Abstract
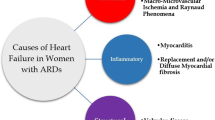
Factors leading to Cardiovascular Disease (CVD) in Autoimmune Rheumatic Diseases (ARD) include: a) atherosclerosis and macro-microvascular coronary artery disease b) pericardial, myocardial and vascular inflammation c) heart valve disease d) heart failure and e) pulmonary hypertension.
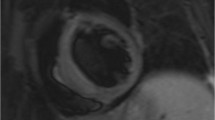
Cardiology utilizes various non-invasive imaging modalities, such as rest/stress Electrocardiogram (ECG), echocardiography, nuclear imaging and more recently Cardiovascular Magnetic Resonance (CMR) to detect ischemic or inflammatory disease in ARD. Exercise ECG is a reliable prognostic test for identification of patients either very unlikely or very likely to have cardiac events. However, this is not the case for intermediate risk patients. In stress echocardiography the diagnostic end point for the detection of myocardial ischemia is the induction of a transient worsening in regional function during stress. It provides similar diagnostic and prognostic accuracy as radionuclide stress perfusion, but at a lower cost and without radiation exposure. Stress Myocardial Perfusion Scintigraphy (MPS) is a non-invasive imaging modality for patients with suspected coronary artery disease, but has important limitations including radiation exposure, imaging artefacts and low spatial resolution, which preclude detection of small myocardial scars commonly found in ARD. By identifying early stages of inflammation and perfusion defects, CMR can shed light on the exact pathophysiologic background of myocardial lesions, even if the underlying ARD seems stable. However, high cost and lack of availability and expertise limit wider adoption.
Hopefully, CMR will not have the same fate as Oedipous, who despite answering the Sphinx’s riddle successfully, finally came to a bitter end; for in the case of CMR overcoming fate is, in fact, in our hands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
In Greek mythology, the Sphinx was a monster with the head of a woman, the wings of a griffin and the body of a lion. The Sphinx stopped travellers on the road to the city of Thebes and asked them a riddle. If they answered wrong they were killed by the Sphinx. Cardiovascular Disease (CVD) is the Sphinx of our times in the management of Autoimmune Rheumatic Diseases (ARD) and the riddle, for which we also seem to lack the answer, is a complex cardiovascular pathophysiology. The systemic manifestations of ARD attract most of the attention of attending rheumatologists, while cardiologists are mostly unaware of CVD in ARD. Lack of diagnostic algorithms for early assessment of ARD, when CVD is still silent only compounds these issues [1]. Furthermore, although use of targeted treatment in ARD has led to a reduction of disease-associated mortality, increased CVD incidence lowers life expectancy compared to the general population [2–7].
The clinical scenarios leading to increased CVD in ARD include:
-
a)
Atherosclerosis and macro-microvascular Coronary Artery Disease (CAD). These are the commonest suspects causing increased CVD and mortality in ARD [8]. Rheumatoid Arthritis (RA) has a 1.5- to 2.0-fold risk of CAD, compared with controls, even before the diagnosis of arthritis [9–11]. Traditional risk assessment usually underestimates CVD risk in ARD [12, 13]. Furthermore, inflammation and autoimmunity, the main characteristics of ARD, promote the development of atherosclerosis [14, 15]. RA, spondyloarthropathies, Systemic Lupus Erythematosus (SLE), Antiphospholipid Syndrome (APS) and systemic vasculitides have all been associated with accelerated atherosclerosis [16–20, 38]. Notably, CAD risk in RA is similar to that of diabetes mellitus and the associated atherosclerotic plaques are more susceptible to instability and rupture, with higher re-infraction rates and worse prognosis [21–23].
