Abstract
Background
The comparison between sedation and general anesthesia (GA) in terms of all-cause mortality remains a subject of ongoing debate. The primary objective of our study was to investigate the impact of GA and sedation on all-cause mortality in order to provide clarity on this controversial topic.
Methods
A systematic review and meta-analysis were conducted, incorporating cohort studies and RCTs about postoperative all-cause mortality. Comprehensive searches were performed in the PubMed, EMBASE, and Cochrane Library databases, with the search period extending until February 28, 2023. Two independent reviewers extracted the relevant information, including the number of deaths, survivals, and risk effect values at various time points following surgery, and these data were subsequently pooled and analyzed using a random effects model.
Results
A total of 58 studies were included in the analysis, with a majority focusing on endovascular surgery. The findings of our analysis indicated that, overall, and in most subgroup analyses, sedation exhibited superiority over GA in terms of in-hospital and 30-day mortality. However, no significant difference was observed in subgroup analyses specific to cerebrovascular surgery. About 90-day mortality, the majority of studies centered around cerebrovascular surgery. Although the overall pooled results showed a difference between sedation and GA, no distinction was observed between the pooled ORs and the subgroup analyses based on RCTs and matched cohort studies. For one-year all-cause mortality, all included studies focused on cardiac and macrovascular surgery. No difference was found between the HRs and the results derived from RCTs and matched cohort studies.
Conclusions
The results suggested a potential superiority of sedation over GA, particularly in the context of cardiac and macrovascular surgery, mitigating the risk of in-hospital and 30-day death. However, for the longer postoperative periods, this difference remains uncertain.
Trial registration
PROSPERO CRD42023399151; registered 24 February 2023.
Similar content being viewed by others
Introduction
Sedation, a type of anesthesia, has seen escalating utilization across various surgical procedures, particularly those involving minimally invasive techniques and catheterization, such as transcatheter aortic valve replacement (TAVR), transcatheter left atrial appendage closure, and endovascular thrombectomy [1,2,3]. Sedation frequently plays an integral part in enhanced recovery after surgery (ERAS) protocols, designed to minimize the reliance on general anesthesia (GA), especially in critically ill patients. Its implementation has been associated with reduced rates of intensive care unit (ICU) admissions and postoperative cognitive impairment [4]. Conversely, these percutaneous procedures are typically performed under GA, which does not appear to yield worse patient outcomes when compared to sedation according to a previous study [5]. GA provides complete intraoperative analgesia and deep sedation, ensuring a stable surgical environment and enhancing safety. Consequently, the choice between GA and sedation in percutaneous procedures remain a topic of ongoing debate, as multiple patient and procedural factors may influence the decision [6].
Of particular importance to surgical patients, especially those in critical condition, is mortality, rendering it the primary outcome of interest when comparing these two anesthesia techniques. Thus, determining whether GA or sedation can affect postoperative mortality has become a clinical concern. However, the impact of different anesthesia approaches on all-cause mortality in patients undergoing percutaneous procedures has produced inconsistent findings. A retrospective study found that monitored anesthesia care (MAC) was associated with lower 30-day mortality and comparable 3-year mortality in patients undergoing TAVR [7]. In contrast, results from a multicenter randomized controlled trial (RCT) investigating the same surgical procedure indicated that GA reduced 30-day mortality compared to sedation [8].
To further clarify the results of this comparison, several systematic reviews and meta-analyses incorporating numerous clinical studies have been conducted. Hung KC et al. identified that sedation was associated with lower risks of 30-day mortality by pooling data from 24 clinical studies on TAVR [9]. In contrast, a meta-analysis of three RCTs involving patients undergoing intracranial mechanical thrombectomy showed no significant difference in mortality [10]. Although variations in percutaneous procedures may contribute to the observed discrepancies, this remains speculative. Therefore, our comprehension of the relationship between the type of anesthesia used and all-cause mortality in patients undergoing percutaneous procedures remains limited.
To address this gap in knowledge, we conducted a systematic review and meta-analysis focusing on sedation alone or combined with local anesthesia and GA, excluding cases where regional nerve block or intraspinal anesthesia was employed. Specifically, our analysis aimed to investigate the impact of GA and sedation on postoperative all-cause mortality by incorporating a larger sample size.
Methods
This meta-analysis was conducted according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [11] and followed a protocol registered on the international prospective register of systematic reviews (PROSPERO: CRD42023399151).
Search strategy
Two investigators conducted an independent and comprehensive search of online databases, namely PubMed, EMBASE, and the Cochrane Library, with no language restrictions, to identify articles on the key terms "general anesthesia," "sedation," and "mortality." The search encompassed articles available up until February 28, 2023. In the event of any discrepancies during the literature search process, a third reviewer was consulted to facilitate a thorough discussion and reach a consensus. The specific search terms employed for each electronic database were detailed in Supplemental Table 1.
Study selection
All references identified through the implemented search strategy were exported to Endnote V.X9 (Thomson Reuters, Philadelphia, USA) and subjected to an independent evaluation by two reviewers. The initial screening involved assessing the titles and abstracts of all retrieved search results, followed by a detailed examination of the full texts of potentially relevant articles. For inclusion in this meta-analysis, studies were required to satisfy the following PICOS criteria: (1) Population: patients undergoing percutaneous procedures with either GA or sedation, (2) Intervention: sedation, encompassing various depths and drug usage, (3) Comparison intervention: GA with tracheal intubation or laryngeal mask ventilation, (4) Outcome: risk estimates of death or mortality at least one postoperative time point, and (5) Study design: RCT and cohort study. Studies that did not meet these criteria, including those with insufficient data, nonhuman studies, abstracts only, and protocols, were excluded. In instances where disputes arose regarding the eligibility of specific papers, a comprehensive discussion was held involving a third reviewer to resolve.
Quality assessment
The risk of bias in RCTs was independently evaluated by two reviewers using the Cochrane risk of bias criteria [12, 13] and the Newcastle–Ottawa Scale (NOS) was employed for cohort studies [14]. The Cochrane risk of bias criteria encompassed several domains, namely random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting, and other biases. Each study was assessed for potential bias, with a rating of "Low," "High," or "Unclear" assigned accordingly. On the other hand, the NOS consisted of eight categories addressing methodological quality, and each study received a score out of a maximum of 9 points. A score ranging from 0 to 6 denoted a low-quality study, while a score of 7 to 9 indicated a high-quality study. The overall certainty of the evidence was evaluated following the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework [15,16,17,18]. Any disagreements about quality assessment were resolved through a full discussion with a third reviewer.
Data extraction
Two reviewers independently conducted the extraction of relevant details from the included studies, with any discrepancies in data extraction resolved through thorough discussion involving a third reviewer. The extracted information encompassed the following: (1) Study characteristics, including the first author's name, study design, publication year, country, and sample size; (2) Participant characteristics, such as age, the proportion of males, and the specific surgeries or procedures included; (3) The number of deaths and survival outcomes reported at all time points for both intervention groups; (4) Adjusted risk estimates, accompanied by 95% CIs, for mortality obtained from any statistical models employed in the studies.
