Abstract
Background
Propofol is use widely used in anesthesia, known for its effectiveness, may lead to cardiopulmonary issues in some patients. Ciprofol has emerged as a possible alternative to propofol because it can achieve comparable effects to propofol while causing fewer adverse events at lower doses. However, no definitive conclusion has been reached yet. This meta-analysis aimed to evaluate the efficacy and safety of ciprofol versus propofol in adult patients undergoing elective surgeries under general anesthesia.
Methods
We searched PubMed, EMBASE, the Cochrane library, Web of Science, and Chinese National Knowledge Infrastructure (CNKI) to identify potentially eligible randomized controlled trials (RCT) comparing ciprofol with propofol in general anesthesia until September 30, 2023. The efficacy outcomes encompassed induction success rate, time to onset of successful induction, time to disappearance of eyelash reflex, and overall estimate means in Bispectral Index (BIS). Safety outcomes were assessed through time to full alertness, incidence of hypotension, incidence of arrhythmia, and incidence of injection-site pain. Continuous variables were expressed as mean difference (MD) with 95% confidence interval (CI), and dichotomous variables were expressed as risk ratio (RR) with 95% CI. Statistical analyses were performed using RevMan 5.4 and STATA 14.0. The quality of the evidence was rated through the grading of recommendations, assessment, development and evaluation (GRADE) system.
Results
A total of 712 patients from 6 RCTs were analyzed. Meta-analysis suggested that ciprofol was equivalent to propofol in terms of successful induction rate, time to onset of successful induction, time to disappearance of eyelash reflex, time to full alertness, and incidence of arrhythmia, while ciprofol was better than propofol in overall estimated mean in BIS (MD: -3.79, 95% CI: -4.57 to -3.01, p < 0.001), incidence of hypotension (RR: 0.63, 95% CI: 0.42 to 0.94, p = 0.02), and incidence of injection-site pain (RR: 0.26, 95% CI: 0.14 to 0.47, p < 0.001). All results were supported by moderate to high evidence.
Conclusions
Ciprofol may be a promising alternative to propofol because it facilitates achieving a satisfactory anesthesia depth and results in fewer hypotension and injection-site pain. However, we still recommend conducting more studies with large-scale studies to validate our findings because only limited data were accumulated in this study.
Trial registration
PROSPERO 2023 CRD42023479767.
Similar content being viewed by others
Background
Propofol is a widely used anesthetic drug, with common applications including conscious sedation [1, 2] and general anesthesia [3, 4]. The efficacy and safety of the use of propofol for conscious sedation and general anesthesia have been supported by solid evidence [5,6,7]; however, studies have shown that propofol can also produce various side effects, such as injection-site pain [8], propofol-related infusion syndrome [9] and an increased risk of infection [10]. As a result, it is imperative to develop a novel anesthetic drug that is as effective as propofol but has fewer side effects [11].
Ciprofol (HSK3486) is a newly developed highly selective γ-aminobutyric acid (GABA) receptor agonist [12], which has become a new type of intravenous sedative anesthetic drug with desirable properties, such as rapid onset of action, fast recovery, minimal pain on injection, and stable cardiopulmonary function [13,14,15]. Clinical studies have shown that 0.4 to 0.5 mg/kg ciprofol is equivalent to 2.0 mg/kg propofol in sedative and anesthetic profile during colonoscopy [16, 17]. All these advantages make ciprofol a promising alternative to propofol in conscious sedation and general anesthesia [15, 17].
Up to date, several randomized controlled trials (RCTs) [18,19,20,21,22,23] have investigated the efficacy and safety of ciprofol compared with propofol in patients undergoing elective surgeries under general anesthesia, but reported conflicting results. Some studies have shown no statistical difference between ciprofol and propofol in the time to onset of successful induction [18, 19, 22, 23] and in the incidence of hypotension [19,20,21, 23], whereas other studies reported conflicting results in terms of the time to onset of successful induction [20, 21] and incidence of hypotension [18, 22]. Furthermore, it is important to note that all these studies included only limited sample size, thus inevitably increasing the risk of producing misleading outcomes.
Therefore, the purpose of this meta-analysis was to systematically evaluate the comparative anesthetic efficacy and safety of ciprofol versus propofol in patients undergoing elective surgeries under general anesthesia, with a view to providing evidence for informing the selection of the optimal anesthetic drug.