Although SLE presents with less “inflammatory reaction” compared with RA and/or spondyloarthropathies, it is characterized by higher incidence of CVD. Atherosclerosis in SLE is associated with older age at diagnosis and longer disease duration, supporting the hypothesis that chronic exposure to SLE immune dysregulation promotes CVD [24]. Early damage of both macro- and microvasculature occurs before or shortly after SLE diagnosis [25, 26]. Macro-or microvascular CAD is also common in SLE, with 54 % of patients having non-calcified coronary plaques and impaired coronary flow reserve, even in patients with seemingly normal coronary arteries [27, 28]. These findings are well correlated with disease activity and disease duration [27, 29–33]. Finally, High Density Lipoprotein (HDL) is decreased in SLE, while Low Density Lipoprotein (LDL), Very Low Density Lipoprotein (VLDL), proinflammatory HDL and triglyceride levels are increased [34, 35]. Therefore, early treatment of immune dysregulation is imperative for CAD prevention.
-
b)
Pericardial, Myocardial and Vascular inflammation. Inflammatory heart disease, despite being considered a rare complication of ARD, can significantly contribute to increases in CVD mortality. The typical appearance of myopericarditis is more common in SLE, but also has notable impact in CVD morbidity of other ARDs, such as RA, mixed collagen diseases, systemic sclerosis, vasculitis, sarcoidosis and inflammatory myopathies [35]. Overt clinical presentation of myopericarditis is typically found only in a minority of SLE patients. In the majority of ARD patients, however, myopericarditis remains silent, leading to delays in diagnosis which in turn can cause deterioration into Heart Failure (HF) and arrhythmias [36].
Additionally, inflammation of large-, medium- and small-sized blood vessel walls, either due to primary or secondary ARD, may lead to stroke, myocardial infarction, visual abnormalities, limb/jaw claudication and digital ulcers [37]. Myocardial vasculitis is common in medium/small vessel vasculitis and in APS; it may cause myocardial ischemia due to involvement of epicardial coronary arteries, or small intramural cardiac vessels, finally leading to Left Ventricular (LV) impairment [38, 39].
-
c)
Heart Valve Disease (HVD). HVD is the commonest cardiac disease in APS, with a prevalence of 30 %. Diagnosis is based on the presence of valvular thickening or vegetations (mainly mitral and aortic) as described by Libman and Sacks for patients with SLE [40]. The coexistence of Antiphospholipid Antibodies (aPL) with SLE carries a 3-fold greater risk of HVD and confirms that the involvement of these antibodies in the pathophysiologic mechanism leads to valve thrombosis secondary to hypercoagulability [41]. Progressive valve disease has also been identified in RA [42, 43]. Transthoracic and/or Transoesophageal Echocardiography (TTE and TEE, respectively) are currently the cornerstone of early and accurate diagnosis [41].
-
d)
Heart Failure (HF). HF in ARD can be caused by ischemic or non-ischemic heart disease and contributes to increased mortality [44]. In RA, the prevalence of HF is 2-fold higher than in general population and remains increased after adjusting for classic CVD risk factors and ischemic heart disease [45, 46]. HF is also seen in SLE, inflammatory myopathies, systemic sclerosis, ankylosing spondylitis and vasculitis, as the endpoint of myocardial inflammation [47–50].
Fibrotic cardiomyopathy, highly prevalent in systemic sclerosis, vasculitis, myositis and sarcoidosis, may also lead to impaired LV function [51]. However, LV systolic function remains normal until the late stages of the disease and only imaging techniques such as Tissue Doppler and Cardiovascular Magnetic Resonance (CMR) can identify these lesions at a preclinical stage [52, 53].
-
e)
Pulmonary Arterial Hypertension (PAH). PAH affects 0.5-15 % of ARD and carries worse prognosis compared with idiopathic PAH. It is most common in systemic sclerosis and is responsible for 30 % of disease-related deaths [54]. Microangiopathy and chronic hypoxemia secondary to interstitial lung fibrosis are the major causes of PAH in SSc [55]. LV dysfunction and thromboembolic disease are additional causes of PAH in ARD and are due to APS and primary Sjogren syndrome respectively [56, 57]. Despite the use of new imaging modalities, more than 50 % of PAH in ARD is missed, and is diagnosed only when the disease is in advanced stages [58]. A schematic expression of various clinical scenarios of CVD in ARD was presented in Table 1.