Statistical analysis
The statistical analyses were conducted utilizing Stata software version 17.0 (Stata Corp.). For dichotomous data, the relevant information extracted from each study included the total number of patients in each group and the number of patients experiencing the outcome of death. Risk ratios (RRs) and their corresponding 95% CIs were synthesized to evaluate the outcome. To calculate the log ORs, HRs, and relative risks, we utilized those (along with their respective 95% CIs) derived from the included articles that compared sedation versus GA. Irrespective of whether the data were dichotomous or in the form of risk estimates, the outcomes were pooled using a random-effects model due to the expected clinical and methodological diversity among the included studies.
To explore statistical heterogeneity among the pooled effects, the Cochran Q statistic was employed, and the extent of heterogeneity was quantified using the I2 metric (significant heterogeneity defined as I2 > 50% and p < 0.05) [19]. For outcomes comprising more than 10 studies, the potential risk of publication bias in the included studies was evaluated through visual examination of funnel plots and quantitative Egger's tests.
Subgroup analyses were conducted to investigate potential sources of significant heterogeneity and assess the impact of important factors on all-cause mortality. These factors included: (1) study design, categorized as RCT, matched cohort study, and non-matched cohort study; and (2) type of surgery or procedure, classified as cardiac and macrovascular, cerebrovascular, or other surgeries. Furthermore, sensitivity analysis was performed by systematically excluding individual studies to assess the influence of each study on the overall pooled estimate.
Results
Search results and study characteristics
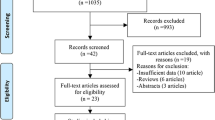
The initial search strategy found 2421 articles, and after excluding papers that were duplicates or did not meet the inclusion criteria, 108 full-text articles of potentially relevant studies were identified. Following a full-text review, an additional 50 articles were excluded. Specifically, to more accurately compare GA and sedation in terms of all-cause mortality, we excluded studies on GA or sedation combined with other types of anesthesia including nerve block and epidural anesthesia. Finally, 57 studies were included in our meta-analysis (Fig. 1), including 8 RCTs, and 49 cohort studies (including 12 matched cohort studies).
Table 1 and Supplemental Table 2 presents the study characteristics. A total of 61,945 patients were involved. 29,843 and 32,102 patients completed surgery under GA and sedation, respectively. The mean or median age of the enrolled patients varied from 6.5 to 85.4 years with the proportion of males ranging from 14.3% to 100%. Of the final included studies, 39 involved cardiac and macrovascular surgery, 18 on cerebrovascular surgery, and one on ERCP. Various medications for GA (total intravenous or intravenous-inhalational anesthesia, intubation, or laryngeal mask general anesthesia) or sedation (e.g., conscious sedation, deep sedation, and MAC) were used across the studies, as detailed in Supplemental Table 2. The NOS score of the 50 cohort studies ranged from 6 to 9 (Table 1), and the risk of bias assessment for 8 RCTs was shown in Supplemental Fig. 1.
GA versus sedation in all-cause mortality
In our analysis, we examined the impact of sedation and GA on all-cause mortality across five distinct time points according to the data included in this study: 24-h mortality, in-hospital mortality, 30-day mortality, 90-day mortality, and 1-year mortality.
Mortality at 24 h postoperatively
Only one study [20] reported mortality 24 h postoperatively, suggesting no significant difference between sedation and GA for cardiac catheterization in children under 2 years old.
In-hospital mortality
Among the studies included in our analysis, a total of 26 articles were examined to assess in-hospital mortality. These articles consisted of 2 RCTs and 13 cohort studies focusing on cardiac and macrovascular surgery, 4 RCTs, and 7 cohort studies pertaining to cerebrovascular surgery.
The pooled results from these studies indicated a significantly lower risk of death associated with the use of sedation compared to GA (RR = 0.68, 95% CI: 0.58 to 0.79, p < 0.001) among a total of 19,245 patients (Fig. 2A). Sensitivity analyses further supported these findings (Supplemental Fig. 2), and the level of heterogeneity was not statistically significant (I2 = 29.54%, p = 0.08).
Subgroup analysis based on study design also demonstrated a lower mortality risk with sedation, regardless of whether it was a non-matched cohort study (RR = 0.67, 95% CI: 0.54 to 0.82) or a matched cohort/RCT (RR = 0.69, 95% CI: 0.54 to 0.86). Notably, the subgroup of matched cohort/RCT showed lower heterogeneity (I2 = 15.61%, p = 0.29) (Fig. 2A). When examining the effect of surgery type, the results indicated that patients undergoing cardiac and macrovascular or cerebrovascular surgery had a lower risk of death with sedation (cardiac and macrovascular: RR = 0.56, 95% CI: 0.46 to 0.67; cerebrovascular: RR = 0.75, 95% CI: 0.62 to 0.92) (Supplemental Fig. 3). Additionally, Egger’s test showed no significant publication bias (t = 0.55, p = 0.588), and the funnel plot is presented in Supplemental Fig. 4.
Moreover, five articles provided adjusted effect sizes in the form of odds ratios (ORs) for in-hospital mortality [47, 49, 55, 58, 70]. The pooled results of the five adjusted OR values demonstrated that sedation was associated with a lower risk of in-hospital death compared to GA (RR = 0.69, 95% CI: 0.56 to 0.85, p < 0.001) among a total of 20,675 patients, although there was significant heterogeneity (I2 = 58.03%, p = 0.05) (Fig. 3A). Notably, subgroup analysis limited to non-matched cohort studies showed consistent results (RR = 0.69, 95% CI: 0.55 to 0.86, I2 = 68.50%) (Fig. 3A). However, the subgroup analysis specific to cerebrovascular surgery revealed an approximately non-significant result (RR = 0.71, 95% CI: 0.50 to 1.00, p = 0.05, I2 = 61.12%) (Fig. 3B).
A Pooled result of adjusted effect size (OR) of sedation on the risk of in-hospital death with the subgroup analysis of study design; B Pooled result of adjusted effect size (OR) of sedation on the risk of in-hospital death with the subgroup analysis of type of surgery; C Pooled result of adjusted effect size (OR) of sedation on the risk of 30-day death with the subgroup analysis of study design; D Pooled result of adjusted effect size (OR) of sedation on the risk of 30-day death with the subgroup analysis of type of surgery; E Pooled result of adjusted effect size (HR) of sedation on the risk of 30-day death
30-day mortality
A total of 30 articles were included in our analysis to investigate 30-day mortality in the comparison between sedation and GA. These articles consisted of 27 cohort studies focusing on cardiac and macrovascular surgery, 1 RCT and 1 cohort study pertaining to cerebrovascular surgery, and 1 non-matched cohort study on ERCP surgery.
The pooled analysis revealed a significantly lower risk of death within 30 days postoperatively in the sedation group compared to the GA group (RR = 0.63, 95% CI: 0.47 to 0.77, p < 0.001), involving a total of 42,888 patients. There was no significant heterogeneity observed among the included studies (I2 = 28.23%, p = 0.08) (Fig. 2B). Sensitivity analyses, conducted by sequentially excluding individual studies, consistently supported these findings, indicating the robustness of the evidence (Supplemental Fig. 5).
