Abstract
Background
Dexmedetomidine and propofol are common sedatives in intensive care units and for interventional procedures. Both may compromise sinus node function and atrioventricular conduction.
The objective of this prospective, randomized study is to compare the effect of dexmedetomidine with propofol on sinus node function and atrioventricular conduction.
Methods
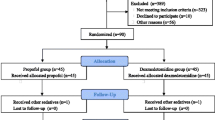
In a tertiary care center in Switzerland we included from September 2019 to October 2020 160 patients (65 ± 11 years old; 32% female) undergoing first ablation for atrial fibrillation by cryoballoon ablation or by radiofrequency ablation.
Patients were randomly assigned to deep sedation with dexmedetomidine (DEX group) versus propofol (PRO group). A standard electrophysiological study was performed after pulmonary vein isolation with the patients still deeply sedated and hemodynamically stable.
Results
Eighty patients each were randomized to the DEX and PRO group. DEX group patients had higher baseline sinus cycle length (1022 vs. 1138 ms; p = 0.003) and longer sinus node recovery time (SNRT400; 1597 vs. 1412 ms; p = 0.042). However, both corrected SNRT and normalized SNRT did not differ. DEX group patients had longer PR interval (207 vs. 186 ms; p = 0.002) and AH interval (111 vs. 95 ms, p = 0.008), longer Wenckebach cycle length of the atrioventricular node (512 vs. 456 ms; p = 0.005), and longer atrioventricular node effective refractory period (390 vs. 344 ms; p = 0.009). QRS width and HV interval were not different. An arrhythmia, mainly atrial fibrillation, was induced in 33 patients during the electrophysiological study, without differences among groups (20% vs. 15%, p = 0.533).
Conclusions
Dexmedetomidine has a more pronounced slowing effect on sinus rate and suprahissian AV conduction than propofol, but not on infrahissian AV conduction and ventricular repolarization. These differences need to be taken into account when using these sedatives.
Trial registration
ClinicalTrials.gov number NCT03844841, 19/02/2019
Similar content being viewed by others
Introduction
With the evolution of medical technologies, medicine has witnessed a shift from traditional, surgical interventions to interventional procedures. The latter are often performed on an outpatient basis and may involve complex and long-lasting interventions. General anesthesia is usually avoided during such procedures and mild to deep sedation is applied instead. Depending on local policy, sedation may be performed by the interventionalists themselves without anesthesiologist support. Traditionally, benzodiazepines (such as midazolam) and opioids have been used for sedation. However, to optimize patient comfort and procedural safety, propofol and dexmedetomidine are frequently employed nowadays. These drugs are used for interventional procedures in various settings, including cardiology, gastroenterology, pulmonology, neurosurgery, ophthalmology, intensive care and many more.[1,2,3,4,5,6,7,8,9,10].
Besides effects on hemodynamics and ventilation, both drugs may also influence cardiac chronotropy and dromotropy. Dexmedetomidine, which is an alpha-2 adrenergic agonist, does in particular affect heart rate and atrioventricular nodal conduction and may induce sinus arrest or complete heart block in susceptible patients. Bradycardia on the other side has also been described after propofol administration. No study to date has directly compared the electrophysiological effects of these two drugs in a randomized controlled trial.
Materials and methods
In this study, patients with atrial fibrillation undergoing pulmonary vein isolation were randomized to sedation with propofol (PRO group) versus dexmedetomidine (DEX group). The primary study outcome was a composite endpoint of inefficient sedation, respiratory depression and hemodynamic changes. The methods and main results of the study have been published elsewhere.[11] In brief, a total of 160 patients undergoing first pulmonary vein isolation for atrial fibrillation by cryoballoon ablation or by radiofrequency ablation were randomized 1:1 to procedural sedation with dexmedetomidine versus propofol.
Patient characteristics were collected on patient inclusion and a standard 12-lead ECG was performed the day before the procedure. On the 12-lead ECG, RR, PR and QT intervals were measured in leads II or V5. These measurements were repeated on the 12-lead ECG during the electrophysiological study. The Bazett formula was used to correct the QT interval for heart rate (QTc).