Table 1 Clinical scenarios of CVD in ARD
Cardiovascular diagnosis in the era of multimodality imaging
Cardiology uses various non-invasive imaging modalities, such as rest/stress Electrocardiogram (ECG), echocardiography, nuclear techniques and more recently CMR, to detect ischemic or inflammatory disease in ARD. Exercise ECG (ExECG) is a reliable prognostic test for identification of patients who are either very unlikely or very likely to have cardiac events. However, this is not the case for intermediate risk patients or patients with small vessel vasculitis, who may be missed both by routine non-invasive as well as invasive assessment [59]. ECGs can be indicative of myocarditis, and there is an association between transmural LV myocardial oedema detected by CMR and T wave inversion in clinically suspected acute myocarditis. However, this is an expression of reversible myocardial oedema during the acute disease phase and can not be used as a predictor of LV systolic dysfunction during follow-up [60].
Echocardiography is the cornerstone imaging technique to assess cardiac morphology in everyday practice. The addition of new techniques, such as speckle tracking echocardiography and in particular longitudinal deformation, has been successfully used for the assessment of subclinical LV dysfunction and monitoring of the effects of anakinra, an interleukin-1 receptor antagonistant, in treatment on LV function in ARD patients, particularly when CAD coexists [61–64].
Stress echocardiography is defined as a combination of 2D echocardiography either with a physical or pharmacological stress. The diagnostic end point for the detection of myocardial ischemia is the induction of a transient worsening in regional function during stress. It has similar diagnostic and prognostic accuracy as radionuclide stress perfusion tests, but at a lower cost, without environmental impact and with no biohazards for patients and physicians. Additionally, the assessment of coronary flow reserve by Doppler echocardiography can reveal microvascular disease in ARD [65] and also subclinical coronary artery disease [66]. Despite being an operator dependent technique, unable to perform tissue characterisation, echocardiography is the most cost-effective approach for the non-invasive exclusion and/or evaluation of the CAD possibility [67].
Stress Myocardial Perfusion Scintigraphy (MPS) is a non-invasive imaging modality used in patients with suspected CAD, commonly found in RA and SLE [68]. However, MPS has serious limitations including radiation exposure, imaging artefacts and low spatial resolution, which do not permit detection of subendocardial ischemia and small scars seen in ARD [69]. The CE-MARC study currently supports wider adoption of CMR for CAD assessment, owing to concerns regarding carcinogenesis risks associated with medical use of ionising radiation [70]. In a study comparing of Single Photon Emission Tomography (SPECT), Positron Emission Tomography (PET), and CMR, all techniques were shown to have high sensitivity, with a broad range for specificity observed in CMR [71].
Conclusions
To correlate Greek mythology with more contemporary terms, CMR now takes the place of Oedipus in our paradigm. Like its “predecessor”, CMR is capable of successfully answering the riddle of CVD in ARD, by increasing our diagnostic capabilities and detecting cardiac pathology before any change in systolic or diastolic indexes takes place [1]. Additionally, by identifying early stages of inflammation and perfusion defects, CMR can shed light on the exact pathophysiologic background of myocardial lesions even if the underlying ARD appears stable. It also provides the additive value of being able to assess myocardial status independently of systemic inflammatory processes, leading to individualization of cardiac treatment, irrespective of ARD status [1]. Finally, stress perfusion CMR allows for early detection of microvascular disease, a common finding in ARD, commonly missed by nuclear imaging techniques due to their low spatial resolution [69].
