Abstract
Background
An increasing number of individuals undergo total knee arthroplasty (TKA), which can result in pain, limited motor function and adverse complications such as infection, nausea and vomiting. Glucocorticoids have been shown anti-inflammatory and antiemetic effects, but can also elevate blood glucose levels and increase the risk of wound infection. Thus, it is essential to investigate the efficacy and safety of glucocorticoid usage in TKA.
Method
A comprehensive systematic search of PubMed, Medline, EMBASE, Cochrane databases, to identify relevant randomized controlled trials (RCTs) of glucocorticoid application in TKA. The primary outcomes assessed were the postoperative pain assessment. Secondary outcomes included the range of motion in knee joint, levels of inflammatory cytokines, adverse complications, and the length of hospital stay.
Results
Thirty-six randomized controlled trials were included in the final analysis. The glucocorticoid group exhibited significant reduction in the resting VAS scores on postoperative days 1, 2 (POD1, 2)and postoperative 3 months (POM3), as well as decreased morphine consumption on POD1 and increased range of motion (ROM) in knee joint on POD1, 3. Additionally, the glucocorticoid group exhibited decreased levels of postoperative inflammatory cytokines and the incidence of PONV along with a shorter length of hospital stay. The blood glucose concentration was significantly increased in the glucocorticoid group on POD1 compared with the control group. While the blood glucose on POD2 and occurrence of postoperative adverse complications were similar between two groups including wound infection and venous thrombosis. The periarticular injection analgesia (PIA) group demonstrated lower VAS scores on POD2 comparing to the systemic administration (SA) group according to two studies. However, there was no significant difference of the resting VAS on POD1 and POD2 between PIA and SA group across all studies.
Conclusion
Perioperative glucocorticoids treatment in TKA significantly reduced short-term pain score and opioid-use which was probably not patient relevant. The application of glucocorticoids in TKA implied a beneficial trend in analgesic, anti-inflammatory, and antiemetic effects, as well as improved range of motion and shortened hospital stay. While it will not increase the risk of continued high glucose, postoperative wound infection and venous thrombosis.
Similar content being viewed by others
Introduction
Total knee arthroplasty (TKA) is a highly effective surgical treatment for severe knee arthritis which could reduce pain and maintain motor function [1, 2]. However, many patients still experience moderate to severe pain following TKA and require increased analgesic use postoperatively [3]. Various analgesic methods, including nerve blocks, local anesthetic infiltration, and intravenous opioids, are utilized in these patients [4,5,6]. Unfortunately, patients often report dissatisfaction with pain management and are prone to complications such as nausea and vomiting [7]. Besides, surgical intervention triggers a local inflammatory response, which further leads to systemic inflammatioty response through the release of inflammatory factors [8]. Inflammation can further exacerbate postoperative joint pain, restrict knee joint range of motion, impede rapid postoperative rehabilitation, prolong hospital stay [9].
Glucocorticoids are commonly employed in TKA surgery due to their anti-inflammatory and antiemetic properties [10]. Some studies have indicated that glucocorticoids can effectively alleviate postoperative pain, reduce opioid consumption and mitigate complications such as nausea and vomiting in TKA patients. However, other studies have raised concerns about glucocorticoids usage, including potential adverse effects such as elevated blood glucose levels, infection, and impaired wound healing, particularly in patients receiving long-term glucocorticoid therapy [11]. Therefore, further investigation is required to determine the efficacy and safety of glucocorticoids in total knee arthroplasty. Additionally, the optimal medication, types, and number of doses of glucocorticoids administered perioperatively in TKA remain uncertain. The objective of this systematic review is to evaluate the available randomized controlled trials that investigate the efficacy and safety associated with glucocorticoid use in total knee arthroplasty. The main purposes of this paper include the following: (1) To explore the effect of glucocorticoids on postoperative rest pain and morphine consumption in patients after TKA; (2) To explore the effect of glucocorticoids on range of knee’s motion and length of hospital stay in patients after TKA; (3) To explore the effect of glucocorticoids on peripheral inflammation in patients after TKA; (4) To explore the adverse complications of glucocorticoids in patients after TKA, including PONV, high blood glucose, wound infection and venous thrombosis. Meanwhile, to investigate the impact of glucocorticoid dosage on PONV and compare the effect of the glucocorticoid by periarticular injection and system administration.
Materials and methods
This systematic review and meta-analysis was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [12]. And this systemic review has been registered (PROSPERO ID: CRD42023435063).
Literature search and selection of studies
PubMed, Medline, EMBASE, Cochrane databases, were searched to identify relevant studies from inception to January 22, 2022. To include all the articles on steroid supplementation for TKA, the string used for the literature search included all the synonyms of TKA and the different drugs that could be supplemented with the following search terms (total knee arthroplasty OR TKA OR total knee replacement OR TKR) AND (Dexamethasone OR steroid OR glucocorticoid OR corticosteroid OR Methylprednisolone OR betamethasone OR cortisone OR hydrocortisone OR hexadecadrol OR prednisolone OR triamcinolone) without putting restrictions on language. After the removal of duplicates, all the records collected were then screened by title and abstract and, when necessary, by reading the full text article. The entire process was completed independently by two authors (LFY, DM). We excluded conference abstracts and case reports unless they had subsequently been published as full articles.
Studies were selected based on the following inclusion criteria: 1) randomized clinical trials; 2) comparison of two or more glucocorticoid interventions in primary TKA; 3) postoperative evaluation included VAS, morphine consumption, inflammatory response (C-reactive protein and Interleukin-6), ROM, adverse effects and LOS. The exclusion criteria were: 1) studies included knee and hip arthroplasty without discrete data for TKA; 2) overall sample size fewer than ten patients; 3) unicompartmental knee arthroplasty (UKA) or revision of total knee arthroplasty.
Data extraction
Two reviewers independently extracted data, and the third reviewer checked the consistency between them. A standard form was used; the extracted items included author, study design, sample size, publishing date, country, case number, age, gender, intervention method, dosages and type of anesthesia and follow-up term. The primary outcome was the analgesic effect, including resting VAS on the 1st, 2nd day and 3rd month after TKA and morphine consumption on the 1st day after operation. Secondary outcome measures included inflammatory response (C-reactive protein and Interleukin-6), ROM during the 3 days postoperatively, adverse effects and LOS.
Assessment of methodological quality
Two reviewers (LFY, DM) independently assessed the methodological quality of the included studies which were performed by the Cochrane Collaboration for Systematic Reviews, including assessment of random sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcomes assessment, incomplete outcome data, selective reporting, and other bias. Quantitative synthesis was conducted using Review Manager (RevMan 5.3). As the guidelines set out by the GRADE (Grading of Recommendations, Assessment, Development and Evaluations), all scores for each measured outcome were converted to a common scale [13].The overall methodological quality of each included study was characterized as "low risk of bias", "high risk of bias", or "unclear risk of bias". Differences will be resolved by consensus after discussion and, if necessary, a third reviewer will be consulted.