Ethics
Ethical approval for this study (N° 2018–02128) was provided by the Ethical Committee of the Kanton of Bern (Kantonale Ethikkommission, Murtenstrasse 31, Hörsaaltrakt Pathologie, Eingang 43A, Büro H372, 3010 Bern; Chairperson Prof Robert Greif) on 12 February 2019. All patients provided written informed consent to participate.
Procedural sedation
Upon arrival of the patient at the operating room, 50 µg fentanyl and 1 mg of midazolam were given. Patients in the dexmedetomidine arm additionally received 4 mg of ondansetron. After five minutes, sedation with propofol or dexmedetomidine was started if the patient was stable.
For propofol sedation we used a target-controlled infusion (TCI) pump using the Schnider pharmacokinetic model with an effect-site propofol concentration initially set to 1.2 µg/ml, unless the patient was already sedated by the initial fentanyl and midazolam dose, in which case an effect-site propofol concentration of 0.8–1.0 µg/ml was chosen. During the procedure, the effect-site propofol concentration was adjusted stepwise (by 0.2 µg/ml) to reach a target score of 3 on the “Modified Observer’s Assessment of Alertness/Sedation” (MOAA/S) scale.
For dexmedetomidine sedation we infused a dexmedetomidine loading dose of 0.8 µg/kg over 3 min via a perfusor pump, which was automatically continued at a maintenance dose of 0.8 µg/kg/h after loading. If the patient was already sedated by the premedication, no dexmedetomidine loading dose was administered and dexmedetomidine initiated at a maintenance dose of 0.4–0.8 µg/kg/h. During the procedure, the dexmedetomidine maintenance dose was adjusted stepwise (using steps of 0.2 µg/ml) to reach a target score of 3 on the MOAA/S scale.
All patients in both arms received an additional bolus of fentanyl (20–50 µg) just before beginning of ablation, and additional fentanyl was administered bolus-wise (10–30 µg) at the discretion of the treating electrophysiologist, as necessary. Addition of 1–2 mg of midazolam during the procedure was allowed in case of anxious or agitated patients. Vasoactive agents were not allowed, since they would have altered the endpoints of the main study. If patients were in atrial fibrillation we performed electrical cardioversion after deepening sedation to a target score of 2 on the MOAA/S scale. To achieve this, either the effect-site propofol concentration was temporarily increased in PRO group patients, or propofol boluses of 20 mg were added stepwise in DEX group patients. The electrophysiological study was performed during the waiting period after isolation of the pulmonary veins with the patients in sinus rhythm and hemodynamically stable. Deep sedation with propofol or dexmedetomidine was continued unchanged during the electrophysiological study.
Electrophysiological study
A decapolar catheter was placed at the His for measurements of atrial—His- (AH) and His – ventricular (HV) intervals. To assess the Wenckebach cycle length of the atrioventricular (AV) node, we paced the atrium with decreasing cycle length (in steps of 10 ms) until we observed loss of 1:1 AV conduction. To measure the effective refractory period of the atrium and AV node we delivered a drive train (S1) of six paced beats with a cycle length of 600 ms followed by a premature, paced beat (S2) at a programmed coupling interval. The coupling interval of S2 was decreased in steps of 10 ms for each consecutive drive train until loss of atrial activation or AV conduction occurred, respectively. The effective refractory period of the atrium and AV node was the longest coupling interval of S2, which failed to elicit atrial activation or AV node conduction, respectively. To determine the sinus node recovery time (SNRT) we paced the atrium for 30 s at cycle lengths of 600, 500 and 400 ms, and then stopped pacing abruptly. The SNRT was the pause induced by this overdrive suppression until the first sinus beat (interval following the cessation of pacing). If an ectopic beat occurred after cessation of pacing, the test was repeated. To calculate the corrected SNRT (cSNRT), we subtracted the sinus cycle length (SCL) from the SNRT. The SCL was measured just before pacing the atrium for 30 s at the respective cycle lengths. To calculate the normalized SNRT (nSNRT) we divided the SNRT by the SCL. Finally, we assessed whether atrial fibrillation or any other supraventricular tachycardia occurred during the electrophysiological study.
