Abstract
Background
Early extubation (EEx) is defined as the removal of the endotracheal tube within 8 h postoperatively. The present study involved determining the availability and threshold of the vasoactive-inotropic score (VIS) for predicting EEx in adults after elective rheumatic heart valve surgery.
Methods
The present study was designed as a single-center retrospective cohort study which was conducted with adults who underwent elective rheumatic heart valve surgery with CPB. The highest VIS in the immediate postoperative period was used in the present study. The primary outcome, the availability of VIS for EEx prediction and the optimal threshold value were determined using ROC curve analysis. The gray zone analysis of the VIS was performed by setting the false negative or positive rate R = 0.05, and the perioperative risk factors for prolonged EEx were identified by multivariate logistic analysis. The postoperative complications and outcomes were compared between different VIS groups.
Results
Among the 409 patients initially screened, 379 patients were ultimately included in the study. The incidence of EEx was determined to be 112/379 (29.6%). The VIS had a good predictive value for EEx (AUC = 0.864, 95% CI: [0.828, 0.900], P < 0.001). The optimal VIS threshold for EEx prediction was 16.5, with a sensitivity of 71.54% (65.85–76.61%) and a specificity of 88.39% (81.15–93.09%). The upper and lower limits of the gray zone for the VIS were determined as (12, 17.2). The multivariate logistic analysis identified age (OR, 1.060; 95% CI: 1.017–1.106; P = 0.006), EF% (OR, 0.798; 95% CI: 0.742–0.859; P < 0.001), GFR (OR, 0.933; 95% CI: 0.906–0.961; P < 0.001), multiple valves surgery (OR, 4.587; 95% CI: 1.398–15.056; P = 0.012), and VIS > 16.5 (OR, 12.331; 95% CI: 5.015–30.318; P < 0.001) as the independent risk factors for the prolongation of EEx. The VIS ≤ 16.5 group presented a greater success rate for EEx, a shorter invasive ventilation support duration, and a lower incidence of complications than did the VIS > 16.5 group, while the incidence of reintubation was similar between the two groups.
Conclusion
In adults, after elective rheumatic heart valve surgery, the highest VIS in the immediate postoperative period was a good predictive value for EEx, with a threshold of 16.5.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Rheumatic heart disease (RHD) affects more than 30 million people worldwide, causing approximately 300,000 deaths and 10 million disabilities each year [1]. In low-income and developing countries such as China, RHD is a major cause of morbidity and mortality [2, 3]. The development of strategies to improve the prognosis of patients with RHD is highly important.
Early extubation (EEx) was defined as the removal of the endotracheal tube within 8 h of admission to the intensive care unit (ICU) after the completion of surgery [4]. Previous studies suggest the close association of the EEx with the lower intensive care unit (ICU) and length of hospital stay (LOS), decreased hospitalization expenses, and reduced mortality in patients undergoing cardiac pulmonary bypass (CPB) surgery [5,6,7]. EEx reportedly does not increase the risk of reintubation after aortic valve replacement surgery [8]. These findings have led to the widespread use of EEx protocols among surgeons, anesthesiologists, and ICUs for patients undergoing coronary artery bypass grafting and valve surgery [9, 10].
Gaies et al. proposed the use of the vasoactive-inotropic score (VIS), which is a weighted sum of all administered inotropes and vasoconstrictors, including dopamine, dobutamine, epinephrine, norepinephrine, milrinone, vasopressin, and levosimendan, for accurate measurement of cardiovascular dysfunction and prediction of outcomes in infants after cardiopulmonary bypass [11]. Recent studies have reported that the VIS is correlated with mortality in patients with sepsis and cardiac surgery [12, 13]. Moreover, the highest VIS in the immediate postoperative period was revealed a predictive factor for poor outcomes [14]. However, to the best of our knowledge, no study thus far has used the highest VIS in the immediate postoperative period for predicting EEx in the ICU for adult patients with elective rheumatic heart valve bypass surgery.
In this context, the present retrospective cohort study aimed to evaluate whether the highest VIS in the immediate postoperative period is an independent predictor of EEx and to determine the diagnostic threshold value of the VIS among adult patients who underwent elective rheumatic heart valve bypass surgery.