Unfortunately, lack of availability and expertise, insufficient collaboration between cardiologists and radiologists, unfamiliarity of rheumatologists with the advantages of CMR in ARD and significant associated costs, especially when a stress study is added, are serious obstacles preventing more widespread adoption of CMR in ARD monitoring. Hopefully, this excellent diagnostic tool will not have the same fate as Oedipous, who despite successfully answering the Sphinx’s riddle, finally came to a bitter end; for in the case of CMR, overcoming fate is, in fact, in our hands.
References
Mavrogeni SI, Kitas GD, Dimitroulas T, Sfikakis PP, Seo P, Gabriel S, Patel AR, Gargani L, Bombardieri S, Matucci-Cerinic M, Lombardi M, Pepe A, Aletras AH, Kolovou G, Miszalski T, van Riel P, Semb A, Gonzalez-Gay MA, Dessein P, Karpouzas G, Puntmann V, Nagel E, Bratis K, Karabela G, Stavropoulos E, Katsifis G, Koutsogeorgopoulou L, van Rossum A, Rademakers F, Pohost G, Lima JA. Cardiovascular magnetic resonance in rheumatology: Current status and recommendations for use. Int J Cardiol. 2016;217:135–48.
Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–7.
Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol. 2006;2:99–106.
Kitas GD, Gabriel SE. Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis. 2011;70:8–14.
Hollan I, Meroni PL, Ahearn JM, Cohen Tervaert JW, Curran S, Goodyear CS, Hestad KA, Kahaleh B, Riggio M, Shields K, Wasko MC. Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev. 2013;12(10):1004–15.
Björnådal L, Yin L, Granath F, Klareskog L, Ekbom A. Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964-95. J Rheumatol. 2004;31(4):713–9.
Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol. 2011;7(7):399–408.
Tanasescu C, Jurcut C, Jurcut R, Ginghina C. Vascular disease in rheumatoid arthritis: from subclinical lesions to cardiovascular risk. Eur J Intern Med. 2009;20:348–54.
Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;14:722–32.
Solomon DH, Goodson NJ, Katz JN, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis. 2006;65:1608–12.
Chung CP, Giles JT, Petri M, Szklo M, Post W, Blumenthal RS, Gelber AC, Ouyang P, Jenny NS, Bathon JM. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41(4):535–44.
Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, Côte R, Grover SA, Fortin PR, Clarke AE, Senécal JL. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2331–7.
Arts EE, Popa C, Den Broeder AA, Semb AG, Toms T, Kitas GD, van Riel PL, Fransen J. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2014. doi: 10.1136/annrheumdis-2013-204024
Abou-Raya A, Abou-Raya S. Inflammation: a pivotal link between autoimmune diseases and atherosclerosis. Autoimmun Rev. 2006;5:331–7.
Libby P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am J Med. 2008;121(10 Suppl 1):S21–31.
Sandoo A, Veldhuijzen van Zanten JJ, Metsios GS, Carroll D, Kitas GD. Vascular function and morphology in rheumatoid arthritis: a systematic review. Rheumatology (Oxford). 2011;50(11):2125–39.
Azevedo VF, Pecoits-Filho R. Atherosclerosis and endothelial dysfunction in patients with ankylosing spondylitis. Rheumatol Int. 2010;30:1411–6.
Kimhi O, Caspi D, Bornstein NM, Maharshak N, Gur A, Arbel Y, Comaneshter D, Paran D, Wigler I, Levartovsky D, Berliner S, Elkayam O. Prevalence and risk factors of atherosclerosis in patients with psoriatic arthritis. Semin Arthritis Rheum. 2007;36(4):203–9.
Schoenfeld SR, Kasturi S, Costenbader KH. The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: a systematic review. Semin Arthritis Rheum. 2013;43(1):77–95.
Djokovic A, Stojanovich L, Stanisavljevic N, Bisenic V, Radovanovic S, Soldatovic I, Simic DV. Does the presence of secondary antiphospholipid syndrome in patients with systemic lupus erythematodes accelerate carotid arteries intima-media thickness changes? Rheumatol Int. 2014;34(3):321-7.