Statistical analysis
We used RevMan 5.3 to conduct this meta-analysis. For dichotomous data, RRs with 95% CIs were used to express the effect sizes, while mean difference and 95% CIs were used for continuous data. Firstly, we conducted a heterogeneity test to evaluate the extent of heterogeneity in combination with the I2-test [14]. A fixed-effects model was used to conduct the meta-analysis if no heterogeneity (p > 0.1 and I2 < 50%) was observed among the studies. If significant heterogeneity (p ≤ 0.1 or I2 ≥ 50%) was observed, then a random-effects model was used for the meta-analysis. The Z-test was used to determine the significance of the pooled effect size, and p < 0.05 was considered statistically significant. Publication bias was assessed using the funnel plots, Egger’s regression test [15], and Begg’s adjusted rank correlation [16], which were conducted with the Stata software (Stata Corp., TX, USA; version 15.0).
Results
Search results
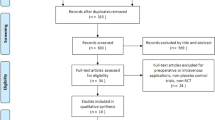
In the recent study, a total of 1,313 studies were identified based on the inclusion criteria. After removing 366 duplicates, 947 records remained. From these, 902 articles were excluded based on the title and abstract screening. 36 RCTs were ultimately enrolled after browsing the whole text and 9 studies were excluded for specific reasons (Fig. 1).
The sample size of the included studies ranged from 23 to 323 participants. Among the included studies, a total of 3,048 patients who underwent primary unilateral TKA were represented in thirty-one RCTs. Additionally, one study by Morales-Munoz et al. included 54 patients who underwent knee replacement surgery, and four studies encompassed 211 patients who underwent bilateral TKA. The follow-up period across the studies varied from 24 h to 1 year. In terms of glucocorticoid administration, 1,188 patients received systemic glucocorticoid administration either before anesthesia induction or after surgery, while 663 patients had been implemented with glucocorticoid in the cocktail protocol for intraoperative periarticular infiltration. The specific drugs utilized in the studies included dexamethasone in twenty studies, methylprednisolone in six studies, triamcinolone acetonide in five studies, hydrocortisone in three studies, betamethasone in two studies, and prednisone in one study. The basic characteristics of the studies are described in the Table 1 [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. The risk of bias assessment of RCT studies is presented in Fig. 2.
Postoperative resting VAS
The Visual Analogue Scale (VAS) is a tool commonly used to assess pain scales. It is an approximately 10 cm long ruler, divided into 10 equal scores ranging from 0 (pain-free end) to 10 (most severe pain). The patient select the appropriate scale on the ruler to indicate their level of pain. In the analysis, twenty-five studies reported the resting VAS on postoperative day 1 (POD1) following TKA, including 1,302 patients in the glucocorticoid group and 1,292 patients in the control group. Furthermore, twenty-three trials recorded the resting VAS on postoperative day 2 (POD2), with 1,243 patients in the glucocorticoid group and 1,236 patients in the control group. Six studies measured the resting VAS score on postoperative 3 months (POM3), including 272 patients in the glucocorticoid group and 271 patients in the control group. The results indicated that the use of glucocorticoids significantly reduced the postoperative resting VAS (Fig. 3, POD1: MD = -0.59; 95%CI: -0.78, -0.39; P < 0.000; Fig. 4, POD2: MD = -0.18; 95%CI: -0.31, -0.05; P = 0.006; Fig. 5, POM3: MD = -0.09; 95%CI: -0.14, -0.04; P = 0.001).
Morphine consumption
Eleven records provided data of morphine consumption within 24 h following TKA. The unit of morphine is milligram (mg). These studies involved 636 patients in the glucocorticoid group and 635 patients in the control group. Morphine consumption was significantly reduced in the glucocorticoid group (Fig. 6, MD = -2.89; 95% CI: -4.79 -1.00; P = 0.003) compared to the control group.
Postoperative Range of Motion
ROM refers to the range of motion of the knee in flexion, which is normally 0–130 angles. ROM in the knee joint was assessed on POD1 and postoperative days 3 (POD3). Eleven studies provided ROM data on POD1 with 481 patients in the glucocorticoid group and 467 patients in the control group. Ten studies assessed ROM on POD3, with 479 patients in the glucocorticoid group and 473 patients in the control group. The overall analysis demonstrated that systemic administration (SA) and periarticular injection analgesia (PIA) of glucocorticoids improved the ROM comparing to the control group after TKA (Fig. 7, POD1: MD = 5.22; 95%CI: 3.40, 7.04; P < 0.000; Fig. 8, POD3: MD = 3.50; 95%CI: 0.86, 6.15; P = 0.009).
Postoperative inflammatory response
Eleven trials evaluated the C-reactive protein (CRP mmol/L) concentration on POD1, involving 487 patients in the glucocorticoid group and 482 patients in control group. Additionally, eleven trials recorded the CRP concentration on POD2, with 526 patients vs. 521 patients in two groups respectively. Seven studies reported the CRP concentration on POD3, encompassing 322 patients in the glucocorticoid group and 323 patients in the control group. The results showed a significant reduction of CRP in the glucocorticoid group compared to the control group (Supplementary Fig. 1, POD1: MD = -18.75; 95% CI: -23.36, -14.15; P < 0.000; Supplementary Fig. 2, POD2: MD: -47.05; 95% CI: -59.80, -34.29; P < 0.000; Supplementary Fig. 3, POD3: MD = -37.79; 95% CI: -49.45, -26.12; P < 0.000).
In addition, eight trials assessed the Interleukin-6 (IL-6 mmol/L) concentration on POD1, with 369 patients in the glucocorticoid group and 368 patients in the control group. The IL-6 concentration was significantly lower in the glucocorticoid group on POD1 (Supplementary Fig. 4, MD = -60.02; 95% CI: -71.02, -49.02; P < 0.000).
Postoperative adverse effects
Data from seven studies were analyzed to investigate the incidence of postoperative nausea and vomiting (PONV) on POD1, while thirteen studies reported the PONV incidence during the entire postoperative period (from post-surgery to discharge). The results showed that perioperative systemic or periarticular injection glucocorticoid reduced the incidence of PONV not only on POD1 but also during total postoperative period (Supplementary Fig. 5, POD1: RR = 0.51; 95% CI: 0.43, 0.62; P < 0.000; Supplementary Fig. 6, Total: RR = 0.64; 95% CI: 0.51, 0.81; P = 0.0002).