Methods
Study procedure and patients
The present study was designed as a single-center, retrospective, cohort study conducted between January 2017 and December 2021. The study procedure was approved by the Medical Ethics Committee of the Affiliated Hospital of North Sichuan Medical College. The study is registered with the Chinese Registry of Clinical Trials (http://www.chictr.org.cn) (ChiCTR2300071659; 22/05/2023) and was conducted in accordance with the Declaration of Helsinki (2013). The requirement for obtaining written informed consent from each participant was exempted from the Ethics Committee due to the retrospective nature of the study, which ensured no exposure risks to the patients due to any additional intervention. The inclusion criteria for participation in the study were age > 18 years and a history of elective rheumatic heart valve surgery with CPB. The exclusion criteria included unplanned tracheal intubation prior to surgery, preoperative EF < 30%, persistent heart failure prior to surgery, preoperative plasma creatinine > 2.0 mg/dL, cardiac arrest prior to CPB, multiple aortic clampings, additional coronary artery bypass grafting (CABG) procedures, and redo surgery until discharge from the hospital. During this period, 409 consecutive adult patients who underwent elective rheumatic heart valve surgery were screened in the present study. Among these, 30 (7.3%) patients were excluded for the following reasons: tracheal intubation or tracheotomy prior to arrival in the operating room (n = 2), multiple aortic clamps during surgery (n = 5), redo surgery (n = 1), or incomplete data (n = 22). Finally, 379 patients were enrolled in the study for analysis. The flow chart of the study procedure is depicted in Fig. 1. The eligible patients were divided into two groups, namely, the success group for EEx (+) and the prolongation group for EEx (–) group, based on whether the duration to extubation exceeded 8 h or not. The postoperative complications and outcomes were compared between the different VIS groups by dividing the patients into two groups based on the optimal VIS threshold determined via ROC curve analysis.
Data collection
Patient demographics, intraoperative data, and postoperative variables were extracted from the medical records available at our hospital (details are shown in Table 1).
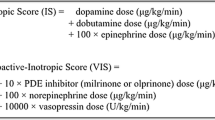
After the patients were weaned from CPB, vasoactive-inotropic agents were titrated to maintain the desired hemodynamic stability. After surgery, the VIS was measured every 5 min or more frequently, as indicated by the change in dose or the addition of drugs from the medical records. The highest VIS in the immediate postoperative period was selected for analysis in the present study. VIS was calculated as described in a previous report [15], using the following formula:
VIS = dopamine (µg/kg/min) + dobutamine (µg/kg/min) + 100 × epinephrine (µg/kg/min) + 100 × norepineprine (µg/kg/min) + 10 × milrinone (µg/kg/min) + 10,000 × vasopressin (units/kg/min) + 50 × levosimendan (µg/kg/min).
Extubation plan
After rheumatic heart valve surgery, patients were sent to the cardiac ICU, where they were placed on continuous invasive ventilation. Patients were managed according to the enhanced recovery protocol, which included lower-dose opioids, limited perioperative fluids, and accelerated physical rehabilitation on the morning after the operation [16]. The patient was considered ready for extubation immediately upon fulfilling the extubation criteria. The following extubation criteria were used, according to previous studies [17, 18]:
(1) Hemodynamic stability with reasonable urination and warm peripheral extremities;
(2) PaO2 ≥ 80 mmHg and PaCO2 ≤ 50 mmHg with adequate spontaneous respiration, FiO2 ≤ 0.4, and end-expiratory pressure ≤ 5 cm H2O;
(3) Awake and able to respond to commands, with no new neurological symptoms observed;
(4) No active bleeding, with stable hemoglobin levels and no requirement for volume replacement;
(5) Patients had no reasonable fear of reintubation.
Outcome measures
The primary outcome of the present study was the availability and threshold of the VIS for predicting EEx. The gray zone analysis of the VIS was performed, and the perioperative risk factors for prolonged EEx were identified by multivariate logistic analysis. Postoperative complications and outcomes, including pneumonia, cardiac dysfunction, IABP, cardiopulmonary resuscitation (CPR), acute kidney injury (AKI), death at the hospital, reintubation, ICU stay, and length of stay (LOS) after surgery, were compared between the different VIS groups.