Statistical analyses
Continuous variables are expressed as means with standard deviations or medians with interquartile ranges (IQR), and categorical variables as frequencies with percentages. Continuous variables were compared using the Mann–Whitney U test or t-test in case of two-group comparison. Differences in proportions were tested with Pearson’s χ2 test or Fisher’s exact test, as appropriate. The relationship between the continuous variables (RR-, PR-, and AH-interval as well as Wenckebach CL of the AV node) and selected demographic and clinical variables were computed using a linear regression model accounting for robust standard errors. To account for possible associations among variables, a backward selection considering the most predictive variables in the univariable results (with selection of p-value lower than 0.2) was performed. Consequently, multivariable models were estimated to measure the influence of the adjusted effects considering only the most informative covariates, where the AIC was applied to select the most predictive variables. All analyses were performed using Stata 17.0 (StataCorp. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC).
Results
Patient characteristics
In total, 160 patients (mean age 65 years; 32% females) were enrolled in the study from September 2019 to October 2020. Of these, 80 patients (50%) received dexmedetomidine and 80 (50%) propofol sedation regimen. Patient characteristics are shown in Table 1. In 79 patients, point by point radiofrequency ablation was used and in 81 patients, cryoballoon ablation was used.
Sedation characteristics
A mean of 231 ± 111 mcg dexmedetomidine was administered in DEX group patients, and a mean of 657 ± 356 mg of propofol in PRO group patients. Table 2. gives all details regarding sedatives administered during the procedure for both groups. Mean and minimal MOAA/S score were not different among groups. At the end of the procedure, when the electrophysiological study was performed, the majority of all patients had a MOAA/S score ≤ 3.[11].
Sinus Node Function according to the type of sedation used
During the electrophysiological study, mean sinus CL was higher in DEX group compared to PRO group patients (1138 vs. 1022 ms; p = 0.003; Table 3). In DEX group patients, absolute SNRT was longer when pacing at cycle lengths of 600 ms and 400 ms and with a trend towards a longer SNRT when pacing at cycle length 500 ms. Both cSNRT and nSNRT were not different among groups for all three pacing cycle lengths (Table 3). There was a trend towards a longer atrial ERP in DEX group patients (270 vs. 255 ms; p = 0.051). The number of atrial arrhythmias induced during the electrophysiological study was not different among groups (26% vs. 17%; p = 0.174), and atrial fibrillation was the most frequent arrhythmia induced in both groups (16 in DEX group [20%] vs. 12 in PRO group [15%]; p = 0.533).
AV Node function according to the type of sedation used
PR interval (207 vs. 186 ms; p = 0.002), AH interval (111 vs. 95 ms; p = 0.008), the ERP of the AV node (390 vs. 344 ms; p = 0.009) and the Wenckebach cycle length of the AV node (512 vs. 456 ms; p = 0.005) were all longer in DEX group patients compared to PRO group patients (Table 3 and Fig. 1).
Venticular De- and repolarization according to the type of sedation used
QRS duration and HV interval were not different among groups. The QT interval was longer (447 vs. 417 ms; p < 0.001; Table 3) in DEX group patients. However, the corrected QT interval was not different among groups (424 vs. 416 ms; p = 0.226).
Predictors of sinus node and AV-Node properties
In univariable analysis, the RR interval correlated with DEX group assignment, older age and larger LAVI (Table 4). In multivariable analysis, these variables remained in the model, and absence of coronary artery disease was added as another predictive variable (Table 5). Regarding PR and AH interval, DEX group assignment and older age were predictive for both in univariable analysis, as well as hypertension and larger LAVI for PR interval (Table 4). In multivariable analysis, dexmedetomidine group assignment and age remained in both models, with LAVI added to predict PR interval and LVEF for prediction of AH interval (Table 5). Group assignment to dexmedetomidine and LAVI were also correlated to the Wenckebach cycle length of the AV node in univariable analysis (Table 4). In multivariable analysis, hypertension and betablocker use were added as explaining variables (Table 5).
Discussion
Deep sedation with dexmedetomidine, in comparison to propofol, affects suprahissian conduction. It results in prolongation of the PR and AH interval and increases the Wenckebach cycle length of the atrioventricular node. Dexmedetomidine also lowers sinus rate compared to propofol, with prolongation of SNRT, but both corrected as well as normalized SNRT are not different.