Subgroup analysis focused on matched cohort or RCTs (RR = 0.68, 95% CI: 0.53 to 0.86, I2 = 0%) (Fig. 2B) and subgroup analysis specific to cardiac and macrovascular surgery (RR = 0.66, 95% CI: 0.56 to 0.77, I2 = 0%) (Supplemental Fig. 6) revealed no significant heterogeneity and provided insights into the possible sources of significant heterogeneity observed in the overall analysis. Egger’s test (t = 2.68, p = 0.012) and the funnel plot (Supplemental Fig. 7) suggested the presence of publication bias concerning the outcome of 30-day mortality. However, the subgroup of cerebrovascular surgery, involving only two studies, revealed no difference between the two groups (RR = 0.56, 95% CI: 0.27 to 1.18, I2 = 0%).
Regarding the adjusted effect size for the risk of 30-day mortality, 8 articles reported adjusted effect sizes, with four articles [26, 49, 60, 70] presenting OR values and four articles [7, 30, 50, 51] presenting HR. The pooled analysis of the OR values indicated a significant difference between the sedation and GA groups (RR = 0.64, 95% CI: 0.56 to 0.74, p < 0.001), involving a total of 28,787 patients, with non-significant heterogeneity (I2 = 0%, p = 0.69) (Fig. 3C). The subgroup analysis limited to cohort studies consistently showed significant differences (RR = 0.64, 95% CI: 0.56 to 0.74) (Fig. 3C). In the subgroup analysis of the types of surgery, the number of literatures for each subgroup was small, and more than one literature was integrated only in the subgroup of cardiac and macrovascular surgery, showing that patients with sedation had a lower risk of death (RR = 0.64, 95% CI: 0.56 to 0.74) (Fig. 3D). Meanwhile, the pooled analysis of the HR values demonstrated a lower risk of death in the sedation group compared to the GA group (RR = 0.59, 95% CI: 0.46 to 0.76, p < 0.001), involving 7,889 patients, with non-significant heterogeneity (I2 = 0%, p = 0.57) (Fig. 3E).
90-day mortality
In our analysis examining the influence of sedation compared to GA on the risk of 90-day mortality, a total of 12 studies were incorporated. It is noteworthy that the majority of these studies focused on cerebrovascular surgery, encompassing 6 randomized RCTs and 4 cohort studies. However, there was limited representation for other surgical procedures, with only one cohort study investigating the comparison of cardiac and macrovascular surgery, and another cohort study reporting on the comparison of ERCP surgery.
The pooled results revealed a significant difference in the risk of postoperative 90-day death between the two groups (RR = 0.73, 95% CI: 0.56 to 0.96, p = 0.02), involving a total of 19,052 patients, with significant heterogeneity (I2 = 55.55%, p = 0.01) (Fig. 4A). Subgroup analysis based on study design demonstrated disparate outcomes between cohort and matched cohort/RCTs groups (cohort: RR = 0.49, 95% CI: 0.36 to 0.67; matched cohort/RCT: RR = 0.84, 95% CI: 0.68 to 1.04), indicating non-significant heterogeneity within both groups (Fig. 4A). Furthermore, subgroup analysis specific to cerebrovascular surgery highlighted a difference in the risk of postoperative 90-day death between the two groups (RR = 0.80, 95% CI: 0.66 to 0.98, I2 = 0%) (Supplemental Fig. 8). However, sensitivity analysis revealed that the removal of two studies [22, 27] could impact the conclusion (Supplemental Fig. 9). Additionally, the funnel plot (Supplemental Fig. 10) and Egger’s test (t = 2.65, p = 0.024) indicated the presence of publication bias, highlighting the limitations of the evidence.
Four articles [56, 60, 65, 70] reported the adjusted effect size regarding the relationship between sedation/GA and 90-day mortality, all of which provided OR values. In contrast to the pooled results of deaths, the pooled analysis of the effect values showed no significant difference between the two groups (RR = 0.68, 95% CI: 0.41 to 1.16, p = 0.16), involving a total of 18,061 patients, with non-significant heterogeneity (I2 = 0%, p = 0.79). Subgroup analyses based on study design and surgery type were consistent with these results (Fig. 5A, B).
A Pooled result of adjusted effect size (OR) of sedation on the risk of 90-day death with the subgroup analysis of study design; B Pooled result of adjusted effect size (OR) of sedation on the risk of 90-day death with the subgroup analysis of type of surgery; C Pooled result of adjusted effect size (HR) of sedation on the risk of one-year death with the subgroup analysis of study design
One-year mortality
In our analysis investigating the relationship between sedation and one-year mortality, a total of 8 articles were included. It is important to note that all of these articles focused exclusively on cohort studies pertaining to cardiac and macrovascular surgery. While the available evidence provides insights into the impact of sedation on one-year mortality in this specific surgical context, there is currently limited data available for other types of surgeries.
The pooled results revealed a correlation between sedation and a lower risk of one-year death compared to GA (RR = 0.84, 95% CI: 0.75 to 0.93, p < 0.001), involving a total of 8,989 patients, with non-significant heterogeneity (I2 = 0%, p = 0.60) (Fig. 4B). While the cohort study design consistently showed a lower risk of one-year mortality with sedation compared to GA (RR = 0.73, 95% CI: 0.62 to 0.86, I2 = 0%), the effect became non-significant for matched cohort/RCT in the subgroup analysis (RR = 0.91, 95% CI: 0.80 to 1.03, I2 = 0%) (Fig. 4B). However, data pooling was not possible for the analysis of surgery-type subgroups.
The adjusted effect size between sedation and the risk of one-year death was examined in 3 studies. The pooled result of these 3 articles, all of which provided HR values, demonstrated no significant difference in the risk of one-year death between the two groups (RR = 0.85, 95% CI: 0.69 to 1.04, p = 0.11), involving a total of 7,689 patients, with non-significant heterogeneity (I2 = 28.62%, p = 0.24). Subgroup analysis of cohort studies also found consistent results (RR = 0.80, 95% CI: 0.55 to 1.17, I2 = 36.76%) (Fig. 5C). However, no data pooling was possible for the effect size analysis of surgery-type subgroups.
Certainty of evidence
The level of certainty regarding the evidence for each outcome was presented in Supplementary Table 3. The overall certainty of the evidence was classified as low for three outcomes, namely the risk of in-hospital mortality based on pooled number of cases, the risk of one-year mortality based on pooled number of cases, and the effect values. For the remaining outcomes, the overall certainty was considered very low. The primary factors contributing to the downgrade in evidence included: (a) the inclusion of observational data; (b) a high I2 value exceeding 30%; and (c) the presence of significant publication bias.
Discussion
This systematic review and meta-analysis, including both RCTs and cohort studies and pooling outcomes of mortality data and effect size (ORs and HRs), compared the all-cause mortality after sedation versus GA. In-hospital mortality: Sedation was associated with a reduced risk of in-hospital death, regardless of whether patients underwent percutaneous cardiac and macrovascular surgery or cerebrovascular surgery. 30-day mortality: Sedation showed lower risks of death within 30 days postoperatively in patients undergoing percutaneous cardiac and macrovascular surgery, but not in those undergoing cerebrovascular surgery. 90-day and one-year mortality: Discrepancies were observed between the results from pooled mortality and those from effect size analysis (ORs and HRs) for both 90-day and one-year mortality. Subgroup analyses based on different study types and surgery types also yielded varying results (Fig. 6).