In order to investigate the impact of glucocorticoid dosage on PONV, we conducted a reanalysis of the relevant data after applying dexamethasone equivalents. The results revealed that the majority of studies examining total PONV predominantly utilized low-dose glucocorticoid with dexamethasone equivalents maximum limit of 15 mg during entire perioperative period, 3 studies utilized high-dose dexamethasone equivalents. Both high-dose and low-dose glucocorticoid therapy can improve the incidence of total PONV (Supplementary Fig. 7, high-dose: RR = 0.42, P < 0.000; low-dose: RR = 0.72, P = 0.006), with a significant lower incidence of PONV in the high-dose group (Supplementary Fig. 7, P = 0.03).
Eight records provided data on blood glucose concentration on POD1, and five of them reported the blood glucose concentration on POD2 as well. The analysis showed that blood glucose concentration (mmol/L) was significantly increased in the glucocorticoid group on POD1 compared with the control group (Supplementary Fig. 8, MD = 0.47; 95%CI: 0.25, 0.68; P < 0.000). However, the difference in blood glucose concentration (mmol/L) between the two groups was reduced on POD2 (Supplementary Fig. 9, MD = -0.16; 95%CI: -0.55, 0.23; P = 0.42).
Fourteen studies evaluated the occurrence of wound infection postoperatively, while two studies reported the occurrence of venous thrombosis. The analysis did not show a significant increase in the risk of postoperative wound infection (Supplementary Fig. 10, RR = 0.98; 95% CI: 0.64, 1.51; P = 0.93) and venous thrombosis in the glucocorticoid group (Supplementary Fig. 11, RR = 1.47; 95% CI: 0.25, 8.68; P = 0.67).
Length of hospital stay
Nineteen trials reported the duration of hospitalization after TKA which including 807 patients in the glucocorticoid group and 801 patients in the control group. The overall pooled outcomes showed that perioperative glucocorticoid administration significantly decreased the days of LOS comparing to the control group (Supplementary Fig. 12, MD = -0.27; 95% CI: -0.44, -0.09; P = 0.003).
Comparison of efficacy between systemic administration and periarticular infiltration in glucocorticoid group
In the comparison between systemic administration (SA) and periarticular infiltration analgesia (PIA) of glucocorticoids, two studies with 95 patients in each group were included. The results showed that the postoperative resting VAS was significantly lower in periarticular infiltration group compare to the systemic administration group on POD2 (Supplementary Fig. 13, MD = -0.16; 95% CI: -0.29, -0.02; P = 0.02). However, the plasma CRP level was significantly lower in the systemic administration group compare to the periarticular infiltration group on POD1 and POD2 (Supplementary Fig. 14, POD1: MD = 10.30; 95% CI: 4.74, 15.85; P = 0.0003; Supplementary Fig. 15, POD2: MD = 13.62; 95% CI: 2.19, 25.05; P = 0.02).
In order to better explore the effects of different glucocorticoids administration routes, apart from the two aforementioned RCTs directly comparing the postoperative pain and peripheral blood inflammatory factors, we conducted subgroup analysis between the SA group and PIA group. Comparing to the control group, both subgroups showed a significant reduction in pain scores on POD1 without Inter-group difference (Supplementary Fig. 16–1, P = 0.38).The results also revealed that the PIA group exhibited a significant alleviation of postoperative resting VAS on POD2 (Supplementary Fig. 16–2, MD = -0.2; 95% CI: -0.36, -0.04; P = 0.01). Meanwhile, there was no statistically significant difference in the SA group on POD2. Totally, the reduction of resting VAS on POD2 was without Inter-group difference between SA and PIA group (Supplementary Fig. 16–2, P = 0.77). The comparison of inflammation cytokines levels between subgroups did not yield accurate results, primarily due to the substantial difference in sample sizes between the two subgroups.
Publication bias
Publication bias was evaluated using funnel plots and Begg’s and Egger’s tests for VAS on POD1, 2, 3, which showed no evidence of publication bias (Supplementary Figs. 17, 18 and 19, Begg: Pr >|z|= 0.624, 0.635, 0.976, Egger’s: P = 0.491, 0.251, 0.733). And the publication bias for VAS on POM3 and morphine consumption were not assessed due to the small number of included studies.
Discussion
The most important finding of the study is that the glucocorticoids can significantly reduce acute pain and morphine consumption within 24 h, and decrease the postoperative level of CRP and IL-6 and the incidence of PONV, and achieve a better ROM and shorter LOS, without increasing continued high blood glucose and the risk of postoperative wound infection and venous thrombosis.
Many patients experience severe pain after TKA surgery, and a significant number continue to suffer from chronic pain even after 3 months [53, 54]. To address this issue, numerous studies have investigated various postoperative analgesia protocols, such as the use of gabapentin [55] or dexmedetomidine [56] as adjuncts, as well as different combinations of nerve blocks [57]. Glucocorticoids have gained increasing attention as adjuvants for acute postoperative pain treatment in TKA [58]. In the current study, both intravenous and periarticular injection of glucocorticoids resulted in a significant decrease of 0.59 mean difference (MD) in resting pain scores on POD1 after surgery, which was consistent with previous studies [59,60,61,62]. While the MD of improvement of the resting VAS on POM3 was 0.09. However, similar MD in improvement of resting VAS scores showed no statistically significant difference in other studies [61, 62]. Therefore the effect of glucocorticoids on chronic pain need to be further explored. Morphine consumption was also reduced in the glucocorticoid group on POD1 which was similar to the morphine reduction of 3.4 mg in Wang et al. study [61]. These findings are consistent with a meta-analysis conducted by De Oliveira et al., which concluded that intermediate and high doses of perioperative dexamethasone can effectively reduce postoperative pain and the need for additional painkillers [63]. They also found that preoperative dexamethasone was more effective in pain control compared to intraoperative administration. Similarly, Waldron et al. demonstrated the pain-relieving effect of perioperative dexamethasone in various surgical procedures, showing that patients who received dexamethasone required fewer postoperative opioids, had a longer time to first analgesic dose, needed less rescue analgesia, and had shorter stays in the recovery room [64]. In conclusion, glucocorticoid applications are effective for short-term pain control but long-term control needs to be explored further.