Statistics
A 44.3% incidence of EEx was assumed based on a previous study [19]. In addition, a 5% acceptable margin of error and a 95% confidence interval (CI) were used. The appropriate sample size for the study was calculated to be [1.962*0.443*(1-0.443)]/0.052= 379. Categorical variables are expressed as the frequency (n) and the corresponding percentage (%) and were analyzed using the two-sided Fisher exact test or chi-squared test, as appropriate. Continuous variables are expressed as the mean ± standard deviation (SD) and were evaluated using the two-sample Student’s t-test. The availability of the VIS to predict EEx and the optimal threshold value were determined using receiver operating characteristic (ROC) curve analysis.
Due to the wide range of VIS, it is quite difficult for some patients to predict perfectly EEx (+) or EEx (–). To address this problem, it is necessary to propose a three-zone partition (negative, positive, and the gray zone between them), which was constructed from the cutoff value of the VIS [20]. In the gray zone, VIS cannot be precisely predicted as EEx (+) or EEx (–). The upper or lower limit of the gray zone was determined by setting the false negative or positive rate R = 0.05.
The risk factors with P < 0.05 revealed in the univariate logistic analysis were subjected to multivariate logistic analysis to identify the risk factors for prolonged EEx. Statistical analysis was conducted using SPSS 25.0 software (Statistical Program for Social Sciences, SPSS, Inc., Chicago, Illinois, USA), considering P < 0.05 as the threshold of statistical significance.
Results
Patient characteristics
As presented in Table 1, patients in the EEx (+) group were younger than those in the EEx (–) group (50.4 ± 11.1 vs. 58.6 ± 9.8 years, P < 0.001). With respect to preoperative factors, EEx (+) patients were more likely to have a lower ASA classification, greater EF%, and higher GFR compared to the EEx (–) patients.
Considering the intraoperative factors, in the EEx (+) group, compared to those in the EEx (–) group patients, the proportion of moderate-severe stenosis or regurgitation of the mitral or aortic valve were lower, and those were less likely to undergo isolated mitral valve replacement and multiple valve surgery. No differences were observed between the two groups with regard to the proportion of patients who underwent isolated mitral valve repair or isolated aortic replacement surgery. Among the additional procedures, tricuspid valve repair, MAZE, and pacemaker implantation were performed less frequently in the EEx (+) group compared to the EEx (–) group. Moreover, other factors, such as ultrafiltration, ACC duration, CPB duration, highest Lac level, and hemorrhage, were lower in the EEx (+) group. The VIS was much lower in the EEx (+) group compared to the EEx (–) group (13.9 ± 2.2 vs. 20.3 ± 8.0, P < 0.001).
Determining the optimal VIS threshold for predicting EEx
ROC curve analysis of the VIS for EEx prediction was performed for all patients. As depicted in Fig. 2, the VIS had a good predictive value for EEx (AUC = 0.864, 95% CI: [0.828, 0.900], P < 0.001). The optimal VIS threshold for EEx prediction was determined to be 16.5, with a sensitivity of 71.54% (65.85–76.61%) and a specificity of 88.39% (81.15–93.09%). To explore the effect of different valvular pathologies on the prediction value of EEx, we further performed subgroup analyses according to isolated mitral valve surgery, isolated aortic valve surgery, and multiple valve surgery. Similarly, the VIS had good predictive values for EEx among all subgroups (isolated mitral valve surgery: AUC = 0.8808, 95% CI: [0.8366, 0.9250], P < 0.001, cutoff = 16.5; isolated aortic valve surgery: AUC = 0.7631, 95% CI: [0.6184, 0.9078], P = 0.0012, cutoff = 17.5; multiple valve surgery: AUC = 0.8689, 95%CI: [0.7878, 0.9501], P = 0.001, cutoff = 15.5) (Shown in supplementary material1-3). Next, the patients were classified into two groups based on the optimal VIS threshold: the VIS ≤ 16.5 group (n = 175) and the VIS >16.5 group (n = 204).