Our search strategy led us to focus on three specific procedures: cardiac and macrovascular surgery, cerebrovascular surgery, and ERCP. These were chosen due to the prevalence of sedation or general anesthesia (GA) usage in endovascular and gastrointestinal interventional procedures. The decision to administer sedation or GA is influenced by various factors, including procedural accuracy, anesthesiologist-rated risk, surgeon preference, and procedure-specific considerations. Our study's results suggest a potential slight superiority of sedation over GA in terms of in-hospital, and 30-day all-cause mortality. However, the advantage of sedation over GA at long-term postoperative time points remains unclear or ambiguous.
Prior meta-analyses have predominantly focused on specific surgery types, such as cardiovascular [76,77,78,79] or cerebrovascular surgery [10, 80,81,82,83], or specific research designs, such as RCTs [82,83,84]. While these approaches allowed for a high-quality and targeted comparison between sedation and GA, they often suffer from limitations due to a smaller number of included articles and limited data, resulting in an incomplete comparison between them.
Two previous meta-analyses [77, 85] specifically examined the risk of in-hospital death in transcatheter heart valve surgery (TAVI or TAVR) and consistently reported no significant difference in mortality rates between sedation and GA. However, our pooled results, encompassing a broader range of studies and incorporating articles published after the cutoff date of those meta-analyses, demonstrated a lower risk of death associated with sedation compared to GA. We speculate that advancements in surgical techniques and anesthesia technology, along with the inclusion of high-quality studies, may contribute to the discrepancies between our findings and previous analyses. Additionally, the broad inclusion and exclusion criteria employed in these studies aimed to increase the number of eligible articles, but this may have led to comparisons that did not strictly adhere to sedation versus GA standards. For instance, the meta-analysis of Ehret C et al. [85] included clinical studies [86, 87] that compared surgical methods rather than anesthesia type, despite both procedures being performed under sedation and GA. Furthermore, our sensitivity analysis and consistent results from overall and subgroup analyses provide robustness to our findings.
About 30-day mortality, previous meta-analyses primarily focused on transcatheter heart valve surgery (TAVI or TAVR) and yielded conflicting findings. Some studies reported no significant difference between minimal anesthesia care and GA, while others suggested a lower overall 30-day mortality with sedation. For instance, a meta-analysis encompassing seven observational studies and a total of 1,542 patients reported no significant difference in overall 30-day mortality between MAC and GA (RR = 0.77, 95% CI 0.38 to 1.56; P = 0.46) based on a literature search spanning from January 1, 2005, to January 31, 2013 [78]. Similarly, other meta-analyses conducted around the time of publication of the aforementioned study produced consistent results [85, 88].
However, two recent meta-analyses demonstrated that the use of sedation for TAVR was associated with a lower overall 30-day mortality (RR = 0.73, 95% CI: 0.57 to 0.93; P = 0.01) through the pooling of mortality data [79, 89]. In alignment with these findings, our pooled analysis of the number of deaths also revealed a reduced risk of death in the sedation group compared to the GA group. The discrepancy between these findings could be attributed to advancements in surgical techniques and anesthesia technology over time. Furthermore, in a more accurate approach than previous studies, we integrated risk estimates based on ORs and HRs, indicating consistent evidence regarding the lower risk of 30-day mortality of sedation. However, it is important to note that our subgroup analysis specific to cerebrovascular surgery did not reveal a difference in in-hospital mortality between sedation and GA. Given the limited number of studies available for this analysis, caution should be exercised when interpreting these results.
Regarding 90-day mortality, previous meta-analyses focused on transcatheter cerebrovascular surgery consistently showed no reduced risk of mortality with sedation compared to GA [83, 84, 90]. Our subgroup analysis of matched cohorts or RCTs also supported this conclusion, as did the pooling of OR values. However, our overall analysis of pooled the number of deaths did reveal a difference in 90-day mortality. Nonetheless, the effect size was minimal, and significant heterogeneity and serious publication bias limited the interpretation of this difference. Therefore, caution should be exercised when interpreting these findings.
To our knowledge, this is the first meta-analysis conducting the comparison in one-year mortality, involving only 8 articles focusing on cardiac and macrovascular surgery. However, we observed inconsistencies between the results obtained from different pooling methods: number of deaths and HR. Given that the included papers consisted mainly of retrospective observational studies, with only two matched cohort studies, the presence of numerous confounding factors is inevitable. Consequently, the adjusted effect values (HR) provided a more reliable indication of no difference between sedation and GA, aligning with the majority of clinical studies [45, 53, 66, 75].
Although the mechanism by which sedation reduces the risk of postoperative death remains uncertain, previous studies offered some insights. Firstly, sedation leads to a reduction in the dosage of anesthetic drugs, thereby mitigating cardiac depression and periprocedural hemodynamic instability [42]. This reduction may decrease the risk of permanent neurological deficits, myocardial ischemia, and renal impairment during the surgical period [91, 92]. Secondly, sedation obviates the need for tracheal intubation, which is associated with an increased risk of intraoperative complications, particularly pulmonary complications [55, 93, 94]. Furthermore, avoiding tracheal intubation eliminates the need to transfer intubated patients to the ICU [74, 95], thereby reducing the patient's susceptibility to postoperative infections.
However, these clues are more closely related to early postoperative mortality, rather than mid- or long-term postoperative mortality. Just like the indication of our results, the effect of the type of anesthesia on these terms was more ambiguous, especially 90-day and one-year mortality. As everyone knows, the risk of postoperative long-term death is related to more factors compared to early one [22, 96], such as home nursing, work, and living habits after discharge, compliance with medical instructions, etc., and anesthesia choice is only a point-in-time intervention, it is hard to imagine how the choice of a single point can affect long-term outcomes. At the same time, no previous studies have established a real and reliable association between anesthesia choice and the risk factors for long-term postoperative death. In our analysis, a lower risk of one-year death was associated with sedation compared to GA, however, all studies included in this analysis were cohort studies, and the inconsistency between the results of the matched cohort subgroup analysis and the main analysis suggested no difference between sedation and GA. For randomized controlled studies, follow-up of up to one year is difficult, which also contributes to the lack of such studies. We seem to get some clues from similar previous RCTs that in elderly patients having hip fracture surgery with spinal anesthesia supplemented with propofol sedation, heavier intraoperative sedation was not associated with significant differences in mortality or return to pre-fracture ambulation up to one year after surgery [97].
Strengths and limitations
This meta-analysis provides a comprehensive synthesis of the available evidence on the association between sedation and GA with all-cause mortality. We have examined a broad spectrum of postoperative mortality outcomes, spanning various time points (24 h postoperatively, in-hospital, 30-day, 90-day, and one-year), and have employed diverse statistical analysis methods including ORs and HRs. Through separate pooling of these outcomes, our aim is to move beyond single time point analyses and individual outcomes, allowing for a nuanced exploration of the relationship between anesthesia type and all-cause mortality, thus facilitating a comprehensive comparison between sedation and GA.
