lower rate of ACE-inhibitors prescription in the no-β-group at discharge. Mean systolic blood pressure at discharge was higher in the β-group. However, the groups did not differ in risk factor profile prior to admission, mean age or final diagnosis. Both groups were to a large extent prescribed statins, ASA and ADP inhibitors.
In the present study, the main significant clinical differences between the groups, were likely associated with the absence or presence of β-blocker at discharge. Lower mean heart rate and mean systolic blood pressure at admission in the no-β group was expected since a common reason to why many patients were not prescribed a β-blocker according to the medical records was bradycardia (n = 54, (38%)), and to a lesser extent hypotension (n = 13, (9%)). At discharge, the groups did not differ in mean heart rate, and instead there was lower mean blood pressure in the β-group. An explanation might be that the β-group also had a significantly higher prescription of ACE inhibitors at discharge, which gives a more potent blood pressure treatment. The higher prevalence of heart failure in the β-group, or the higher prevalence of hypotension in the no-β-group, may explain the higher prescription rate of ACE inhibitors in the β-group. The higher rate of calcium channel blocker prescription in the no-β-group is probably as an antihypertensive and anti-ischemic drug, as an alternative to of ACE inhibitors and β-blockers.
The fact that the patient group characteristics in the present study differs from that in other observational studies on β-blockers post-MI might depend on the differences in inclusion criteria previously described. It might also depend on differences in local guidelines with regard to which patients should receive β-blockers. Additionally, these differences in patient characteristics between the two groups possibly illustrates a clinical approach that reflects the current scientific evidence, suggesting that β-blockers are more beneficial in patients with chronic heart failure, left ventricular dysfunction and larger infarcts [27].
Considering these difficulties in interpreting the results of observational registry-based studies, and the necessity to determine the place of β-blockers in contemporary acute coronary care, a clinical trial of β-blockers after MI is warranted. There is a study currently ongoing within the Riks-HIA registry, REDUCE-SWEDEHEART, where 7000 patients are going to be randomized to either treatment with β-blocker or no β-blocker, with 3500 patients in each arm [28]. (ClinicalTrials.gov identifier: NCT03278509).
Study limitations
The intention to answer the question whether the absence of β-blocker treatment post-MI is associated with a higher rate of death and readmission to hospital for cardiovascular events, is hampered by several study limitations.
First of all, this being a retrospective observational study, the most important study limitation consists of the selection bias, since the majority of the patients were treated with or without a β-blocker after taking into account their individual clinical characteristics. Another problem with observational studies is the issue of adjusting for all possible confounders, which is not possible [29]. Important confounders not measured in this study would be other major risk factors for cardiovascular disease, such as low socioeconomic status, lack of physical activity, family history of coronary artery disease, autoimmune and inflammatory diseases [30]. In the present study, no statistical adjustment for measured confounder were done, partially since the patient groups did not differ in the measured aspects of risk factor profile and was thus considered comparable. No statistical adjustments such as propensity scores was calculated due to a small number of patients.
Moreover, the small number of patients in the present study renders a low statistical power. Statistical power is also affected by the expected effect of the treatment. In this case, to illustrate the effect of β-blocker treatment post-MI, the number needed to treat to avoid one death for long-term β-blockers have in meta-analysis of RCTs conducted in the pre-reperfusion era been 82 [31]. This indicates the need for a large number of patients in a study with this research question. On the other hand, the relatively small number of included patients enabled validation of register data and additional information from the medical records.
Conclusion
A majority of the patients in the no-β-group had a justified absence of a β-blocker. β-blocker treatment post-MI showed a trend to fewer readmissions for MI. But as illustrated by this study, patient specific factors not visible in registry data affects whether a patient were prescribed a β-blocker post-MI or not. This complicates the interpretation of the quality index (Table 1) and will affect the quality and interpretation of studies conducted based solely on registry data, since important information is lacking to draw firm conclusions.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding the first author and author HW on request.
Abbreviations
- MI:
-
Myocardial infarction
- RCTs:
-
Randomized controlled trials
- PCI:
-
Percutaneous intervention
- ESC:
-
European society of cardiology
- STEMI:
-
ST-elevation myocardial infarction
- NSTEMI:
-
Non ST-elevation myocardial infarction
- LVDF:
-
Left ventricular systolic dysfunction
- Riks-Hia:
-
Swedish national registry of cardiac intensive care
- CCU:
-
Coronary care unit
- ICD:
-
International classification of disease
- ACE I:
-
Angiotensin-converting-enzyme inhibitor
- ARB:
-
Angiotensin receptor blocker
- ASA:
-
Acetylsalicylic acid
- LBBB:
-
Left bundle branch block
- LMWH:
-
Low-molecular-weight heparin
- AV-block:
-
Atrioventricular block
- COPD:
-
Chronic obstructive pulmonary disease
References
Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27(5):335–71.