In this study, the beneficial effects of glucocorticoid administration extended beyond pain relief. It was observed that perioperative glucocorticoids administration significantly improved ROM and decreased the LOS, which is consistent with previous studies [65]. Patients with total knee osteoarthritis often have decreased ROM due to joint stiffness and pain. ROM is a significant parameter evaluating postoperative functional recovery for patients after TKA [66]. Better ROM is more benefit to different activities of daily living [67]and has a positive impact on TKA outcomes [68]. In our study, the glucocorticoid group presented about 5.22 degrees on POD1 and 3.50 degrees on POD3 increased in the ROM after TKA. Though these results were also confirmed in some studies [40, 59], a study from Xu B et indicated that the ROM on POD3 was not significantly increasing after applying glucocorticoids [30]. Glucocorticoid was found no significant improvement in long-term keen function of TKA patients due to it short-lasting pain relief [59]. In general, Glucocorticoids could improve short-term ROM contributing to better functional recovery [69]. Because many other variables may influence postoperative ROM, including surgical technique, implant design, and preoperative training as well as postoperative care, further investigation is required, including follow up for long-term ROM.
LOS is deemed as an important outcome for its economics burden [70]. Our study has showed that glucocorticoids shorten postoperative LOS by 0.27 day, which is lessen than 1.2 days in the study from Backes et al. [35] LOS is affected by many factors, including Enhanced Recovery After Surgery (ERAS), persistent postoperative pain, nausea and vomiting, wound infection, as well as the administration times and dose of glucocorticoids. As studies attention intensifies on ERAS, encompassing routine education, smoking and alcohol cessation, optimized fasting periods, standardized multimodal anesthesia protocols, alongside the utilization of local infiltrative analgesia, tranexamic acid, and multimodal prophylactic treatment for postoperative nausea, vomiting, and analgesia, a notable reduction in LOS by 5.4 days following TKA is achievable [71]. Therefore, further studies are warranted to substantiate the efficacy of combining glucocorticoids with ERAS in reducing Length of Stay.
Our study also found that periarticular injection analgesia group had lower pain score on POD2 but higher CRP level on POD 1 and POD2 compared to the systemic administration group in the two comparative studies. And in a subgroup analysis of the route of glucocorticoid administration across included studies, PIA group indicated decreased resting VAS on POD2, while the SA group didn’t. Previous studies have used glucocorticoids via different routes, with intravenous or topical administration being commonly used and proven to accelerate recovery after TKA [72, 73]. The different effects of intravenous or topical glucocorticoids in TKA patients may be explained by the analgesic mechanism of glucocorticoids. Glucocorticoids exert their analgesic effect by inhibiting phospholipase, which blocks the cyclooxygenase and lipoxygenase pathways in the inflammatory chain reaction [74,75,76]. This leads to a decrease in the release of pain-causing substances such as bradykinin [74] from tissues and neuropeptides from nerve endings [75]. From this perspective, topical glucocorticoids directly inhibit the production of inflammatory factors, resulting in a faster and more targeted reduction of pain-causing substances such as bradykinin, compared to intravenous glucocorticoids [45, 51]. However, the advantages of locally administered glucocorticoids following TKA have not been conclusively demonstrated. Some studies have indicated that the use of local glucocorticoids results in prolonged pain relief and improved active knee flexion after surgery [77,78,79]. On the other hand, a study by Christensen et al. [50] did not observe improvements on pain scores, range of knee motion, or narcotic consumption with local glucocorticoid administration. As similar in our study, there were insignificant difference of the resting VAS on POD1 and POD2 between PIA and SA group across all studies. These conflicting results may be attributed to differences in disease severity among the study groups, variations in treatments prior to TKA, differences in the type of steroid used, concomitant analgesic drugs, and variations in postoperative physical therapy approaches, which can influence the range of motion outcomes.
Indeed, TKA is associated with a profound inflammatory response due to the deep surgical site, osteotomy, massive blood loss, soft tissue injury, and surgical-related stress response. This led to increased levels of various inflammatory cytokines including CRP, IL-1β, IL-6, and TNF-a [80, 81]. The systemic and local inflammatory responses contribute to hyperalgesia [82, 83] and pain sensitization after TKA, which can result in severe pain even with a slight stimuli [30, 84]. Furthermore, the persistent inflammatory response can contribute to the development of chronic pain in the postoperative period [85]. Glucocorticoid had been wildly used in TKA to enhance recovery due to their potent anti-inflammatory effects [30, 72, 86]. The use of glucocorticoids can reduce CRP and IL6 level in the blood, as observed in our study. The analgesic effects of preoperative glucocorticoids can be partly attributed to their reduction of inflammation [87]. Glucocorticoids exert their anti-inflammatory response by suppressing the transcription of AP-1, NF-kB, and STAT3, which leads to the inhibition of proinflammatory cytokine production [88]. However, topical administration of glucocorticoid group showed higher CRP level postoperatively compared to system administration of glucocorticoid group in our study. The administration route we chose were closely associated with local or systemic inflammatory response. Intravenous corticosteroids have been proved to reduce systemic CRP and IL-6 effectively [30, 72, 86], while topical corticosteroids predominantly improve local inflammation at the surgical site in arthroplasty [45] and have limited effects on system inflammation after TKA [73]. However, these findings need to be confirmed through additional clinical studies, as there are only two comparative studies presented in this paper. Study by Ugras et al. proposed that amelioration of local inflammation is more significant in the analgesia of glucocorticoid than system inflammation [80], which is consistent with our results that topical administration of glucocorticoid has lower pain score on POD2. However, more studies are required to support it.
PONV commonly occur in TKA patients and can hinder participation in physiotherapy and early mobilization [89], leading to prolonged hospital stays and increasing the dissatisfaction of patients and health care costs [90, 91]. Glucocorticoids have been utilized in the prevention of PONV in many studies [92]. We found that the glucocorticoids have anti-emetic function after TKA not only in system administration but in local usage. The anti-emetic effect of glucocorticoids may be mediated through the inhibition of prostaglandin synthesis or the inhibition of endogenous opioid release [93]. Meanwhile, surgical trauma, anesthesia, and analgesics [94] are all factors associated with PONV, and surgical trauma can trigger the release of systemic inflammatory biomarkers, thereby increasing the occurrence of PONV [95]. The potent anti-inflammatory effects of glucocorticoids may contribute to their anti-emetic function. Though the anti-emetic of glucocorticoid has been well documented and supported in our study, the application dosage is still not clarified clearly. Therefore, we performed subgroup analysis of glucocorticoid dose and concluded that low dosage (≤ 15 mg dexamethasone) and high dosage (> 15 mg dexamethasone) both reduced the incidence of PONV, with high dosage reducing PONV more significantly. And pre-emptive low dose (10 mg) dexamethasone has been proved to reduce postoperative emesis [36]. Though higher dosage of glucocorticoid is more beneficial in reducing PONV, we cannot ignore higher risk of complications with it. It has so far been proposed multiple low dosage of glucocorticoid is more effective on decrease PONV and early rehabilitation [59, 86]. However, a single high dose of 20 mg dexamethasone is more preferable to multiple low dose application by an opposite study [96]. It may be related to the type of glucocorticoid, as they have different times of action and should therefore be used specifically according to the type of glucocorticoid in clinical.