Receiver operating characteristic (ROC) curve analysis of the ability of the VIS to predict EEx.
Note: The AUC for the prediction of EEx was 0.864, 95% CI: [0.828, 0.900], P < 0.001. The optimal VIS threshold for EEx prediction was 16.5, with a sensitivity of 71.54% (65.85–76.61%) and a specificity of 88.39% (81.15–93.09%)
Analysis of the upper and lower limits of the gray zone for VIS
The sensitivity, specificity, false positive rate, and false negative rate of the VIS values near the upper and lower limits of the gray zone are shown in Table 2. The upper limit of the gray zone is clearly between 11.5 (P1) and 12.5 (P2) because the false negative rate 0.05 (R) is located between 0.0449 (R1) and 0.0562 (R2); correspondingly, the upper limit (P) of the gray zone is calculated to be 12 based on the linear interpolation method: (R1-R2)/(R1-R)=(P1-P2)/(P1-P). Similarly, the lower limit of the gray zone is between 16.5 and 17.5 because the false positive rate 0.05(R) is located between 0.1161 and 0.0268. The lower limit of the gray zone was determined to be 17.2. The gray zone is depicted in Fig. 3, and 187 (49.3%) patients were located in the gray zone.
Perioperative risk factors associated with prolonged EEx
To determine the perioperative risk factors associated with prolonged EEx, a univariate logistic analysis was performed, which revealed age, ASA classification, EF%, GFR, mitral valve stenosis classification, mitral valve regurgitation classification, isolated mitral valve replacement, multiple valve surgery, tricuspid valve repair, MAZE, pacemaker implantation, ACC duration > 90 min, CPB duration > 142 min, highest lactate level, hemorrhage > 450 mL, and VIS > 16.5 as the significant variables. These identified significant variables were subsequently subjected to multivariate logistic analysis, which revealed age (OR, 1.060; 95% CI: 1.017–1.106;P = 0.006), EF% (OR, 0.798; 95% CI: 0.742–0.859; P < 0.001), GFR (OR, 0.933; 95% CI: 0.906–0.961; P < 0.001), multiple valves surgery (OR, 4.587; 95% CI: 1.398–15.056; P = 0.012), and VIS > 16.5 (OR, 12.331; 95% CI: 5.015–30.318; P < 0.001) as the independent risk factors for the prolongation of EEx (Table 3).
Comparisons of postoperative complications and outcomes between the different VIS groups
The complications and other outcomes after surgery were compared between the different VIS groups. The patients were divided into two groups based on the optimal VIS threshold: the VIS ≤ 16.5 group (n = 175) and the VIS > 16.5 group (n = 204). As presented in Table 4, the percentage of successful EEx was significantly greater in the VIS ≤ 16.5 group than in the VIS > 16.5 group (88.4% vs. 11.6%, P < 0.001). Moreover, the duration of extubation was shorter in the VIS ≤ 16.5 group than in the VIS > 16.5 group (8.8 ± 2.5 h vs. 19.5 ± 23.3 h, P < 0.001). With regard to complications, the VIS ≤ 16.5 group presented lower incidences of pneumonia, cardiac dysfunction, IABP, CPR, and AKI; death at the hospital; prolonged ICU stay; and longer LOS after surgery than did the VIS > 16.5 group. On the other hand, the incidence of reintubation was similar between the two groups.
Discussion
EEx following cardiac surgery was demonstrated to improve outcomes, including pneumonia incidence, ICU stay, LOS, healthcare costs, morbidity, and mortality [16]. Currently, EEx is widely considered the key objective of enhanced recovery in patients who underwent heart valve surgery with CPB [21]. Hiromoto et al. reported that the incidence of EEx was 44.3% for valve surgery patients receiving CPB [19]. In the aforementioned study, the percentages of patients who underwent multiple valve surgeries (3.2% vs. 8.9%), MAZE (9.7% vs. 25.0%), and tricuspid valve repair (22.6% vs. 65.2%) in the EEx (+) group were significantly lower than those in the present study; these differences could have led to the prolongation of ACC and CPB durations, thereby increasing the risk of lung damage by initiating a systemic inflammatory response, hemodilution, intrapulmonary shunt, ischemic damage, ischemia-reperfusion, and atelectasis [22, 23], resulting in the prolongation of mechanical ventilation support. These findings might explain why the incidence of EEx in the present study (29.6%) was lower than that reported by Hiromoto et al [19].