Bradycardia is a well-described phenomenon of dexmedetomidine sedation. Cases of cardiac arrest of up to 4 min duration have been described during dexmedetomidine infusion, necessitating cardiopulmonary resuscitation.[12, 13] Several meta-analysis confirmed an increased risk of bradycardia during dexmedetomidine sedation.[14] Cases of prolonged, complete AV block have also been reported with dexmedetomidine sedation, and age > 50 years and cardiac comorbidities described as risk factors.[15, 16] Likewise, the occurrence of complete AV block has been reported during propofol administration,[17,18,19] and propofol infusion does also increase the risk of bradycardia.[20].
So far, no head-to-head comparison of the electrophysiological effects of dexmedetomidine versus propofol has been performed. However, previous electrophysiological studies showed that dexmedetomidine has an impact on both AV and sinus node function. Sairaku randomized 215 patients to receive or not to receive dexmedetomidine during an electrophysiological study. They reported a longer, corrected SNRT, Wenckebach cycle length of the AV node, AV nodal ERP and AH interval in patients with dexmedetomidine sedation.[21] Poyhia performed an electrophysiological study in 11 patients before and after dexmedetomidine infusion.[22] They found a prolonged cSNRT, higher Wenckebach cycle length of the AV node and ERP of the AV node. Sinus cycle length and SNRT were not different in their study. Ergul et al. showed in 20 children that dexmedetomidine increased sinus rate and prolonged SNRT, cSNRT, AV Wenckebach cycle length of the AV node and AV nodal ERP.[23] However, they found no effect of dexmedetomidine on AH interval.
Previous electrophysiological studies on the effect of propofol on sinus and AV node function show a less consistent pattern. In a cross-over study, Warpechowski investigated the effect of propofol on AV nodal conduction properties in 12 patients with AV nodal reentry tachycardia. [24] They found no effect of propofol on AH and HV interval and on ERP of both the fast (antegrade and retrograde) and slow (antegrade) pathways. Similarly, Sharpe et al. demonstrated in 12 patients with Wolff-Parkinson-White syndrome that propofol, compared to alfentanyl and midazolam, did not affect the ERP of the AV node and sinus node function. In a pig model, propofol was shown to decrease sinus rate, to prolong cSNRT and to increase the HV interval in a dose-dependent way.[25] Matsushima recently reported that high-dose propofol in 23 pediatric patients prolonged the HV interval, but was without effect on AH interval and SNRT.[26].
Our randomized controlled study, directly comparing the electrophysiological effects of both drugs, clearly confirms a more pronounced effect of dexmedetomidine on sinus rate as well as on suprahissian conduction of the AV node. Age was the most important modifying factor besides group allocation regarding both sinus rate, PR and AH interval. Also importantly, we did not find a difference of the two drugs on infrahissian conduction, as both HV interval and QRS were not different among groups. Likewise, after correction of heart rate, we did not observe any difference in ventricular repolarization between the two sedatives. Our findings have clinical implications. Although both drugs have proven safe for deep sedation in various settings, propofol may be preferred over dexmedetomidine for deep sedation in patients with sinus bradycardia or AV conduction abnormalities. In particular in clinical settings, in which emergency cardiac pacing is not available.
Arrhythmia inducibility is another important point to consider during electrophysiological studies. To this regard it is reassuring that we did not find any difference in arrhythmia inducibility among groups. Similarly, in the study by Sairaku et al., atrial fibrillation inducibility was not different among patients with versus without dexmedetomidine administration.[21] Previous studies already reported that inducibility of supraventricular tachycardia is not compromised by both drugs. In 326 patients undergoing electrophysiological study for paroxysmal, supraventricular tachycardia, arrhythmia inducibility was retrospectively investigated by Slupe et al.[27] Compared to fentanyl and midazolam alone, the addition of dexmedetomidine did not affect arrhythmia inducibility. In the study by Matsushima described above, sustained reciprocating tachycardia were inducible in 8 of 12 patients, and propofol had no effect on electrophysiological properties.[26] However, because of the effect of dexmedetomidine on suprahissian conduction, propofol sedation may be preferred over dexmedetomidine sedation in patients with AV nodal reentry tachycardia. Our study is limited by the small number of patients and the results cannot be generalized to all patients, as patients with advanced conduction abnormalities or bradycardia and patients with impaired left ventricular function were excluded. Some DEX group patients had additional propofol administration for electrical cardioversion during the procedure, which may have affected the results of the EP study. However, the median dose of propofol administered was only 20 mg and the EP study was performed at least 10–15 min after propofol administration.