In addition, referring to the concern of hyperglycemia caused by the administration of corticosteroids, previous studies indicated that the fasting blood glucose of patients treated with topical and intravenous glucocorticoid was significantly increased after TKA [52, 97, 98], which was similar to significant elevation of blood glucose in the glucocorticoid group on POD1 in the study. Hyperglycemia, especially when levels exceed 200 mg/dl has been associated with an increased risk of surgical site infection and wound complications [99, 100]. However, other studies reported that the intravenous administration of dexamethasone did not increase fasting blood glucose levels postoperatively following TKA [35, 101], which were consistent with the insignificant increase blood glucose in patients of glucocorticoid group after TKA on POD2. Different results may be related to the determination time of blood glucose and Hormone dosage. It also indicated that preoperative intravenous administration of methylprednisolone resulted in a transient postoperative increase in plasma glucose and insulin resistance, which were normalized 48 h postoperatively [97].
The other complications in terms of wound drainage, delayed wound healing, intramuscular venous thrombosis, which were not significantly different between the two groups. To our knowledge, most important possible risks with steroid during post-operative period, including gastric ulcers [102], impaired wound healing [103] or wound infections [11] were exclusively associated with chronic glucocorticoid use not with single dose glucocorticoid use. And the side effects from glucocorticoid use are proportional to the duration and intensity of therapy [104]. Theses similar results were presented in many articles [105]. Even though, no studies have confirmed that a short term use of glucocorticoid has not harm effect on patients of TKA. In addition, it may be small samples and low evidenced articles that result in negative adverse outcomes in single dose of glucocorticoid use [106]. Therefore, further high-quality studies with larger sample sizes is needed to establish the safety of perioperative glucocorticoid administration in TKA patients.
We have investigated the clinical efficacy of most glucocorticoid, but the optimal corticosteroids administration regimen regarding of dosage, times, drug combination are still undetermined. In concern of the comparison between topical and systemic glucocorticoid, there need more studies to explore their different effect on pain and system inflammation. Additionally, the lack of long-term follow-up data highlights the need for further studies to explore and validate the results over an extended period of time.
Conclusion
This systematic review and meta-analysis suggest that glucocorticoids may exert pain relief within 24 h, although its clinical relevance to patients may be limited. The application of glucocorticoids provided antiemetic effects, as well as facilitate functional recovery, accompanied by the inhibition of inflammatory factors releasing after TKA, including plasma CRP and IL-6. Importantly, these effects are observed without elevating blood glucose levels persistently, increasing the risks of wound infection and venous thrombosis.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000;380:85–90.
Sun YQ, Yang B, Tong SL, Sun J, Zhu YC. Patelloplasty versus traditional total knee arthroplasty for osteoarthritis. Orthopedics. 2012;35(3):e343-348.
Jiao JH, Liu L, Sun XF. Evidence of drug-free interventions for postoperative pain management after total knee arthroplasty. JAMA Surg. 2018;153(4):392.
Jæger P, Zaric D, Fomsgaard JS, Hilsted KL, Bjerregaard J, Gyrn J, et al. Adductor canal block versus femoral nerve block for analgesia after total knee arthroplasty: a randomized, double-blind study. Reg Anesth Pain Med. 2013;38(6):526–32.
Ramamoorthy KG. Local infiltration analgesia following total knee arthroplasty. Indian J Anaesth. 2012;56(2):208–9.
Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803–8.
Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89(3):652–8.
Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193(3):237–44.
Yang X, Dong J, Xiong W, Huang F. Early postoperative pain control and inflammation for total knee arthroplasty: a retrospective comparison of continuous adductor canal block versus single-shot adductor canal block combined with patient-controlled intravenous analgesia. Emerg Med Int. 2022;2022:1351480.
Hannon CP, Fillingham YA, Mason JB, Sterling RS, Casambre FD, Verity TJ, et al. The efficacy and safety of corticosteroids in total joint arthroplasty: a direct meta-analysis. J Arthroplasty. 2022;37(10):1898-1905.e1897.
Cutolo M, Seriolo B, Pizzorni C, Secchi ME, Soldano S, Paolino S, et al. Use of glucocorticoids and risk of infections. Autoimmun Rev. 2008;8(2):153–5.
Hutton B, Wolfe D, Moher D, Shamseer L. Reporting guidance considerations from a statistical perspective: overview of tools to enhance the rigour of reporting of randomised trials and systematic reviews. Evid Based Ment Health. 2017;20(2):46–52.
Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013;66(2):173–83.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Gasbjerg KS, Hägi-Pedersen D, Lunn TH, Laursen CC, Holmqvist M, Vinstrup L, et al. Effect of dexamethasone as an analgesic adjuvant to multimodal pain treatment after total knee arthroplasty: randomised clinical trial. BMJ. 2022;376:e067325.
Lei Y, Huang Z, Huang Q, Pei F, Huang W. Dose optimization of intravenous dexamethasone for total knee arthroplasty: when two is not better than one. Arch Orthop Trauma Surg. 2022;142(4):665–72.
Cheng X, Wang Z, Zhang Y, Zhang X. Oral administration of prednisone effectively reduces subacute pain after total knee arthroplasty. Orthop Traumatol Surg Res. 2021;107(3):102770.
Zhang S, Xu H, Xie J, Cao G, Lei Y, Pei F. Tranexamic acid attenuates inflammatory effect and modulates immune response in primary total knee arthroplasty: a randomized, placebo-controlled, pilot trial. Inflammopharmacology. 2020;28(4):839–49.
Tammachote N, Kanitnate S. Intravenous dexamethasone injection reduces pain from 12 to 21 hours after total knee arthroplasty: a double-blind, randomized. Placebo-Controlled Trial J Arthroplasty. 2020;35(2):394–400.
Kim JK, Ro DH, Lee HJ, Park JY, Han HS, Lee MC. Efficacy of systemic steroid use given one day after total knee arthroplasty for pain and nausea: a randomized controlled study. J Arthroplasty. 2020;35(1):69–75.
Chan TCW, Cheung CW, Wong SSC, Chung AYF, Irwin MG, Chan PK, et al. Preoperative dexamethasone for pain relief after total knee arthroplasty: a randomised controlled trial. Eur J Anaesthesiol. 2020;37(12):1157–67.
Yu Y, Lin H, Wu Z, Xu P, Lei Z, Mayr J. Perioperative combined administration of tranexamic acid and dexamethasone in total knee arthroplasty - benefit versus harm? Medicine (United States). 2019;98(34):e15852.