To the best of our knowledge, the present study is the first to explore the availability and threshold of the VIS and its cutoff for the prediction of EEx in adults who underwent elective rheumatic heart valve surgery. The results of the present study indicated that a VIS > 16.5 in the immediate postoperative period was a good indicator of sensitivity and specificity, with an AUC = 0.864, for the independent prediction of EEx in the ICU. This finding is similar to that of Haque et al., who demonstrated that a VIS > 20 was an independent predictive factor for mortality in children with septic shock [24]. However, the gray zone of the VIS was determined to be (12, 17.2), and 49.3% of the patients were located in the gray zone, which indicates that the predicted VIS for EEx was relatively limited. Therefore, VIS located in the gray zone for predicting EEx should be treated conservatively, while perioperative factors, including age, EF%, GFR, and multiple valve surgeries, should be taken into consideration comprehensively to predict EEx.
As vasoplegic syndrome (VS) is frequently observed as a common complication after weaning from CPB, the occurrence of VS varies from 5 to 25% in low-risk cardiac patients; however, in high-risk cardiac patients, vasopressor administration before CPB; prolonged duration of CPB; preoperative low EF; and preoperative administration of beta-blockers or angiotensin-converting enzyme inhibitors, the incidence of VS can increase to a range of 30–50% [25,26,27]. VS represents a type of distributive shock that is characterized by low systemic vascular resistance and normal-to-high cardiac output, resulting in severe hypotension and increased consumption of vasopressors to maintain adequate systemic perfusion. In addition, in the early postoperative period after being separated from CPB, patients require vasoactive-inotropic agents to support cardiac function, as a high VIS usually represents poor cardiovascular function [14]. Therefore, the VIS directly reflects overall heart dysfunction and low systemic vascular resistance after weaning from CPB in the present study. Due to cardiovascular dysfunction, blood may accumulate in the lungs, facilitating intrapulmonary shunts and impairing gas exchange and pulmonary ventilation function. Moreover, decreased cardiac output may limit perfusion to the coronary, systemic, and microcirculatory systems, resulting in acidosis and inadequate perfusion in multiple organs, including the heart, forming a vicious cycle. Collectively, these factors could explain why a high VIS could be an independent predictor of prolonged EEx. Likewise, patients with a high VIS in the immediate postoperative period should also be allowed to extubate timely in the ICU if their respiratory status is good, the hemodynamics are stable, and meet the extubation criteria [28]. However, caution should be taken when considering that after extubation, persistent high VIS in the ICU may increase the risk of reintubation [29].
The multivariable logistic analysis conducted in the present study indicated that age, EF%, GFR, multiple valve surgery, and VIS > 16.5 were the independent risk factors for EEx failure. It is generally recognized that elderly patients with a decreased ‘physiological reserve’ in multiple organs after valve bypass surgery find it difficult to overcome the perioperative period [30]. In addition, the preoperative GFR was revealed to be an independent predictor of EEx in cardiac surgery patients [31, 32]. The reason for this could be that poor renal function facilitates the release of inflammatory cytokines and impairs endothelial function, leading to an increase in the postoperative burden of vasoactive-inotropic agents and the prolongation of EEx [33, 34]. According to previous studies, age, low EF, and complex procedures were revealed to be correlated with the prolongation of invasive mechanical ventilation support after cardiac bypass surgery [35, 36]. Multiple valve surgery prolonged the duration of CPB, which could have led to increased capillary permeability, resulting in pulmonary edema and prolonged mechanical ventilation, even indirectly affecting the postoperative ICU stay and mortality, which is consistent with the findings of previous reports [37,38,39].