Methods
We strictly followed the Cochrane handbook to conduct this meta-analysis [24]. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 statement was cited as the guidance for reporting this meta-analysis [25]. Institutional review approval and informed consent were not required because we collected data directly from previously published studies.
Selection criteria
Studies were eligible if (a) adult patients underwent non-emergency, non-cardiothoracic, and non-neurological elective surgeries under general anesthesia, with American Society of Anesthesiologists (ASA) status I/II; (b) anesthesia induction in the study group was performed using ciprofol (0.4–0.5 mg/kg); (c) anesthesia induction in the control group was performed using propofol (2.0 mg/kg); (d) they reported at least one of the following outcomes, including induction success rate, onset of successful induction, time to disappearance of eyelash reflex, time to fully alertness, overall estimated mean in bispectral index (BIS), and incidence of hypotension, arrhythmia and injection-site pain; and (e) only randomized controlled trials (RCTs) were considered to meet inclusion criteria.
Studies were excluded if they (a) used ineligible study designs, such as animal study, single-arm trial, case report, and review; (b) conference abstract without essential data for statistical analysis; (c) evaluated the synthetic effect of ciprofol combined with propofol rather than effect of individual anesthetic drug; (d) repeated report of the same population.
Search strategy
A systematic search was conducted in PubMed, EMBASE, the Cochrane library, Web of Science and Chinese National Knowledge Infrastructure (CNKI) to retrieve potentially eligible studies that compared ciprofol with propofol in adult patients underwent elective surgery under general anesthesia. The latest date to update search was September 30, 2023. We used “ciprofol,” “propofol,” and “random”, as well as their analogs as search terms, and the strategy of combining full text and medical subject heading (MeSH) was adopted as the principle for constructing search strategy. Supplementary Table 1 summarized the detailed search strategies of all target databases. Additional studies were also retrieved using manual search of the reference lists of eligible studies and reviews that investigated the same topic.
Selection processes
Two authors (Wanwei Jiang and Dilireba Ainiwaer) independently performed study selection following three steps. First, we removed duplicate studies using EndNote software. Second, we excluded irrelevant studies based on title and abstract screening. Third, we identified studies that met our selection criteria based on full-text screening. Consensus was employed to resolve disagreements between the two authors.
Data collection
Data were collected independently by two authors using a pre-designed standard data extraction form based on MS Excel 2022 (Microsoft Corporation, the USA). Specifically, we collected the following data from all eligible studies: study characteristics (the first author’s name, country, year of publication, surgical procedure, protocol of administration of ciprofol and propofol, general anesthesia protocol, and muscle relaxant), patient characteristics (sample size, the number of female patients, average age, body mass index [BMI], ASA status, and operative duration), outcomes data, and information for methodological quality.
Outcome definition
Induction success rate was defined as the percentage of successful induction cases in each group, with successful induction defined as not requiring any alternative sedative or anesthetic drug or requiring > 2 top-up study drug doses after the start of study drug administration. The time to onset of successful induction refers to the time from the initiation of study drug treatment until the patient achieved a Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score of ≤ 1. The time to disappearance of eyelash reflex was defined as the time before the eyelash reflex is completely lost. The overall estimated mean in BIS was the difference between the two groups in the overall estimated value in BIS after achieving sedation. The time to full alertness refers to the time from drug withdrawal to extubation (MOAA/S of 5 for three consecutive assessments). The definition of hypotension was left to each study [26]. Arrhythmia was the composite of bradycardia and tachycardia in this meta-analysis. Bradycardia and tachycardia refer to a heart rate < 50 beats/min and heart rate > 100 beats/min with a duration of > 30s, respectively. Injection site pain as detected by a withdrawal response or a numeric rating scale value of ≥ 3.
Risk of bias assessment
The risk of bias for each eligible study was independently assessed by two authors (Wanwei Jiang and Dilireba Ainiwaer) using the revised Cochrane risk of bias (RoB) tool [27]. The tool was designed with 5 domains including randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Each domain would be rated as ‘low risk,’ ‘some concerns,’ or ‘high risk’ based on the actual information provided by each study. The overall risk of an individual study was rated as low if 5 domains were marked as low risk, and if one or more domains were rated as high risk, the overall risk was rated as high. In addition, the overall risk of an individual study was rated as having some concerns if there was one or more domains of some concerns but no domain of high risk.