Wu Y, Lu X, Ma Y, Zeng Y, Bao X, Xiong H, et al. Perioperative multiple low-dose Dexamethasones improves postoperative clinical outcomes after Total knee arthroplasty 11 Medical and Health Sciences 1103 Clinical Sciences. BMC Musculoskelet Disord. 2018;19(1):428.
Xu H, Zhang S, Xie J, Lei Y, Cao G, Pei F. Multiple doses of perioperative dexamethasone further improve clinical outcomes after total knee arthroplasty: a prospective, randomized. Controlled Study J Arthroplasty. 2018;33(11):3448–54.
Cheng BLY, So EHK, Hui GKM, Yung BPK, Tsui ASK, Wang OKF, et al. Pre-operative intravenous steroid improves pain and joint mobility after total knee arthroplasty in Chinese population: a double-blind randomized controlled trial. Eur J Orthop Surg Traumatol. 2019;29(7):1473–9.
Li D, Zhao J, Yang Z, Kang P, Shen B, Pei F. Multiple low doses of intravenous corticosteroids to improve early rehabilitation in total knee arthroplasty: a randomized clinical trial. J Knee Surg. 2019;32(2):171–9.
Dissanayake R, Du HN, Robertson IK, Ogden K, Wiltshire K, Mulford JS. Does dexamethasone reduce hospital readiness for discharge, pain, nausea, and early patient satisfaction in hip and knee arthroplasty? A randomized. Controlled Trial J Arthroplasty. 2018;33(11):3429–36.
Xu B, Ma J, Huang Q, Huang ZY, Zhang SY, Pei FX. Two doses of low-dose perioperative dexamethasone improve the clinical outcome after total knee arthroplasty: a randomized controlled study. Knee Surg Sports Traumatol Arthrosc. 2018;26(5):1549–56.
Lindberg-Larsen V, Kehlet H, Pilely K, Bagger J, Rovsing ML, Garred P. Preoperative methylprednisolone increases plasma Pentraxin 3 early after total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. Clin Exp Immunol. 2018;191(3):356–62.
Lee S, Rooban N, Vaghadia H, Sawka AN, Tang R. A randomized non-inferiority trial of adductor canal block for analgesia after total knee arthroplasty: single injection versus catheter technique. J Arthroplasty. 2018;33(4):1045–51.
Morales-Muñoz C, Sánchez-Ramos JL, Díaz-Lara MD, González-González J, Gallego-Alonso I, Hernández-Del-Castillo MS. Analgesic effect of a single-dose of perineural dexamethasone on ultrasound-guided femoral nerve block after total knee replacement. Rev Esp Anestesiol Reanim. 2017;64(1):19–26.
McLawhorn AS, Beathe J, YaDeau J, Buschiazzo V, Purdue PE, Ma Y, et al. Effects of steroids on thrombogenic markers in patients undergoing unilateral total knee arthroplasty: a prospective, double-blind, randomized controlled trial. J Orthop Res. 2015;33(3):412–6.
Backes JR, Bentley JC, Politi JR, Chambers BT. Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: a prospective, randomized controlled trial. J Arthroplasty. 2013;28(8 Suppl):11–7.
Koh IJ, Chang CB, Lee JH, Jeon YT, Kim TK. Preemptive low-dose dexamethasone reduces postoperative emesis and pain after TKA: a randomized controlled study. Clin Orthop Relat Res. 2013;471(9):3010–20.
Lunn TH, Kristensen BB, Andersen LØ, Husted H, Otte KS, Gaarn-Larsen L, et al. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: A randomized, placebo-controlled trial. Br J Anaesth. 2011;106(2):230–8.
Jules-Elysee KM, Lipnitsky JY, Patel N, Anastasian G, Wilfred SE, Urban MK, et al. Use of low-dose steroids in decreasing cytokine release during bilateral total knee replacement. Reg Anesth Pain Med. 2011;36(1):36–40.
Peng H, Wang W, Lin J, Weng X, Qian W, Wang W. Local efficacy of corticosteroids as an adjuvant for periarticular cocktail injection in simultaneous bilateral total knee arthroplasty: a prospective randomized double-blind controlled trial. Pain Res Manag. 2021;2021:5595095.
Wang Q, Tan G, Mohammed A, Zhang Y, Li D, Chen L, et al. Adding corticosteroids to periarticular infiltration analgesia improves the short-term analgesic effects after total knee arthroplasty: a prospective, double-blind, randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2021;29(3):867–75.
El-Boghdadly K, Short AJ, Gandhi R, Chan V. Addition of dexamethasone to local infiltration analgesia in elective total knee arthroplasty: double-blind, randomized control trial. Reg Anesth Pain Med. 2021;46(2):130–6.
Chan VWK, Chan PK, Yan CH, Henry CH, Chan CW, Chiu KY. Effect of steroid in local infiltration analgesia in one-stage bilateral total knee arthroplasty: a paired-randomized controlled study. J Knee Surg. 2022;35(3):317–22.
Tsukada S, Wakui M, Hoshino A. The impact of including corticosteroid in a periarticular injection for pain control after total knee arthroplasty: a double-blind randomised controlled trial. Bone Joint J. 2016;98-b(2):194–200.
Kim TW, Park SJ, Lim SH, Seong SC, Lee S, Lee MC. Which analgesic mixture is appropriate for periarticular injection after total knee arthroplasty? Prospective, randomized, double-blind study. Knee Surg Sports Traumatol Arthrosc. 2015;23(3):838–45.
Ikeuchi M, Kamimoto Y, Izumi M, Fukunaga K, Aso K, Sugimura N, et al. Effects of dexamethasone on local infiltration analgesia in total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(7):1638–43.
Kwon SK, Yang IH, Bai SJ, Han CD. Periarticular injection with corticosteroid has an additional pain management effect in total knee arthroplasty. Yonsei Med J. 2014;55(2):493–8.
Yue DB, Wang BL, Liu KP, Guo WS. Efficacy of multimodal cocktail periarticular injection with or without steroid in total knee arthroplasty. Chin Med J (Engl). 2013;126(20):3851–5.
Chia SK, Wernecke GC, Harris IA, Bohm MT, Chen DB, Macdessi SJ. Peri-articular steroid injection in total knee arthroplasty: a prospective, double blinded, randomized controlled trial. J Arthroplasty. 2013;28(4):620–3.
Seah VWT, Chin PL, Chia SL, Yang KY, Lo NN, Yeo SJ. Single-dose periarticular steroid infiltration for pain management in total knee arthroplasty: A prospective, double-blind, randomised controlled trial. Singapore Med J. 2011;52(1):19–23.