Conclusions
Dexmedetomidine, compared to propofol, has a more pronounced effect on sinus rate and suprahissian conduction of the AV node. No differences among the two drugs were observed on infrahissian conduction and ventricular repolarization. In patients with sinus or AV node disease propofol may be preferred over dexmedetomidine for deep sedation.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AH:
-
atrial - His
- HV:
-
His - ventricular
- AV:
-
atrioventricular
- CL:
-
cycle length
- cSNRT:
-
corrected sinus node recovery time
- ERP:
-
effective refractory period
- nSNRT:
-
normalized sinus node recovery time
- SCL:
-
sinus cycle length
- SNRT:
-
sinus node recovery time
- MOAA/S:
-
modified observer’s assessment of alertness/sedation
- TCI:
-
targedt controlled infusion
References
Pertzov B, Krasulya B, Azem K, et al. Dexmedetomidine versus propofol sedation in flexible bronchoscopy: a randomized controlled trial. BMC Pulm Med. 2022;22:87.
Salukhe TV, Willems S, Drewitz I, et al. Propofol sedation administered by cardiologists without assisted ventilation for long cardiac interventions: an assessment of 1000 consecutive patients undergoing atrial fibrillation ablation. Europace. 2012;14:325–30.
Servatius H, Hofeler T, Hoffmann BA, et al. Propofol sedation administered by cardiologists for patients undergoing catheter ablation for ventricular tachycardia. Europace. 2016;18:1245–51.
Dere K, Sucullu I, Budak ET, et al. A comparison of dexmedetomidine versus midazolam for sedation, pain and hemodynamic control, during colonoscopy under conscious sedation. Eur J Anaesthesiol. 2010;27:648–52.
Venn RM, Bradshaw CJ, Spencer R, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–42.
Alizadehasl A, Sadeghpour A, Totonchi Z, Azarfarin R, Rahimi S, Hendiani A. Comparison of sedation between dexmedetomidine and propofol during transesophageal echocardiography: A randomized controlled trial. Ann Card Anaesth. 2019;22:285–90.
Chen YT, Sun CK, Wu KY, et al. The use of propofol versus dexmedetomidine for patients receiving drug-induced sleep endoscopy: a meta-analysis of randomized controlled trials. J Clin Med. 2021;10:10.
Constantin JM, Momon A, Mantz J, et al. Efficacy and safety of sedation with dexmedetomidine in critical care patients: a meta-analysis of randomized controlled trials. Anaesthesia, critical care & pain medicine. 2016;35:7–15.
Goettel N, Bharadwaj S, Venkatraghavan L, Mehta J, Bernstein M, Manninen PH. Dexmedetomidine vs propofol-remifentanil conscious sedation for awake craniotomy: a prospective randomized controlled trial. Br J Anaesth. 2016;116:811–21.
Ter Bruggen F, Ceuppens C, Leliveld L, Stronks DL, Huygen F. Dexmedetomidine vs propofol as sedation for implantation of neurostimulators: A single-center single-blinded randomized controlled trial. Acta Anaesthesiol Scand. 2019;63:1321–9.
Servatius H, Kuffer T, Baldinger SH, et al. Dexmedetomidine versus propofol for operator-directed nurse-administered procedural sedation during catheter ablation of atrial fibrillation: A randomized controlled study. Heart Rhythm. 2022;19:691–700.
Amaniti A, Dalakakis I, Gkinas D, Sapalidis K, Grosomanidis V, Papazisis G. Corrigendum to “Bradycardia Leading to Asystole Following Dexmedetomidine Infusion during Cataract Surgery: Dexmedetomidine-Induced Asystole for Cataract Surgery.” Case Rep Anesthesiol. 2019;2019:7254218.
Banc-Husu AM, Badke CM, Sanchez-Pinto LN, Alonso EM. Dexmedetomidine leading to profound bradycardia in a pediatric liver transplant recipient. Pediatr Transplant. 2021;25:e13895.
Lewis K, Alshamsi F, Carayannopoulos KL, et al. Dexmedetomidine vs other sedatives in critically ill mechanically ventilated adults: a systematic review and meta-analysis of randomized trials. Intensive Care Med. 2022;48:811–40.