However, it is crucial to acknowledge the limitations of our meta-analysis. Firstly, to comprehensively evaluate all-cause mortality between sedation and GA, we included studies with diverse designs, including retrospective observational studies, which inherently contribute to a lower level of evidence for our findings, all of which were graded as very low to low certainty. Furthermore, despite employing various analytical approaches, we encountered significant heterogeneity and conflicting results, particularly in the assessment of 90-day and one-year postoperative mortality. Thirdly, our study focused on patients undergoing percutaneous procedures, with a predominant emphasis on endovascular surgery. Therefore, the generalizability of our conclusions to all surgical patients eligible for sedation or GA is limited. Lastly, the studies included in our analysis employed different anesthesia techniques (e.g., total intravenous or intravenous-inhalational anesthesia, intubation or laryngeal mask general anesthesia) or sedation techniques (e.g., conscious sedation, deep sedation, and MAC), which could further increase heterogeneity and robustness of pooled outcomes. Consequently, future research should aim to explore the comparison between sedation and GA of different types across a broader range of postoperative outcomes, particularly in non-endovascular surgery and long-term outcomes, through high-quality clinical studies.
Conclusion
The currently available evidence, graded as very low to low certainty, suggests a possible slight advantage of sedation over GA in reducing the risk of in-hospital mortality, specifically in cardiac and macrovascular surgery. However, the extent of this difference is not clearly evident or uncertain in the medium and long-term postoperative periods. Furthermore, the comparison between sedation and GA in cerebrovascular surgery and other surgical patient populations also yields uncertain results, despite the limited number of studies included in the subgroup analysis.
Availability of data and materials
All data relevant to the study are included in the article or uploaded as supplementary information.
Abbreviations
- GA:
-
General anesthesia
- TAVR:
-
Transcatheter aortic valve replacement
- MAC:
-
Monitored anesthesia care
- RCTs:
-
Randomized controlled trials
- ORs:
-
Odds ratios
- HRs:
-
Hazard ratios
- RRs:
-
Risk ratios
- CIs:
-
Confidence interval
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses statement
References
Yang L, Hennis L, Patel K, Saccocci MA. Laparoscopic adrenalectomy of pheochromocytoma following management of severe aortic stenosis with transcatheter aortic valve replacement under monitored anesthesia care sedation: a case report. BMC Anesthesiol. 2023;23(1):18.
Wang A, Abramowicz AE. Endovascular thrombectomy in acute ischemic stroke: new treatment guide. Curr Opin Anaesthesiol. 2018;31(4):473–80.
Reid C, Meineri M, Riva T, Pilgrim T, Räber L, Luedi MM. Anaesthesia for minimally invasive cardiac procedures in the catheterization lab. Curr Opin Anaesthesiol. 2021;34(4):437–42.
Tagliari AP, Taramasso M. New practices in Transcatheter aortic valve implantation: how I do it in 2023. J Clin Med. 2023;12(4).
Campbell D, Butler E, Barber PA. End the confusion: general anaesthesia improves patient outcomes in endovascular thrombectomy. Br J Anaesth. 2022;129(4):461–4.
Gruenbaum SE, Gruenbaum BF, Bertasi RAO, Bertasi TGO, Zlotnik A. Intraoperative management of thrombectomy for acute ischemic stroke: Do we need general anesthesia? Best practice & research Clinical anaesthesiology. 2021;35(2):171-9.
Sammour Y, Kerrigan J, Banerjee K, Gajulapalli RD, Lak H, Chawla S, et al. Comparing outcomes of general anesthesia and monitored anesthesia care during transcatheter aortic valve replacement: the Cleveland Clinic Foundation experience. Catheter Cardiovasc Interv. 2021;98(3):E436–43.
Thiele H, Kurz T, Feistritzer HJ, Stachel G, Hartung P, Lurz P, et al. General Versus Local Anesthesia with conscious sedation in transcatheter aortic valve implantation: the Randomized SOLVE-TAVI trial. Circulation. 2020;142(15):1437–47.
Hung KC, Chen JY, Hsing CH, Chu CC, Lin YT, Pang YL, et al. Conscious sedation/monitored anesthesia care versus general anesthesia in patients undergoing transcatheter aortic valve replacement: a meta-analysis. Front Cardiovasc Med. 2022;9:1099959.
Zhang Y, Jia L, Fang F, Ma L, Cai B, Faramand A. General Anesthesia Versus Conscious Sedation for Intracranial Mechanical Thrombectomy: a systematic review and Meta-analysis of Randomized clinical trials. J Am Heart Association. 2019;8(12):e011754.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed). 2021;372:n71.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed). 2011;343:d5928.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed). 2019;366:l4898.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Res ed). 2008;336(7650):924–6.
Brozek JL, Akl EA, Alonso-Coello P, Lang D, Jaeschke R, Williams JW, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64(5):669–77.
Brozek JL, Akl EA, Jaeschke R, Lang DM, Bossuyt P, Glasziou P, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines: part 2 of 3. The GRADE approach to grading quality of evidence about diagnostic tests and strategies. Allergy. 2009;64(8):1109–16.
Brożek JL, Akl EA, Compalati E, Kreis J, Terracciano L, Fiocchi A, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines part 3 of 3. The GRADE approach to developing recommendations. Allergy. 2011;66(5):588–95.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142.
Mikus M, Welchowski T, Schindler E, Schneider M, Mini N, Vergnat M. Sedation versus General Anesthesia for Cardiac Catheterization in infants: a retrospective, monocentric, cohort evaluation. J Clin Med. 2021;10(23).
Zaouter C, Smaili S, Leroux L, Bonnet G, Leuillet S, Ouattara A. Transcatheter aortic valve implantation: General anesthesia using transesophageal echocardiography does not decrease the incidence of paravalvular leaks compared to sedation alone. Ann Card Anaesth. 2018;21(3):277–84.
Weyland CS, Chen M, Potreck A, Jäger LB, Seker F, Schönenberger S, et al. Sedation Mode during Endovascular Stroke Treatment in the posterior Circulation-Is conscious sedation for eligible patients feasible? Front Neurol. 2021;12:711558.
Neumann FJ, Redwood S, Abdel-Wahab M, Lefèvre T, Frank D, Eltchaninoff H, et al. General Anesthesia or Conscious Sedation for Transfemoral Aortic Valve Replacement with the SAPIEN 3 Transcatheter Heart Valve. Int Heart J. 2020;61(4):713–9.
Liu HY, Yang LL, Dai XY, Li ZP. Local anesthesia with sedation and general anesthesia for the treatment of chronic subdural hematoma: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2022;26(5):1625–31.
Liang Y, Wang W, Wang X, Liu M, Hei F, Guan Y. General Anesthesia increased the risk of Atrial Fibrillation and Acute kidney Injury in Transcatheter aortic valve replacement. Heart Surg Forum. 2021;24(1):E082–100.