Ample research has indicated that high VIS was closely associated with poor outcomes, including extended ICU and LOS stay, prolonged mechanical ventilation, requirement for renal replacement therapy, and mortality [11, 14]. Consistently, in the present study, the VIS ≤ 16.5 group presented a greater success rate of EEx; shorter invasive ventilation support duration; and lower incidences of complications, including pneumonia, cardiac dysfunction, IABP, CPR, AKI, death at the hospital, prolonged ICU stay, and extended LOS after surgery, than did the VIS > 16.5 group. However, the incidence of reintubation was similar between the two groups. These results indicated that a VIS ≤ 16.5 was associated with better postoperative outcomes without an increase in the incidence of reintubation.
Like with all related research, the present study has certain limitations. First, the study was retrospective and was therefore susceptible to selection bias and confounding factors. In addition, although overall adherence to optimal vasopressor strategies was ensured to achieve optimal hemodynamics, the selection of vasopressor and inotropic agents, fluid resuscitation strategies, etc., was based on the decisions of different anesthesiologists and ICU doctors, which could impact the VIS and clinical outcomes. Third, the preoperative pulmonary function test (PFT) was not performed in the present study. Preoperative PFT may be important because it could be a confounding factor of EEx; however, its value in predicting the need for mechanical ventilation is controversial [40,41,42]. Many hospitals, including ours, do not routinely perform PFT before cardiac surgery, especially for patients without a history of pulmonary disease. However, we included the preoperative resting PaO2, PaCO2, and COPD diagnosis to assess preoperative lung function. Finally, the study was conducted in a single center at a tertiary care hospital, which limits the scope of generalizing the obtained results.
Conclusion
In adults, after elective rheumatic heart valve surgery, the highest VIS in the immediate postoperative period is a good predictive value for EEx, with a threshold of 16.5.
Data availability
The data used and/or analyzed during the current study are also available from the corresponding author upon reasonable request.
References
Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015. N Engl J Med. 2017;377(8):713–22.
Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet. 2012;379(9819):953–64.
Coffey S, Cairns BJ, Iung B. The modern epidemiology of heart valve disease. Heart. 2016;102(1):75–85.
Zhu F, Lee A, Chee YE. Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database Syst Rev. 2012;10:CD003587.
Guller U, Anstrom KJ, Holman WL, Allman RM, Sansom M, Peterson ED. Outcomes of early extubation after bypass surgery in the elderly. Ann Thorac Surg. 2004;77(3):781–8.
Cheng DC, Karski J, Peniston C, Raveendran G, Asokumar B, Carroll J, David T, Sandler A. Early tracheal extubation after coronary artery bypass graft surgery reduces costs and improves resource use. A prospective, randomized, controlled trial. Anesthesiology. 1996;85(6):1300–10.
van Mastrigt GAPG, Maessen JG, Heijmans J, Severens JL, Prins MH. Does fast-track treatment lead to a decrease of intensive care unit and hospital length of stay in coronary artery bypass patients? A meta-regression of randomized clinical trials. Crit Care Med. 2006;34(6):1624–34.
Brovman EY, Tolis G, Hirji S, Axtell A, Fields K, Muehlschlegel JD, Urman RD, Deseda GAC, Kaneko T, Karamnov S. Association between Early Extubation and Postoperative Reintubation after Elective Cardiac surgery: a bi-institutional study. J Cardiothorac Vasc Anesth. 2022;36(5):1258–64.
Fitch ZW, Debesa O, Ohkuma R, Duquaine D, Steppan J, Schneider EB, Whitman GJR. A protocol-driven approach to early extubation after heart surgery. J Thorac Cardiovasc Surg. 2014;147(4):1344–50.
Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM, et al. 2011 ACCF/AHA Guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice guidelines. Circulation. 2011;124(23):e652–735.
Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, Charpie JR, Hirsch JC. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234–8.
Song J, Cho H, Park DW, Moon S, Kim JY, Ahn S, Lee S-G, Park J. Vasoactive-inotropic score as an early predictor of mortality in adult patients with Sepsis. J Clin Med 2021, 10(3).
Demirhan S, Topcuoglu S, Karadag N, Ozalkaya E, Karatekin G. Vasoactive inotropic score as a predictor of mortality in neonatal septic shock. J Trop Pediatr 2022, 68(6).