Christensen CP, Jacobs CA, Jennings HR. Effect of periarticular corticosteroid injections during total knee arthroplasty. A double-blind randomized trial. J Bone Joint Surg Am. 2009;91(11):2550–5.
Li D, Wang Q, Zhao X, Luo Y, Kang P. Comparison of intravenous and topical dexamethasone for total knee arthroplasty: a randomized double-blinded controlled study of effects on dexamethasone administration route and enhanced recovery. J Arthroplasty. 2021;36(5):1599–606.
Hatayama K, Terauchi M, Oshima A, Kakiage H, Ikeda K, Higuchi H. Comparison of intravenous and periarticular administration of corticosteroids in total knee arthroplasty: a prospective, randomized controlled study. J Bone Joint Surg Am. 2021;103(4):319–25.
Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. 2015;114(4):551–61.
Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566–72.
Clarke HA, Katz J, McCartney CJL, Stratford P, Kennedy D, Pagé MG, et al. Perioperative gabapentin reduces 24 h opioid consumption and improves in-hospital rehabilitation but not post-discharge outcomes after total knee arthroplasty with peripheral nerve block. Br J Anaesth. 2014;113(5):855–64.
Chassery C, Marty P, Rontes O, Chaubard M, Vuillaume C, Basset B, et al. Total knee arthroplasty under quadruple nerve block with ropivacaine 0.32%: effect of addition of intravenous dexmedetomidine to intravenous dexamethasone on analgesic duration. Reg Anesth Pain Med. 2021;46(2):104–10.
Chen J, Zhou C, Ma C, Sun G, Yuan L, Hei Z, et al. Which is the best analgesia treatment for total knee arthroplasty: Adductor canal block, periarticular infiltration, or liposomal bupivacaine? A network meta-analysis. J Clin Anesth. 2021;68:110098.
Nielsen RV, Siegel H, Fomsgaard JS, Andersen JDH, Martusevicius R, Mathiesen O, et al. Preoperative dexamethasone reduces acute but not sustained pain after lumbar disk surgery: a randomized, blinded, placebo-controlled trial. Pain. 2015;156(12):2538–44.
Wu L, Si H, Li M, Zeng Y, Wu Y, Liu Y, et al. The optimal dosage, route and timing of glucocorticoids administration for improving knee function, pain and inflammation in primary total knee arthroplasty: a systematic review and network meta-analysis of 34 randomized trials. Int J Surg. 2020;82:182–91.
Køppen KS, Gasbjerg KS, Andersen JH, Hägi-Pedersen D, Lunn TH, Mathiesen O. Systemic glucocorticoids as an adjunct to treatment of postoperative pain after total hip and knee arthroplasty. Eur J Anaesthesiol. 2023;40(3):155–70.
Wang Q, Tan G, Mohammed A, Zhang Y, Li D, Chen L, et al. Adding corticosteroids to periarticular infiltration analgesia improves the short-term analgesic effects after total knee arthroplasty: a prospective, double-blind, randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2020;29(3):867–75.
Wu C, Luo D, Zhu Y, Zhao Q, Wang J, Dai Y. Efficacy of combining intravenous and topical dexamethasone against postoperative pain and function recovery after total knee arthroplasty: a prospective, double-blind, randomized controlled trial. J Orthop Surg. 2023;31(2):10225536231189782.
De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–88.
Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth. 2013;110(2):191–200.
Wu Y, Lu X, Ma Y, Zeng Y, Bao X, Xiong H, et al. Perioperative multiple low-dose Dexamethasones improves postoperative clinical outcomes after Total knee arthroplasty. BMC Musculoskelet Disord. 2018;19(1):428.
Lei YT, Xu B, Xie XW, Xie JW, Huang Q, Pei FX. The efficacy and safety of two low-dose peri-operative dexamethasone on pain and recovery following total hip arthroplasty: a randomized controlled trial. Int Orthop. 2018;42(3):499–505.
Laubenthal KN, Smidt GL, Kettelkamp DB. A quantitative analysis of knee motion during activities of daily living. Phys Ther. 1972;52(1):34–43.
Matsuda S, Kawahara S, Okazaki K, Tashiro Y, Iwamoto Y. Postoperative alignment and ROM affect patient satisfaction after TKA. Clin Orthop Relat Res. 2013;471(1):127–33.
Sean VW, Chin PL, Chia SL, Yang KY, Lo NN, Yeo SJ. Single-dose periarticular steroid infiltration for pain management in total knee arthroplasty: a prospective, double-blind, randomised controlled trial. Singapore Med J. 2011;52(1):19–23.
Sibia US, MacDonald JH, King PJ. Predictors of hospital length of stay in an enhanced recovery after surgery program for primary total hip arthroplasty. J Arthroplasty. 2016;31(10):2119–23.
Lee WC, Neoh EC, Wong LP, Tan KG. Shorter length of stay and significant cost savings with ambulatory surgery primary unilateral total knee arthroplasty in Asians using enhanced recovery protocols. J Clin Orthop Trauma. 2024;50:102379.
Li D, Wang C, Yang Z, Kang P. Effect of intravenous corticosteroids on pain management and early rehabilitation in patients undergoing total knee or hip arthroplasty: a meta-analysis of randomized controlled trials. Pain Pract. 2018;18(4):487–99.
Kurosaka K, Tsukada S, Ogawa H, Nishino M, Nakayama T, Yoshiya S, et al. Addition of corticosteroid to periarticular injections reduces postoperative pain following total hip arthroplasty under general anaesthesia: a double-blind randomized controlled trial. Bone Joint J. 2020;102-b(10):1297–302.
Hargreaves KM, Costello A. Glucocorticoids suppress levels of immunoreactive bradykinin in inflamed tissue as evaluated by microdialysis probes. Clin Pharmacol Ther. 1990;48(2):168–78.
Hong D, Byers MR, Oswald RJ. Dexamethasone treatment reduces sensory neuropeptides and nerve sprouting reactions in injured teeth. Pain. 1993;55(2):171–81.
Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89.
Pang HN, Lo NN, Yang KY, Chong HC, Yeo SJ. Peri-articular steroid injection improves the outcome after unicondylar knee replacement: a prospective, randomised controlled trial with a two-year follow-up. J Bone Joint Surg Br. 2008;90(6):738–44.
Mullaji A, Kanna R, Shetty GM, Chavda V, Singh DP. Efficacy of periarticular injection of bupivacaine, fentanyl, and methylprednisolone in total knee arthroplasty:a prospective, randomized trial. J Arthroplasty. 2010;25(6):851–7.