Simonsen CZ, Rasmussen M, Schönenberger S, Hendén PL, Bösel J, Valentin JB. General anesthesia during endovascular therapy for acute ischemic stroke: benefits beyond better reperfusion? J Neurointerventional Surg. 2022;14(8):767–71.
Toppen W, Johansen D, Sareh S, Fernandez J, Satou N, Patel KD, et al. Improved costs and outcomes with conscious sedation vs general anesthesia in TAVR patients: time to wake up? PLoS ONE. 2017;12(4):e0173777.
Theron P, Guha K, Mantziari L, Salahuddin S, Sharma R, Jaggar S. General anesthesia versus sedation for implantation of a biventricular pacing device for cardiac resynchronization therapy. J Cardiothorac Vasc Anesth. 2014;28(2):280–4.
Téllez-Alarcón M, Montes FR, Hurtado P, Gutiérrez LP, Cabrales JR, Camacho J, et al. Conscious sedation versus general anesthesia for transcatheter aortic valve implantation: a retrospective study. Brazilian J Anesthesiology (Elsevier). 2022;72(4):539–41.
Stragier H, Dubois C, Verbrugghe P, Jacobs S, Adriaenssens T, Rex S. General Anesthesia Versus monitored Anesthesia Care for Transfemoral Transcatheter aortic valve implantation: a retrospective study in a single Belgian Referral Center. J Cardiothorac Vasc Anesth. 2019;33(12):3283–91.
Shan W, Yang D, Wang H, Xu L, Zhang M, Liu W, et al. General Anesthesia may have similar outcomes with conscious sedation in Thrombectomy patients with Acute ischemic stroke: a Real-World Registry in China. Eur Neurol. 2018;80(1–2):7–13.
Schönenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC, et al. Effect of conscious sedation vs General Anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a Randomized Clinical Trial. JAMA. 2016;316(19):1986–96.
Palermo C, Degnan M, Candiotti K, Salerno T, de Marchena E, Rodriguez-Blanco Y. Monitored Anesthesia Care Versus General Anesthesia: experience with the Medtronic CoreValve. J Cardiothorac Vasc Anesth. 2016;30(5):1234–7.
Renner J, Tesdorpf A, Freitag-Wolf S, Francksen H, Petzina R, Lutter G, et al. A retrospective study of conscious sedation versus general anaesthesia in patients scheduled for transfemoral aortic valve implantation: a single center experience. Health Sci Rep. 2019;2(1):e95.
Ren C, Xu G, Liu Y, Liu G, Wang J, Gao J. Effect of conscious sedation vs. General Anesthesia on outcomes in patients undergoing mechanical thrombectomy for Acute ischemic stroke: a prospective Randomized Clinical Trial. Front Neurol. 2020;11:170.
Rassaf T, Balzer J, Zeus T, Rammos C, Shayganfar S, Hall SV, et al. Safety and efficacy of deep sedation as compared to general anaesthesia in percutaneous mitral valve repair using the MitraClip system. Catheterization Cardiovasc Interventions: Official J Soc Cardiac Angiography Interventions. 2014;84(4):E38–42.
Powers CJ, Dornbos D 3rd, Mlynash M, Gulati D, Torbey M, Nimjee SM, et al. Thrombectomy with conscious sedation compared with General Anesthesia: a DEFUSE 3 analysis. AJNR Am J Neuroradiol. 2019;40(6):1001–5.
Ben-Dor I, Looser PM, Maluenda G, Weddington TC, Kambouris NG, Barbash IM, et al. Transcatheter aortic valve replacement under monitored anesthesia care versus general anesthesia with intubation. Cardiovasc Revascularization Medicine: Including Mol Interventions. 2012;13(4):207–10.
Fröhlich GM, Lansky AJ, Webb J, Roffi M, Toggweiler S, Reinthaler M, et al. Local versus general anesthesia for transcatheter aortic valve implantation (TAVR)--systematic review and meta-analysis. BMC Med. 2014;12:41.
Gao L, Jin B, Chao C, Wang B, Zhang X, Shen J. Comparative efficacy of local and general anesthesia for transcatheter aortic valve implantation: a Meta-analysis and systematic review. Heart Surg Forum. 2022;25(3):E364–73.
Piayda K, Hellhammer K, Nielsen-Kudsk JE, Schmidt B, Mazzone P, Berti S, et al. Clinical outcomes of patients undergoing percutaneous left atrial appendage occlusion in general anaesthesia or conscious sedation: data from the prospective global Amplatzer Amulet Occluder Observational Study. BMJ open. 2021;11(3):e040455.
Patzelt J, Ulrich M, Magunia H, Sauter R, Droppa M, Jorbenadze R et al. Comparison of Deep Sedation with General Anesthesia in patients undergoing percutaneous mitral valve repair. J Am Heart Association. 2017;6(12).
Pani S, Cagino J, Feustel P, Musuku SR, Raja A, Bruno N, et al. Patient selection and outcomes of Transfemoral Transcatheter aortic valve replacement performed with monitored Anesthesia Care Versus General Anesthesia. J Cardiothorac Vasc Anesth. 2017;31(6):2049–54.
Villablanca PA, Mohananey D, Nikolic K, Bangalore S, Slovut DP, Mathew V, et al. Comparison of local versus general anesthesia in patients undergoing transcatheter aortic valve replacement: a meta-analysis. Catheterization Cardiovasc Interventions: Official J Soc Cardiac Angiography Interventions. 2018;91(2):330–42.
Banga S, Hafiz AM, Chami Y, Gumm DC, Banga P, Howard C, et al. Comparing sedation vs. general anaesthesia in transoesophageal echocardiography-guided percutaneous transcatheter mitral valve repair: a meta-analysis. Eur Heart J Cardiovasc Imaging. 2020;21(5):511–21.
Maas EH, Pieters BM, Van de Velde M, Rex S. General or local anesthesia for TAVI? A systematic review of the literature and Meta-analysis. Curr Pharm Design. 2016;22(13):1868–78.
Musuku SR, Capua CAD, Doshi I, Cherukupalli D, Byun Y, Shapeton AD. Outcomes of Transfemoral Transcatheter aortic valve replacement performed with General Anesthesia using a Supraglottic Airway Versus monitored Anesthesia Care. J Cardiothorac Vasc Anesth. 2021;35(6):1760–8.
Goren O, Finkelstein A, Gluch A, Sheinberg N, Dery E, Matot I. Sedation or general anesthesia for patients undergoing transcatheter aortic valve implantation–does it affect outcome? An observational single-center study. J Clin Anesth. 2015;27(5):385–90.
Attizzani GF, Alkhalil A, Padaliya B, Tam CC, Lopes JP, Fares A, et al. Comparison of outcomes of Transfemoral Transcatheter aortic valve implantation using a minimally invasive Versus Conventional Strategy. Am J Cardiol. 2015;116(11):1731–6.
Jobs A, Grund S, de Waha-Thiele S, Ledwoch J, Sievert H, Rassaf T, et al. Deep sedation versus general anaesthesia for transcatheter mitral valve repair: an individual patient data meta-analysis of observational studies. EuroIntervention: J EuroPCR Collab Working Group Interventional Cardiol Eur Soc Cardiol. 2021;16(16):1359–65.