A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Collaborators (907) CAPRIE Steering Committee. Lancet. 1996;348(9038):1329-39.
Antman EM, Wiviott SD, Murphy SA, Voitk J, Hasin Y, Widimsky P, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to assess improvement in therapeutic outcomes by optimizing platelet InhibitioN with Prasugrel-thrombolysis in myocardial infarction) analysis. J Am Coll Cardiol. 2008;51(21):2028–33.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Chen J, Radford MJ, Wang Y, Marciniak TA, Krumholz HM. Are beta-blockers effective in elderly patients who undergo coronary revascularization after acute myocardial infarction? Arch Intern Med. 2000;160(7):947–52.
Ozasa N, Kimura T, Morimoto T, Hou H, Tamura T, Shizuta S, et al. Lack of effect of oral beta-blocker therapy at discharge on long-term clinical outcomes of ST-segment elevation acute myocardial infarction after primary percutaneous coronary intervention. Am J Cardiol. 2010;106(9):1225–33.
Yang JH, Hahn JY, Song YB, Choi SH, Choi JH, Lee SH, et al. Association of beta-blocker therapy at discharge with clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. JACC Cardiovasc Interventions. 2014;7(6):592–601.
Huang BT, Huang FY, Zuo ZL, Liao YB, Heng Y, Wang PJ, et al. Meta-analysis of relation between Oral beta-blocker therapy and outcomes in patients with acute myocardial infarction who underwent percutaneous coronary intervention. Am J Cardiol. 2015;115(11):1529–38.
Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569–619.
Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–73.
Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). 2016;37(3):267–315.
Socialstyrelsen. Nationella riktlinjer för hjärtsjukvård; 2015. p. 120.
Koul S, Hagström E, Smith G. Akut kranskärlssjukdom Rekommendationer för bedömning, behandling och sekundärprevention [Internet]. Svenska Kardiologföreningen; 2015. p. 34. Available from: http://www.cardio.se/nationella-riktlinjer.
arbetsgrupp: FR-Hs, Jernberg T, Bergström O, Held C, Johanson P, Kellerth T, et al. Riks-HIA ska ta fram nytt index för områden med förbättringsutrymme. Läkartidningen. 2012;109(13):672–3.
SWEDEHEART. Hur tas RIKS-HIA:s kvalitetsindex fram?. 2016 [2016 May]. Available from: http://www.ucr.uu.se/swedeheart/index.php/nyheter/137-hur-tas-riks-hias-kvalitetsindex-fram.
Antman EM. ST-segment elevation myocardial infarction: pathology, pathophysiology, and clinical features. In: Bonow RO, Mann DM, Zipes DP, Libby P, Braunwald E, editors. Braunwald's heart disease a textbook of cardiovascular medicine. 2. 9th ed. Philadelphia: Elsevier Saunders; 2012. p. 1087–110.
Eric W W. Bonferroni Correction [Internet]. MathWorld - A Wolfram Web Resource 2016 [2016 May 22]. Available from: http://mathworld.wolfram.com/BonferroniCorrection.html.
Bao B, Ozasa N, Morimoto T, Furukawa Y, Nakagawa Y, Kadota K, et al. Beta-blocker therapy and cardiovascular outcomes in patients who have undergone percutaneous coronary intervention after ST-elevation myocardial infarction. Cardiovasc Interv Ther. 2013;28(2):139–47.
Choo EH, Chang K, Ahn Y, Jeon DS, Lee JM, Kim DB, et al. Benefit of beta-blocker treatment for patients with acute myocardial infarction and preserved systolic function after percutaneous coronary intervention. Heart. 2014;100(6):492–9.
De Luca G, de Boer MJ, Ottervanger JP, van’t Hof AW, Hoorntje JC, Gosselink AT, et al. Impact of beta-blocker therapy at discharge on long-term mortality after primary angioplasty for ST-segment elevation myocardial infarction. Am J Cardiol. 2005;96(6):806–9.
Ellis K, Tcheng JE, Sapp S, Topol EJ, Lincoff AM. Mortality benefit of beta blockade in patients with acute coronary syndromes undergoing coronary intervention: pooled results from the epic, epilog, Epistent, capture and rapport trials. J Interv Cardiol. 2003;16(4):299–305.