Ng YC, Lo NN, Yang KY, Chia SL, Chong HC, Yeo SJ. Effects of periarticular steroid injection on knee function and the inflammatory response following Unicondylar Knee Arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):60–5.
Ugraş AA, Kural C, Kural A, Demirez F, Koldaş M, Cetinus E. Which is more important after total knee arthroplasty: Local inflammatory response or systemic inflammatory response? Knee. 2011;18(2):113–6.
Jules-Elysee KM, Tseng A, Sculco TP, Baaklini LR, McLawhorn AS, Pickard AJ, et al. Comparison of topical and intravenous tranexamic acid for total knee replacement: a randomized double-blinded controlled study of effects on tranexamic acid levels and thrombogenic and inflammatory marker levels. J Bone Joint Surg Am. 2019;101(23):2120–8.
Wegner A, Elsenbruch S, Rebernik L, Roderigo T, Engelbrecht E, Jäger M, et al. Inflammation-induced pain sensitization in men and women: does sex matter in experimental endotoxemia? Pain. 2015;156(10):1954–64.
Benson S, Engler H, Wegner A, Schedlowski M, Elsenbruch S. Elucidating vulnerability to inflammation-induced hyperalgesia: Predictors of increased musculoskeletal pain sensitivity during experimental endotoxemia. Brain Behav Immun. 2020;88:302–7.
Si HB, Yang TM, Zeng Y, Zhou ZK, Pei FX, Lu YR, et al. Correlations between inflammatory cytokines, muscle damage markers and acute postoperative pain following primary total knee arthroplasty. BMC Musculoskelet Disord. 2017;18(1):265.
Azim S, Nicholson J, Rebecchi MJ, Galbavy W, Feng T, Rizwan S, et al. Interleukin-6 and leptin levels are associated with preoperative pain severity in patients with osteoarthritis but not with acute pain after total knee arthroplasty. Knee. 2018;25(1):25–33.
Li D, Zhao J, Yang Z, Kang P, Shen B, Pei F. Multiple low doses of intravenous corticosteroids to improve early rehabilitation in total knee arthroplasty: a randomized clinical trial. J Knee Surg. 2019;32(2):171–9.
Toner AJ, Ganeshanathan V, Chan MT, Ho KM, Corcoran TB. Safety of perioperative glucocorticoids in elective noncardiac surgery: a systematic review and meta-analysis. Anesthesiology. 2017;126(2):234–48.
Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab. 2013;24(3):109–19.
Gan TJ, Alexander R, Fennelly M, Rubin AP. Comparison of different methods of administering droperidol in patient-controlled analgesia in the prevention of postoperative nausea and vomiting. Anesth Analg. 1995;80(1):81–5.
Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59(2):213–43.
McCracken G, Houston P, Lefebvre G. Guideline for the management of postoperative nausea and vomiting. J Obstet Gynaecol Can. 2008;30(7):600-607–8-616.
Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113.
Henzi I, Walder B, Tramèr MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg. 2000;90(1):186–94.
Lichtor JL, Chung F. Nausea and vomiting treatment after surgery: we still can do better. Anesthesiology. 2012;117(3):454–5.
Wehner S, Vilz TO, Stoffels B, Kalff JC. Immune mediators of postoperative ileus. Langenbecks Arch Surg. 2012;397(4):591–601.
Lei Y, Huang Z, Huang Q, Pei F, Huang W. Dose optimization of intravenous dexamethasone for total knee arthroplasty: when two is not better than one. Arch Orthop Trauma Surg. 2022;142(4):665–72.
Lindberg-Larsen V, Kehlet H, Bagger J, Madsbad S. Preoperative high-dose methylprednisolone and glycemic control early after total hip and knee arthroplasty: a randomized, double-blind. Placebo-Controlled Trial Anesth Analg. 2018;127(4):906–13.
Oshima A, Hatayama K, Terauchi M, Kakiage H, Hashimoto S, Chikuda H. The comparison of dexamethasone and triamcinolone periarticular administration in total knee arthroplasty: retrospective cohort study. BMC Musculoskelet Disord. 2022;23(1):120.
Stryker LS, Abdel MP, Morrey ME, Morrow MM, Kor DJ, Morrey BF. Elevated postoperative blood glucose and preoperative hemoglobin A1C are associated with increased wound complications following total joint arthroplasty. J Bone Joint Surg Am. 2013;95(9):808–14, s801-802.
Hwang JS, Kim SJ, Bamne AB, Na YG, Kim TK. Do glycemic markers predict occurrence of complications after total knee arthroplasty in patients with diabetes? Clin Orthop Relat Res. 2015;473(5):1726–31.
Godshaw BM, Mehl AE, Shaffer JG, Meyer MS, Thomas LC, Chimento GF. The effects of peri-operative dexamethasone on patients undergoing total hip or knee arthroplasty: is it safe for diabetics? J Arthroplasty. 2019;34(4):645–9.
Messer J, Reitman D, Sacks HS, Smith H Jr, Chalmers TC. Association of adrenocorticosteroid therapy and peptic-ulcer disease. N Engl J Med. 1983;309(1):21–4.
Wicke C, Halliday B, Allen D, Roche NS, Scheuenstuhl H, Spencer MM, et al. Effects of steroids and retinoids on wound healing. Arch Surg. 2000;135(11):1265–70.
Salerno A, Hermann R. Efficacy and safety of steroid use for postoperative pain relief. Update and review of the medical literature. J Bone Joint Surg Am. 2006;88(6):1361–72.
Rytter S, Stilling M, Munk S, Hansen TB. Methylprednisolone reduces pain and decreases knee swelling in the first 24 h after fast-track unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):284–90.
Lunn TH, Kehlet H. Perioperative glucocorticoids in hip and knee surgery - benefit vs. harm? A review of randomized clinical trials. Acta Anaesthesiol Scand. 2013;57(7):823–34.
Acknowledgements
The authors would like to thank Beijing Municipal Health Commission (Jing2019-2) for its contribution to support the meta-analysis.
Funding
This study was supported by Beijing Municipal Health Commission (Jing2019-2).
Author information
Authors and Affiliations
Contributions
Tianlong Wang, Huiqun Fu, Fangyan Liu and Mei Duan designed the experiment. Fangyan Liu and Mei Duan made substantial contributions to acquisition of data, and analysis and interpretation of data. Fangyan Liu and Mei Duan wrote the manuscript. Fangyan Liu and Mei Duan contributed equally. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, F., Duan, M., Fu, H. et al. The efficacy and safety of perioperative glucocorticoid for total knee arthroplasty: a systematic review and meta-analysis. BMC Anesthesiol 24, 144 (2024). https://doi.org/10.1186/s12871-024-02530-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02530-9