Koponen T, Karttunen J, Musialowicz T, Pietiläinen L, Uusaro A, Lahtinen P. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br J Anaesth. 2019;122(4):428–36.
Koponen T, Karttunen J, Musialowicz T, Pietilainen L, Uusaro A, Lahtinen P. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br J Anaesth. 2019;122(4):428–36.
McCarthy C, Fletcher N. Early extubation in enhanced recovery from cardiac surgery. Crit Care Clin. 2020;36(4):663–74.
London MJ, Shroyer AL, Coll JR, MaWhinney S, Fullerton DA, Hammermeister KE, Grover FL. Early extubation following cardiac surgery in a veterans population. Anesthesiology. 1998;88(6):1447–58.
Walthall H, Robson D, Ray S. Do any preoperative variables affect extubation time after coronary artery bypass graft surgery? Heart Lung. 2001;30(3):216–24.
Hiromoto A, Maeda M, Murata T, Shirakawa M, Okamoto J, Maruyama Y, Imura H. Early extubation in current valve surgery requiring long cardiopulmonary bypass: benefits and predictive value of preoperative spirometry. Heart Lung. 2020;49(6):709–15.
Coste J, Pouchot J. A grey zone for quantitative diagnostic and screening tests. Int J Epidemiol. 2003;32(2):304–13.
García-Delgado M, Navarrete-Sánchez I, Colmenero M. Preventing and managing perioperative pulmonary complications following cardiac surgery. Curr Opin Anaesthesiol. 2014;27(2):146–52.
Imura H, Duncan HP, Corfield AP, Myerscough N, Caputo M, Angelini GD, Wolf AR, Henderson AJ. Increased airway mucins after cardiopulmonary bypass associated with postoperative respiratory complications in children. J Thorac Cardiovasc Surg. 2004;127(4):963–9.
Imura H, Caputo M, Lim K, Ochi M, Suleiman MS, Shimizu K, Angelini GD. Pulmonary injury after cardiopulmonary bypass: beneficial effects of low-frequency mechanical ventilation. J Thorac Cardiovasc Surg. 2009;137(6):1530–7.
Haque A, Siddiqui NR, Munir O, Saleem S, Mian A. Association between vasoactive-inotropic score and mortality in pediatric septic shock. Indian Pediatr. 2015;52(4):311–3.
Levin MA, Lin H-M, Castillo JG, Adams DH, Reich DL, Fischer GW. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation. 2009;120(17):1664–71.
Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345(8):588–95.
Argenziano M, Chen JM, Choudhri AF, Cullinane S, Garfein E, Weinberg AD, Smith CR, Rose EA, Landry DW, Oz MC. Management of vasodilatory shock after cardiac surgery: identification of predisposing factors and use of a novel pressor agent. J Thorac Cardiovasc Surg. 1998;116(6):973–80.
Evans A, McCurdy MT, Weiner M, Zaku B, Chow JH. Use of Angiotensin II for Post Cardiopulmonary Bypass Vasoplegic Syndrome. Ann Thorac Surg. 2019;108(1):e5–7.
Miyata K, Shigematsu S. [Vasoplegic Syndrome after aortic valve replacement]. Masui. 2016;65(1):90–2.
Dell’Amore A, Aquino TM, Pagliaro M, Lamarra M, Zussa C. Aortic valve replacement with and without combined coronary bypass grafts in very elderly patients: early and long-term results. Eur J Cardiothorac Surg. 2012;41(3):491–8.
Nguyen Q, Coghlan K, Hong Y, Nagendran J, MacArthur R, Lam W. Factors Associated with Early Extubation after Cardiac surgery: a retrospective single-center experience. J Cardiothorac Vasc Anesth. 2021;35(7):1964–70.
Youssefi P, Timbrell D, Valencia O, Gregory P, Vlachou C, Jahangiri M, Edsell M. Predictors of failure in fast-track cardiac surgery. J Cardiothorac Vasc Anesth. 2015;29(6):1466–71.
Lebherz-Eichinger D, Klaus DA, Reiter T, Hörl WH, Haas M, Ankersmit HJ, Krenn CG, Roth GA. Increased chemokine excretion in patients suffering from chronic kidney disease. Transl Res. 2014;164(6):433–e443431.