Kernis SJ, Harjai KJ, Stone GW, Grines LL, Boura JA, O'Neill WW, et al. Does beta-blocker therapy improve clinical outcomes of acute myocardial infarction after successful primary angioplasty? J Am Coll Cardiol. 2004;43(10):1773–9.
Konishi M, Haraguchi G, Yoshikawa S, Kimura S, Inagaki H, Isobe M. Additive effects of beta-blockers on renin-angiotensin system inhibitors for patients after acute myocardial infarction treated with primary coronary revascularization. Circul J. 2011;75(8):1982–91.
Nakatani D, Sakata Y, Suna S, Usami M, Matsumoto S, Shimizu M, et al. Impact of beta blockade therapy on long-term mortality after ST-segment elevation acute myocardial infarction in the percutaneous coronary intervention era. Am J Cardiol. 2013;111(4):457–64.
Ponikowski P, Voors AA, Anker SD, Bueno Hc, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975.
Lindahl B. Guldgruvan i svensk kardiologi - Svenska kardiologiska kvalitetsregister. In: Kardiologföreningen S, editor. Svensk kardiologi - en historiebok. Mölndal: Svenska kardiologföreningen; 2014. p. 401–13.
Ko DT, Jackevicius CA. Stopping beta-Blockers After Myocardial Infarction: Not So Fast! Circ Cardiovasc Qual Outcomes. 2018;11(4):e004678.
Jernberg T. Evaluation of Decreased Usage of Betablockers After Myocardial Infarction in the SWEDEHEART Registry (REDUCE-SWEDEHEART). ClinicalTrials.gov identifier: NCT03278509.
Eljertsson G. Korrelation och regression. Statistik för hälsovetenskaperna. 2nd ed. Lund: Studentlitteratur AB; 2012. p. 221–50.
MNilsson P. Kardiovaskulära riskfaktorer, primär och sekundär prevention. In: Fundin B, editor. Kardiovaskulär medicin. Stockholm: Liber AB; 2010. p. 459–75.
Freemantle N, Cleland J, Young P, Mason J, Harrison J. beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ (Clinical research ed). 1999;318(7200):1730–7.
Acknowledgements
Not applicable.
Funding
J. Gustav Smith was supported by grants from the Swedish Heart-Lung Foundation (2016–0134 and 2016–0315), the Swedish Research Council (2017–02554), the European Research Council (ERC-STG-2015-679242), the Crafoord Foundation, Skåne University Hospital, the Scania county, governmental funding of clinical research within the Swedish National Health Service, a generous donation from the Knut and Alice Wallenberg foundation to the Wallenberg Center for Molecular Medicine in Lund, and funding from the Swedish Research Council (Linnaeus grant Dnr 349–2006-237, Strategic Research Area Exodiab Dnr 2009–1039) and Swedish Foundation for Strategic Research (Dnr IRC15–0067) to the Lund University Diabetes Center. The above stated funding bodies did not play any role in the design of the study, collection, analysis and interpretation of data, or in writing the manuscript. Open access funding provided by Lund University.
Author information
Authors and Affiliations
Contributions
TH, SEO and HW: Designed the study, TH: collect, analysed. TH, BMH and HW. interpreted all the patient data in this manuscript. TH: made the first draft of the manuscript, tables and figure. All five authors (TH, SEO, JGS, BMH and HW) worked together with the interpretations of the data and manuscript until it reached its final stage as it is submitted. All authors (TH, SEO, JGS, BMH and HW) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was performed by the first author, within the medical education and since the study was a quality work regarding given treatment recommended in national Swedish and European guidelines, ethical approval is not needed [11,12,13]. All patients gave written and verbal informed consent to participate in the Riks-HIA and SCAAR registries at the time at their admission at the coronary unit or at the coronary catheterization laboratory. Hence Application for ethical approval from the local Swedish Ethical Review Authority at Lund University, was thereby waived (Dnr 2017/885).
Consent for publication
All patients have given written and verbal informed consent to participate in the Riks-HIA and SCAAR registries, which includes the approval for analysis of the data and publication in scientific journals.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hagsund, T., Olsson, SE., Smith, J.G. et al. β-blockers after myocardial infarction and 1-year clinical outcome – a retrospective study. BMC Cardiovasc Disord 20, 165 (2020). https://doi.org/10.1186/s12872-020-01441-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-020-01441-0