Cohen Tervaert JW. Cardiovascular disease due to accelerated atherosclerosis in systemic vasculitides. Best Pract Res Clin Rheumatol. 2013;27:33–44.
Nurmohamed MT, Kitas G. Cardiovascular risk in rheumatoid arthritis and diabetes: how does it compare and when does it start? Ann Rheum Dis. 2011;70(6):881–3.
Aubry MC, Maradit-Kremers H, Reinalda MS, Crowson CS, Edwards WD, Gabriel SE. Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. J Rheumatol. 2007;34:937–42.
Kahlenberg JM, Kaplan MJ. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu Rev Med. 2013;64:249–63.
Roman MJ, Crow MK, Lockshin MD, Devereux RB, Paget SA, Sammaritano L, Levine DM, Davis A, Salmon JE. Rate and determinants of progression of atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2007;56:3412–9.
Rajagopalan S, Somers EC, Brook RD, Kehrer C, Pfenninger D, Lewis E, Chakrabarti A, Richardson BC, Shelden E, McCune WJ, Kaplan MJ. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103:3677–83.
Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic infl ammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30:1837–43.
Ishimori ML, Martin R, Berman DS, Goykhman P, Shaw LJ, Shufelt C, Slomka PJ, Thomson LE, Schapira J, Yang Y, Wallace DJ, Weisman MH, Bairey Merz CN. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging. 2011;4(1):27–33.
El-Magadmi M, Bodill H, Ahmad Y, Durrington PN, Mackness M, Walker M, Bernstein RM, Bruce IN. Systemic lupus erythematosus: an independent risk factor for endothelial dysfunction in women. Circulation. 2004;110:399–404.
Roldan C, Joson J, Sharrar J, Qualls C, Sibbitt W. Premature aortic atherosclerosis in systemic lupus erythematosus: a controlled transesophageal echocardiographic study. J Rheumatol. 2010;37:71–8.
Manzi S, Selzer F, Sutton-Tyrrell K, Fitzgerald SG, Rairie JE, Tracy RP, Kuller LH. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:51–60.
Kiani AN, Vogel-Claussen J, Magder LS, Petri M. Noncalcified coronary plaque in systemic lupus erythematosus. J Rheumatol. 2010;37:579–84.
Pieretti J, Roman MJ, Devereux RB, Lockshin MD, Crow MK, Paget SA, Schwartz JE, Sammaritano L, Levine DM, Salmon JE. Systemic lupus erythematosus predicts increased left ventricular mass. Circulation. 2007;116:419–26.
Borba EF, Bonf E, Vinagre CG, Ramires JA, Maranho RC. Chylomicron metabolism is markedly altered in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1033–40.
McMahon M, Grossman J, Skaggs B, Fitzgerald J, Sahakian L, Ragavendra N, Charles-Schoeman C, Watson K, Wong WK, Volkmann E, Chen W, Gorn A, Karpouzas G, Weisman M, Wallace DJ, Hahn BH. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 2009;60:2428–37.
Mavrogeni S, Dimitroulas T, Kitas GD. Multimodality imaging and the emerging role of cardiac magnetic resonance in autoimmune myocarditis. Autoimmun Rev. 2012;12(2):305–12.
Mavrogeni S, Spargias C, Bratis C, Kolovou G, Markussis V, Papadopoulou E, et al. Myocarditis as a precipitating factor for heart failure: evaluation and 1-year follow-up using cardiovascular magnetic resonance and endomyocardial biopsy. Eur J Heart Fail. 2011;13:830–7.
Mackie SL, Dasgupta B. Vasculitis syndromes: Dealing with increased vascular risk and mortality in GCA. Nat Rev Rheumatol. 2014. doi:10.1038/nrrheum.2014.38
Mavrogeni S, Sfikakis PP, Gialafos E, Karabela G, Stavropoulos E, Sfendouraki E, Panopoulos S, Kolovou G, Kitas GD. Diffuse, subendocardial vasculitis. A new entity identified by cardiovascular magnetic resonance and its clinical implications. Int J Cardiol. 2013;168(3):2971–2.