Takata K, Adachi YU, Suzuki K, Obata Y, Sato S, Nishiwaki K. Dexmedetomidine-induced atrioventricular block followed by cardiac arrest during atrial pacing: a case report and review of the literature. J Anesth. 2014;28:116–20.
Bharati S, Pal A, Biswas C, Biswas R. Incidence of cardiac arrest increases with the indiscriminate use of dexmedetomidine: a case series and review of published case reports. Acta Anaesthesiol Taiwan. 2011;49:165–7.
Noh JI, Lee JH, Woo SY, et al. Complete atrioventricular nodal block after propofol administration in an elderly patient undergoing total knee replacement arthroplasty -A case report. Korean J Anesthesiol. 2013;64:363–6.
Morozowich ST, Saslow SB. Progression of asymptomatic bifascicular block to complete heart block during upper gastrointestinal endoscopy with propofol sedation. Can J Anaesth. 2009;56:83–4.
Gauss A, Hubner C, Radermacher P, Georgieff M, Schutz W. Perioperative risk of bradyarrhythmias in patients with asymptomatic chronic bifascicular block or left bundle branch block: does an additional first-degree atrioventricular block make any difference? Anesthesiology. 1998;88:679–87.
Tramer MR, Moore RA, McQuay HJ. Propofol and bradycardia: causation, frequency and severity. Br J Anaesth. 1997;78:642–51.
Sairaku A, Yoshida Y, Hirayama H, Nakano Y, Ando M, Kihara Y. Procedural sedation with dexmedetomidine during ablation of atrial fibrillation: a randomized controlled trial. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2014;16:994–9.
Poyhia R, Nieminen T, Tuompo VWT, Parikka H. Effects of dexmedetomidine on basic cardiac electrophysiology in adults; a descriptive review and a prospective case study. Pharmaceuticals (Basel). 2022;15:15.
Ergul Y, Unsal S, Ozyilmaz I, Ozturk E, Carus H, Guzeltas A. Electrocardiographic and electrophysiologic effects of dexmedetomidine on children. Pacing and clinical electrophysiology : PACE. 2015;38:682–7.
Warpechowski P, Lima GG, Medeiros CM, et al. Randomized study of propofol effect on electrophysiological properties of the atrioventricular node in patients with nodal reentrant tachycardia. Pacing Clin Electrophysiol. 2006;29:1375–82.
Pires LA, Huang SK, Wagshal AB, Kulkarni RS. Electrophysiological effects of propofol on the normal cardiac conduction system. Cardiology. 1996;87:319–24.
Matsushima M, Kimura S, Kitaura A, et al. Propofol suppresses the His-ventricular conduction in paediatric patients. J Clin Pharm Ther. 2021;46:433–9.
Slupe AM, Minnier J, Raitt MH, Zarraga IGE, MacMurdy KS, Jessel PM. Dexmedetomidine sedation for paroxysmal supraventricular tachycardia ablation is not associated with alteration of arrhythmia inducibility. Anesth Analg. 2019;129:1529–35.
Acknowledgements
We would like to thank our study nurses Susanne Jenni, Helene Schenk, Lea Streich and Tu Hanh Zarrabi Saffari for their efforts to accomplish this study. Our thanks also goes to the entire staff of the electrophysiology lab for their patience and support of our study.
Funding
Dr. Helge Servatius was supported for this project from the Gottfried and Julia Bangerter-Rhyner-Stiftung and received a CTU-Grant from the Bern University Hospital, Switzerland.