Esteve E, Castro A, López-Bermejo A, Vendrell J, Ricart W, Fernández-Real JM. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care. 2007;30(4):939–45.
Siddiqui MM, Paras I, Jalal A. Risk factors of prolonged mechanical ventilation following open heart surgery: what has changed over the last decade? Cardiovasc Diagn Ther. 2012;2(3):192–9.
Légaré JF, Hirsch GM, Buth KJ, MacDougall C, Sullivan JA. Preoperative prediction of prolonged mechanical ventilation following coronary artery bypass grafting. Eur J Cardiothorac Surg. 2001;20(5):930–6.
Madhavan S, Chan SP, Tan WC, Eng J, Li B, Luo HD, Teoh LK. Cardiopulmonary bypass time: every minute counts. J Cardiovasc Surg (Torino). 2018;59(2):274–81.
Cislaghi F, Condemi AM, Corona A. Predictors of prolonged mechanical ventilation in a cohort of 3,269 CABG patients. Minerva Anestesiol. 2007;73(12):615–21.
Apostolakis E, Filos KS, Koletsis E, Dougenis D. Lung dysfunction following cardiopulmonary bypass. J Card Surg. 2010;25(1):47–55.
Saleh HZ, Mohan K, Shaw M, Al-Rawi O, Elsayed H, Walshaw M, Chalmers JAC, Fabri BM. Impact of chronic obstructive pulmonary disease severity on surgical outcomes in patients undergoing non-emergent coronary artery bypass grafting. Eur J Cardio-thoracic Surgery: Official J Eur Association Cardio-thoracic Surg 2012, 42(1).
Risom EC, Buggeskov KB, Mogensen UB, Sundskard M, Mortensen J, Ravn HB. Preoperative pulmonary function in all comers for cardiac surgery predicts mortality†. Interact Cardiovasc Thorac Surg. 2019;29(2):244–51.
Ivanov A, Yossef J, Tailon J, Worku BM, Gulkarov I, Tortolani AJ, Sacchi TJ, Briggs WM, Brener SJ, Weingarten JA, et al. Do pulmonary function tests improve risk stratification before cardiothoracic surgery? J Thorac Cardiovasc Surg. 2016;151(4):1183–e11891183.
Acknowledgements
We thank the patient’s medical records involved in this study.
Funding
This study was supported by the Project of North Sichuan Medical College (NO. CBY21-QA42, belongs to Yang Zhao) and the Innovation Project of Guangxi Graduate Education (NO. YCBZ2022091, belongs to Yang Zhao).
Author information
Authors and Affiliations
Contributions
Yang Zhao: Conceptualization, Formal analysis, Funding acquisition, Writing an original draft, Software, Validation. Hanlei Zhao: Project administration, Data curation, Software. Jiao Huang: Methodology, Software, Formal Analysis, Supervision. Bo Mei: Methodology, Investigation, Software. Jun Xiang: Methodology, Software. Yizheng Wang: Data curation, Methodology, Software. Jingyan Lin: Software, Validation, Editing. San Huang: Conceptualization, Project administration, Methodology, Writing–review & editing, Visualization, Validation. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate.
The present study was designed as a single-center, retrospective, cohort study conducted for the period between January 2017 and December 2021. The study procedure was approved by the Medical Ethics Committee of the Affiliated Hospital of North Sichuan Medical College. The study is registered with the Chinese Registry of Clinical Trials (http://www.chictr.org.cn) (ChiCTR2300071659; 22/05/2023) and conducted in accordance with the Declaration of Helsinki (2013). The requirement for obtaining written informed consent from each participant was exempted from the Medical Ethics Committee of the Affiliated Hospital of North Sichuan Medical College due to the retrospective nature of the study that ensured no exposure risks to the patients due to any additional intervention.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.



Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, Y., Zhao, H., Huang, J. et al. Availability and threshold of the vasoactive-inotropic score for predicting early extubation in adults after rheumatic heart valve surgery: a single-center retrospective cohort study. BMC Anesthesiol 24, 102 (2024). https://doi.org/10.1186/s12871-024-02489-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02489-7