Mosleh W, Mather JF, Amer MR, Hiendlmayr B, Kiernan FJ, McKay RG. Propensity matched analysis comparing conscious Sedation Versus General Anesthesia in Transcatheter aortic valve implantation. Am J Cardiol. 2019;124(1):70–7.
Miles LF, Joshi KR, Ogilvie EH, Densem CG, Klein AA, O’Sullivan M, et al. General Anaesthesia vs. conscious sedation for transfemoral aortic valve implantation: a single UK centre before-and-after study. Anaesthesia. 2016;71(8):892–900.
McDonald JS, Brinjikji W, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anaesthesia during mechanical thrombectomy for stroke: a propensity score analysis. J Neurointerventional Surg. 2015;7(11):789–94.
Cappellari M, Pracucci G, Forlivesi S, Saia V, Nappini S, Nencini P, et al. General Anesthesia Versus Conscious Sedation and local Anesthesia during Thrombectomy for Acute ischemic stroke. Stroke. 2020;51(7):2036–44.
Schönenberger S, Hendén PL, Simonsen CZ, Uhlmann L, Klose C, Pfaff JAR, et al. Association of General Anesthesia vs Procedural Sedation with Functional Outcome among patients with Acute ischemic stroke undergoing thrombectomy: a systematic review and Meta-analysis. JAMA. 2019;322(13):1283–93.
Mayr NP, Hapfelmeier A, Martin K, Kurz A, van der Starre P, Babik B, et al. Comparison of sedation and general anaesthesia for transcatheter aortic valve implantation on cerebral oxygen saturation and neurocognitive outcome†. Br J Anaesth. 2016;116(1):90–9.
Aslan S, Güner A, Demir AR, Yılmaz E, Aslan AF, Çelik Ö, et al. Conscious sedation versus general anesthesia for transcatheter aortic valve implantation in patients with severe chronic obstructive pulmonary disease. Perfusion. 2023;38(1):186–92.
Campbell D, Diprose WK, Deng C, Barber PA. General Anesthesia Versus Conscious Sedation in Endovascular Thrombectomy for Stroke: a Meta-analysis of 4 randomized controlled trials. J Neurosurg Anesthesiol. 2021;33(1):21–7.
Löwhagen Hendén P, Rentzos A, Karlsson JE, Rosengren L, Leiram B, Sundeman H, et al. General Anesthesia Versus Conscious Sedation for Endovascular Treatment of Acute ischemic stroke: the AnStroke Trial (Anesthesia during Stroke). Stroke. 2017;48(6):1601–7.
Skutecki J, Audibert G, Finitsis S, Consoli A, Lapergue B, Blanc R, et al. General anesthesia or conscious sedation for endovascular therapy of basilar artery occlusions: ETIS registry results. Rev Neurol. 2022;178(8):771–9.
Kleinecke C, Allakkis W, Buffle E, Liu XX, Mohrez Y, Gloekler S, et al. Impact of conscious sedation and general anesthesia on periprocedural outcomes in Watchman left atrial appendage closure. Cardiol J. 2021;28(4):519–27.
Kislitsina ON, Smith D, Sherwani SS, Pham DT, Churyla A, Ricciardi MJ, et al. Comparison of monitored Anesthesia Care and General Anesthesia for Transcatheter aortic valve replacement. Innovations (Philadelphia Pa). 2019;14(5):436–44.
Kiramijyan S, Ben-Dor I, Koifman E, Didier R, Magalhaes MA, Escarcega RO, et al. Comparison of clinical outcomes with the utilization of monitored anesthesia care vs. general anesthesia in patients undergoing transcatheter aortic valve replacement. Cardiovasc Revascularization Medicine: Including Mol Interventions. 2016;17(6):384–90.
Jumaa MA, Zhang F, Ruiz-Ares G, Gelzinis T, Malik AM, Aleu A, et al. Comparison of safety and clinical and radiographic outcomes in endovascular acute stroke therapy for proximal middle cerebral artery occlusion with intubation and general anesthesia versus the nonintubated state. Stroke. 2010;41(6):1180–4.
John S, Thebo U, Gomes J, Saqqur M, Farag E, Xu J, et al. Intra-arterial therapy for acute ischemic stroke under general anesthesia versus monitored anesthesia care. Cerebrovasc Dis. 2014;38(4):262–7.
Jadhav AP, Bouslama M, Aghaebrahim A, Rebello LC, Starr MT, Haussen DC, et al. Monitored Anesthesia Care vs Intubation for Vertebrobasilar Stroke Endovascular Therapy. JAMA Neurol. 2017;74(6):704–9.
Althoff FC, Agnihotri A, Grabitz SD, Santer P, Nabel S, Tran T, et al. Outcomes after endoscopic retrograde cholangiopancreatography with general anaesthesia versus sedation. Br J Anaesth. 2021;126(1):191–200.
Hyman MC, Vemulapalli S, Szeto WY, Stebbins A, Patel PA, Matsouaka RA, et al. Conscious sedation Versus General Anesthesia for Transcatheter aortic valve replacement: insights from the National Cardiovascular Data Registry Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation. 2017;136(22):2132–40.
Husser O, Fujita B, Hengstenberg C, Frerker C, Beckmann A, Möllmann H, et al. Conscious sedation Versus General Anesthesia in Transcatheter aortic valve replacement: the German aortic Valve Registry. JACC Cardiovasc Interventions. 2018;11(6):567–78.
Herrmann HC, Cohen DJ, Hahn RT, Babaliaros VC, Yu X, Makkar R, et al. Utilization, costs, and outcomes of conscious Sedation Versus General Anesthesia for Transcatheter aortic valve replacement. Circulation Cardiovasc Interventions. 2021;14(7):e010310.
Haurand JM, Kavsur R, Ochs L, Tanaka T, Iliadis C, Sugiura A, et al. Deep sedation vs. general anesthesia for transcatheter tricuspid valve repair. Front Cardiovasc Med. 2022;9:976822.
Campbell D, Butler E, Campbell RB, Ho J, Barber PA. General Anesthesia compared with Non-GA in Endovascular Thrombectomy for ischemic stroke: a systematic review and Meta-analysis of Randomized controlled trials. Neurology. 2023;100(16):e1655–63.
Harjai KJ, Bules T, Berger A, Young B, Singh D, Carter R, et al. Efficiency, Safety, and Quality of Life after Transcatheter aortic valve implantation performed with moderate Sedation Versus General Anesthesia. Am J Cardiol. 2020;125(7):1088–95.
Babaliaros V, Devireddy C, Lerakis S, Leonardi R, Iturra SA, Mavromatis K, et al. Comparison of transfemoral transcatheter aortic valve replacement performed in the catheterization laboratory (minimalist approach) versus hybrid operating room (standard approach): outcomes and cost analysis. JACC Cardiovasc Interventions. 2014;7(8):898–904.
Griessenauer CJ, Shallwani H, Adeeb N, Gupta R, Rangel-Castilla L, Siddiqui AH, et al. Conscious sedation Versus General Anesthesia for the treatment of cerebral aneurysms with Flow Diversion: a matched cohort study. World Neurosurg. 2017;102:1–5.