Amigo MC. What do we know about the cardiac valve lesion in the antiphospholipid syndrome (APS)? Lupus. 2014;23(12):1259–61.
Sharma J, Lasic Z, Bornstein A, Cooper R, Chen J. Libman-Sacks endocarditis as the first manifestation of systemic lupus erythematosus in an adolescent, with a review of the literature. Cardiol Young. 2013;23(1):1–6.
Tarkin JM, Hadjiloizou N, Savage HO, Prasad SK, Sheppard MN, Moat NE, Kaddoura S. Severe cardiac failure due to rapidly progressive rheumatoid arthritis-associated valvulopathy. Cardiovasc J Afr. 2012;23(7):e1–3.
Aziz S, Sohail M, Murphy G. Acute aortic regurgitation due to necrotizing granulomatous inflammation of the aortic valve in a patient with rheumatoid arthritis. Circulation. 2012;126(8):e106–7.
Gonzalez A, Maradit Kremers H, Crowson CS, Nicola PJ, Davis 3rd JM, Therneau TM, Roger VL, Gabriel SE. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56(11):3583–7.
Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: a focus on heart failure. Heart Fail Clin. 2014;10(2):339–52.
Nicola PJ, Maradit-Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, Gabriel SE. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52(2):412–20.
Alpaslan M, Onrat E, Evcik D. Doppler echocardiographic evaluation of ventricular function in patients with rheumatoid arthritis. Clin Rheumatol. 2003;22(2):84–8.
Dimitroulas T, Giannakoulas G, Papadopoulou K, Sfetsios T, Karvounis H, Dimitroula H, Parcharidou D, Koliakos G, Garyfallos A, Styliadis I, Settas L. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol. 2010;29(9):957–64.
Buss SJ, Wolf D, Korosoglou G, et al. Myocardial left ventricular dysfunction in patients with systemic lupus erythematosus: new insights from tissue Doppler and strain imaging. J Rheumatol. 2010;37(1):79–86.
Sandoo A, Protogerou AD, Hodson J, Smith JP, Zampeli E, Sfikakis PP, Kitas GD. The role of inflammation, the autonomic nervous system and classical cardiovascular disease risk factors on subendocardial viability ratio in patients with RA: a cross-sectional and longitudinal study. Arthritis Res Ther. 2012;14(6):R258.
Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des. 2014;20(4):536–44.
Mavrogeni S, Bratis K, van Wijk K, Stavropoulos E, Hautemann D, Reiber JH, Kolovou G. Myocardial perfusion-fibrosis pattern in systemic sclerosis assessed by cardiac magnetic resonance. Int J Cardiol. 2012;159(3):e56–8.
Dimitroulas T, Giannakoulas G, Papadopoulou K, Karvounis H, Dimitroula H, Koliakos G, et al. Early detection of cardiac involvement in systemic sclerosis assessed by tissue-Doppler echocardiography: relationship with neurohormonal activation and endothelial dysfunction. J Rheumatol. 2010;37:993–9.
Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66(7):940–4.
Dimitroulas T, Giannakoulas G, Karvounis H, Settas L, Kitas GD. Systemic sclerosis-related pulmonary hypertension: unique characteristics and future treatment targets. Curr Pharm Des. 2012;18(11):1457–64.
Pardos-Gea J, Avegliano G, Evangelista A, Vilardell M, Ordi-Ros J. Cardiac manifestations other than valvulopathy in antiphospholipid syndrome: long-time echocardiography follow-up study. Int J Rheum Dis. 2013;18(1):76-83.
Kobak S, Kalkan S, Kirilmaz B, Orman M, Ercan E. Pulmonary arterial hypertension in patients with Primary Sjögren’s Syndrome. Autoimmune Dis. 2014;2014:710401.
Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum. 2011;41:19–37.
Gibbons RJ, Balady GJ, Beasley JW, et al. ACC/AHA guidelines for exercise testing. J Am Coll Cardiol. 1997;30:260–311.
De Lazzari M, Zorzi A, Baritussio A, Siciliano M, Migliore F, Susana A, Giorgi B, Lacognata C, Iliceto S, Perazzolo Marra M, Corrado D. Relationship between T-wave inversion and transmural myocardial edema as evidenced by cardiac magnetic resonance in patients with clinically suspected acute myocarditis: clinical and prognostic implications. J Electrocardiol. 2016;S0022-0736(16):30016–4.
Ikonomidis I, Tzortzis S, Andreadou I, Paraskevaidis I, Katseli C, Katsimbri P, Pavlidis G, Parissis J, Kremastinos D, Anastasiou-Nana M, Lekakis J. Increased benefit of interleukin-1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ Cardiovasc Imaging. 2014;7(4):619–28.
Ikonomidis I, Tzortzis S, Lekakis J, Paraskevaidis I, Dasou P, Parissis J, Nikolaou M, Markantonis SL, Katsimbri P, Skarantavos G, Andreadou I, Anastasiou-Nana M. Association of soluble apoptotic markers with impaired left ventricular deformation in patients with rheumatoid arthritis. Effects of inhibition of interleukin-1 activity by anakinra. Thromb Haemost. 2011;106(5):959–67.
Ikonomidis I, Tzortzis S, Lekakis J, Paraskevaidis I, Andreadou I, Nikolaou M, Kaplanoglou T, Katsimbri P, Skarantavos G, Soucacos P, Kremastinos DT. Lowering interleukin-1 activity with anakinra improves myocardial deformation in rheumatoid arthritis. Heart. 2009;95(18):1502–7.
Ikonomidis I, Lekakis JP, Nikolaou M, Paraskevaidis I, Andreadou I, Kaplanoglou T, Katsimbri P, Skarantavos G, Soucacos PN, Kremastinos DT. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation. 2008;117(20):2662–9.
Kakuta K, Dohi K, Sato Y, Yamanaka T, Kawamura M, Ogura T, Nakamori S, Fujimoto N, Fujii E, Yamada N, Ito M. Chronic inflammatory disease is an independent risk factor for coronary flow velocity reserve impairment unrelated to the processes of coronary artery calcium deposition. J Am Soc Echocardiogr. 2016;29(2):173–80.
Hirata K, Kadirvelu A, Kinjo M, Sciacca R, Sugioka K, Otsuka R, Choy A, Chow SK, Yoshiyama M, Yoshikawa J, Homma S, Lang CC. Altered coronary vasomotor function in young patients with systemic lupus erythematosus. Arthritis Rheum. 2007;56(6):1904–9.
Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, Voigt JU, Zamorano JL, European Association of Echocardiography. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr. 2008;9(4):415–37.
Faccini A, Kaski JC, Camici PG. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. Eur Heart J. 2016;37(23):1799–806.
Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, Diamond GA. Exercise myocardial perfusion SPECT in patients without known coronary artery disease. Circulation. 1996;93:905–14.
Greenwood JP, Motwani M, Maredia N, Brown JM, Everett CC, Nixon J, Bijsterveld P, Dickinson CJ, Ball SG, Plein S. Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the ce-marc trial. Circulation. 2014;129(10):1129–38.
Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, Nelemans PJ, Schalla S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59(19):1719–28.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
No applicable.
Authors’ contributions
All authors had equally contributed for the construction of the current manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mavrogeni, S., Markousis-Mavrogenis, G. & Kolovou, G. The Sphinx’s riddle: cardiovascular involvement in autoimmune rheumatic disease. BMC Cardiovasc Disord 16, 204 (2016). https://doi.org/10.1186/s12872-016-0381-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-016-0381-5