Author information
Authors and Affiliations
Contributions
HS: (i) substantial contributions to the conception, (ii) design of the work; (iii) the acquisition, analysis, and interpretation of data; and (iv) have drafted the work and substantively revised it and approved the submitted version (and any substantially modified version that involves the author's contribution to the study) and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. TK: (i) design of the work; (ii) the analysis, and interpretation of data; and (iii) substantively revised it and approved the submitted version (and any substantially modified version that involves the author's contribution to the study) and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.: GE: (i) substantial contributions to the analysis, and interpretation of data; and (ii) have substantively revised it and approved the submitted version (and any substantially modified version that involves the author's contribution to the study) and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. JS: (i) the acquisition of data; and (ii) have substantively revised and approved the submitted version (and any substantially modified version that involves the author's contribution to the study) and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. HT: (i) the acquisition of data; and (ii) have substantively revised and approved the submitted version (and any substantially modified version that involves the author's contribution to the study) and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. FN: (i) the acquisition of data; and (ii) have substantively revised and approved the submitted version (and any substantially modified version that involves the author's contribution to the study) and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. AH: (i) substantial contributions to the analysis, and interpretation of data; and (ii) have drafted the work or substantively revised it and approved the submitted version (and any substantially modified version that involves the author's contribution to the study) and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. AM: (i) the acquisition of data; and (ii) have substantively revised and approved the submitted version (and any substantially modified version that involves the author's contribution to the study) and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. MB: (i) substantial contributions to the conception, (ii) the acquisition, analysis, and interpretation of data; and (iv) have drafted the work or substantively revised it and approved the submitted version (and any substantially modified version that involves the author's contribution to the study) and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. SD: (i) the acquisition of data; and (ii) have substantively revised and approved the submitted version (and any substantially modified version that involves the author's contribution to the study) and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. LT: (i) substantial contributions to the conception and (ii) have revised and approved the submitted version (and any substantially modified version that involves the author's contribution to the study) and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. TR: (i) substantial contributions the acquisition, analysis, and interpretation of data; and (ii) have drafted the work or substantively revised it and approved the submitted version (and any substantially modified version that involves the author's contribution to the study) and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. LR: (i) substantial contributions to the conception, (ii) design of the work; (iii) the acquisition, analysis, and interpretation of data (iv) have drafted the work or substantively revised it and approved the submitted version (and any substantially modified version that involves the author's contribution to the study) and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study (N° 2018-02128) was provided by the Ethical Committee of the Kanton of Bern (Kantonale Ethikkommission, Murtenstrasse 31, Hörsaaltrakt Pathologie, Eingang 43A, Büro H372, 3010 Bern; Chairperson Prof Robert Greif) on 12 February 2019. All patients provided written informed consent to participate.
Consent for publication
Not applicable.
Competing interests
Laurent Roten has received speaker/consulting honoraria from Abbott/SJM and from Medtronic and has received a research grant for an investigator-initiated study to the institution from Medtronic. Tobias Reichlin: Research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, and the sitem insel support funds, all for work outside the submitted study. Speaker/consulting honoraria or travel support from Abbott/SJM, Astra Zeneca, Brahms, Bayer, Biosense-Webster, Biotronik, Boston-Scientific, Daiichi Sankyo, Farapulse, Medtronic, Pfizer-BMS and Roche, all for work outside the submitted study. Support for his institution’s fellowship program from Abbott/SJM, Biosense-Webster, Biotronik, Boston-Scientific and Medtronic for work outside the submitted study. Andreas Haeberlin has received travel fees/educational grants from Medtronic, Biotronik, Abbott, and Philips/Spectranetics without impact on his personal remuneration. He serves as a proctor for Medtronic. He has received research grants from the Swiss National Science Foundation, the Swiss Innovation agency Innosuisse, the Swiss Heart Foundation, the University of Bern, the University Hospital Bern, the Velux Foundation, the Hasler Foundation, the Swiss Heart Rhythm Foundation, and the Novartis Research Foundation. He is Co-founder and CEO of Act-Inno AG. Jens Seiler: The spouse of Dr Seiler is an employee of Boston Scientific and stock owner of Boston Scientific and Abbott. Mattia Branca is affiliated with CTU Bern, University of Bern, which has a staff policy of not accepting honoraria or consultancy fees. However, CTU Bern is involved in design, conduct, or analysis of clinical studies funded by not-for-profit and for-profit organizations. In particular, pharmaceutical and medical device companies provide direct funding to some of these studies. For an up-to-date list of CTU Bern’s conflicts of interest see http://www.ctu.unibe.ch/research/declaration_of_interest/index_eng.html. All other authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Servatius, H., Kueffer, T., Erdoes, G. et al. Electrophysiological differences of randomized deep sedation with dexmedetomidine versus propofol. BMC Anesthesiol 24, 263 (2024). https://doi.org/10.1186/s12871-024-02647-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02647-x