Feil K, Herzberg M, Dorn F, Tiedt S, Küpper C, Thunstedt DC, et al. General Anesthesia versus Conscious Sedation in Mechanical Thrombectomy. J Stroke. 2021;23(1):103–12.
Du H, Tong X, Sun X, Shi Z, Liu B, Gao F, et al. Effect of anesthesia strategy during endovascular therapy on 90-day outcomes in acute basilar artery occlusion: a retrospective observational study. BMC Neurol. 2020;20(1):398.
D’Errigo P, Ranucci M, Covello RD, Biancari F, Rosato S, Barbanti M, et al. Outcome after General Anesthesia Versus monitored Anesthesia Care in Transfemoral Transcatheter aortic valve replacement. J Cardiothorac Vasc Anesth. 2016;30(5):1238–43.
Motloch LJ, Rottlaender D, Reda S, Larbig R, Bruns M, Müller-Ehmsen J, et al. Local versus general anesthesia for transfemoral aortic valve implantation. Clin Res Cardiology: Official J German Cardiac Soc. 2012;101(1):45–53.
Yamamoto M, Meguro K, Mouillet G, Bergoend E, Monin JL, Lim P, et al. Effect of local anesthetic management with conscious sedation in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2013;111(1):94–9.
Hoefnagel AL, Yao J, Rao D, Kovacs P, Brzezicki G, Mongan PD, Anesthesia. Blood pressure, and Socioeconomic Status in Endovascular Thrombectomy for Acute Stroke: a single Center Retrospective Case Cohort. J Neurosurg Anesthesiol. 2023;35(1):41–8.
Valente MF, Simões FJ, Mourão J. General anesthesia vs. sedation in transcatheter aortic valve implantation (TAVI): retrospective study of the incidence of acute kidney injury. Rev Esp Anestesiol Reanim (Engl Ed). 2021;68(3):121–7.
Maurice A, Eugène F, Ronzière T, Devys JM, Taylor G, Subileau A, et al. General Anesthesia versus Sedation, both with Hemodynamic Control, during Intraarterial Treatment for Stroke: the GASS Randomized Trial. Anesthesiology. 2022;136(4):567–76.
Ehret C, Rossaint R, Foldenauer AC, Stoppe C, Stevanovic A, Dohms K, et al. Is local anaesthesia a favourable approach for transcatheter aortic valve implantation? A systematic review and meta-analysis comparing local and general anaesthesia. BMJ open. 2017;7(9):e016321.
Kanda H, Takahashi Y, Sugawara A, Takahoko K, Shirasaka T, Saijo Y, et al. Comparing conscious Sedation with Regional Anesthesia Versus General Anesthesia in minimally invasive mitral valve surgery with right-sided minithoracotomy: a retrospective study. J Cardiothorac Vasc Anesth. 2022;36(2):452–60.
Holmes HR, Falasa M, Neal D, Choi CY, Park K, Bavry AA, et al. Monitored Anesthesia Care Versus General Anesthesia for Transcatheter aortic valve replacement. Innovations (Philadelphia Pa). 2022;17(5):401–8.
Liang F, Wu Y, Wang X, Yan L, Zhang S, Jian M, et al. General Anesthesia vs conscious sedation for endovascular treatment in patients with posterior circulation Acute ischemic stroke: an exploratory Randomized Clinical Trial. JAMA Neurol. 2023;80(1):64–72.
Sanders JA, Vaidyanathan A, Sayeed H, Sherdiwala B, Han X, Wyman J, et al. Comparison of Deep Sedation and General Anesthesia with an endotracheal tube for Transcaval Transcatheter aortic valve replacement: a pioneering Institution’s experience. J Cardiothorac Vasc Anesth. 2021;35(9):2607–12.
Monaco F, Barucco G, Licheri M, De Luca M, Labanca R, Rocchi M, et al. Association between Type of Anaesthesia and clinical outcome in patients undergoing endovascular repair of Thoraco-Abdominal aortic aneurysms by Fenestrated and branched endografts. Eur J Vascular Endovascular Surgery: Official J Eur Soc Vascular Surg. 2022;64(5):489–96.
Ouyang F, Chen Y, Zhao Y, Dang G, Liang J, Zeng J. Selection of patients and anesthetic types for Endovascular Treatment in Acute ischemic stroke: a Meta-analysis of Randomized controlled trials. PLoS ONE. 2016;11(3):e0151210.
Aitkenhead AR. Injuries associated with anaesthesia. A global perspective. Br J Anaesth. 2005;95(1):95–109.
Watterson LM, Morris RW, Westhorpe RN, Williamson JA. Crisis management during anaesthesia: bradycardia. Qual Saf Health Care. 2005;14(3):e9.
Cornelissen CG, Dapper J, Dreher M, Müller T. Endobronchial ultrasound-guided transbronchial needle aspiration under general anesthesia versus bronchoscopist-directed deep sedation: a retrospective analysis. Endoscopic Ultrasound. 2019;8(3):204–8.
Wong HM, Woo XL, Goh CH, Chee PHC, Adenan AH, Tan PCS, et al. Chronic Subdural Hematoma Drainage under Local Anesthesia with Sedation versus General Anesthesia and its outcome. World Neurosurg. 2022;157:e276–85.
Kesimci E, Erkiliç E, Gümüş T, Kanbak O. Impact of different anesthetic managements in outcomes of transcatheteraortic valve implantation: the first Turkish experience. Turk J Med Sci. 2016;46(3):742–8.
Feng L, Fu S, Yao Y, Yuan W, Zhao Y, Age. Prognostic Nutritional Index, and Charlson Comorbidity Index Were Independent Risk Factors for Postoperative Long-Term Mortality in Chinese geriatric patients who sustain hip fracture. J Am Med Dir Assoc. 2021;22(12):2602–3.
Sieber F, Neufeld KJ, Gottschalk A, Bigelow GE, Oh ES, Rosenberg PB, et al. Depth of sedation as an interventional target to reduce postoperative delirium: mortality and functional outcomes of the strategy to reduce the incidence of postoperative delirium in Elderly patients randomised clinical trial. Br J Anaesth. 2019;122(4):480–9.
Acknowledgements
Not applicable.
Funding
Organization: This research was funded by the Four “Batches” Innovation Project of Invigorating Medical through Science and Technology of Shanxi Province.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the study and critically revised the final manuscript. Xuesen Su, Wenjie Zhang, and Shouyuan Tian completed the search and determined eligible papers for inclusion. Xin Wang, Xin Yuan, and Shouyuan Tian completed the quality assessment. Xuesen Su, Xin Wang, and Shouyuan Tian completed the data extraction. Xuesen Su, Zixin Zhao, Yihe Tian, Wenjie Zhang, and Shouyuan Tian completed the statistical analyses and drafted the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethical approval is necessary for this systematic review. And all the studies included in this meta-analysis have obtained ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Su, X., Zhao, Z., Zhang, W. et al. Sedation versus general anesthesia on all-cause mortality in patients undergoing percutaneous procedures: a systematic review and meta-analysis. BMC Anesthesiol 24, 126 (2024). https://doi.org/10.1186/s12871-024-02505-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02505-w