Statistical analysis
The estimates for continuous variables were summarized with mean difference (MD) with 95% confidence interval (CI), and estimates for dichotomous variables were expressed as risk ratio (RR) with 95% CI [24]. We assessed statistical heterogeneity between studies by using the Cochrane Q statistic and I2 statistic [28]. Statistically significant heterogeneity was considered if p < 0.1 and I2 ≥ 50%, and the random-effects model was used for meta-analysis [29]. In contrast, statistical heterogeneity was considered as low if p ≥ 0.1 and I2 < 50%, and the fixed-effects model was selected for meta-analysis. We also employed the leave-one-out method to conduct sensitivity analysis. Although the number of included studies was less than ten [30], we still used both funnel plot and Egger’s test to assess publication bias. Review Manager (RevMan) version 5.4 (the Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, Denmark) and STATA 14.0 (StataCorp LP, College Station, USA) [31] were used for all statistical analyses.
The quality of evidence
We used the grading of recommendations, assessment, development and evaluation (GRADE) system to assess the quality of the evidence [32]. Using the GRADE method, the level of evidence for each outcome would be rated as ‘high,’ ‘moderate,’ ‘low,’ or ‘very low’. According to the GRADE method, the initial level of evidence for RCT is the highest level; however, the level of evidence would be downgraded based on limitations in the 5 aspects: risk of bias, consistency, indirectness, imprecision, and publication bias.
Results
Study retrieval
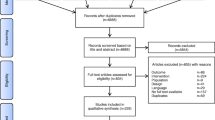
We retrieved a total of 204 potentially eligible studies from five electronic databases, including PubMed (n = 24), EMBASE (n = 26), Cochrane library (n = 89), Web of Science (n = 22), and CNKI (n = 43). After excluding 68 duplicate studies and 119 irrelevant studies, 17 studies were retained for final eligibility assessment. After exclusion of 11 ineligible studies due to ineligible control (n = 1), ineligible patients (n = 7), lack of outcome (n = 1), and unrelated to topic (n = 2), 6 eligible RCTs [18,19,20,21,22,23] were eventually included for data analysis. The detailed process of study screening is depicted in Fig. 1.
Study characteristics
Table 1 summarizes the detailed basic characteristics of eligible studies. All eligible studies [18,19,20,21,22,23] were conducted in China between 2022 and 2023. Three studies [18, 21, 23] recruited patients undergoing elective surgeries under general anesthesia, one study [19] recruited patient undergoing non-emergency, non-cardiothoracic, and non-brain elective surgeries, and other two studies [20, 22] recruited patients who were assigned to receive gynecological ambulatory surgeries. The sample size of individual studies ranged from 40 to 176, with a cumulative total of 712 cases. Five studies [18, 19, 21,22,23] used 0.6 mg/kg Rocuronium as a muscle relaxant, while the other one study [20] used 0.2 mg/kg Mivacurium as a muscle relaxant.
Risk of bias assessment
Five studies [18,19,20,21, 23] were rated as low risk in randomization process, but one study [22] was rated as having some concerns. Two studies [22, 23] were rated as having some concerns regarding deviations from the intended interventions, whereas the other four studies [18,19,20,21] were rated as low risk in this domain. Three studies [19, 22, 23] were rated as having low risk in terms of measurement of the outcome, and the other three studies [18, 20, 21] were rated as having some concerns in this domain. All studies [18,19,20,21,22,23] were rated as having low risk in missing outcome data and selection of the reported result. Finally, three studies [18, 20, 21] were rated as having a low risk of overall bias, while the other studies [19, 22, 23] were rated as having some concerns for overall bias. Detailed results of the risk of bias assessment are showed in Fig. 2.
Meta-analysis of efficacy
Induction success rate
All eligible studies [18,19,20,21,22,23] evaluated successful induction rate, which were 100% in both groups. As shown in Fig. 3a, no significant statistical heterogeneity was detected (p = 1.00, I2 = 0.0%), therefore the fixed-effects model was used for meta-analysis. The merged result showed that both ciprofol and propofol achieved the same successful induction rate (RR: 1.00, 95% CI: 0.99 to 1.01, z = 0.00, p = 1.00), which was supported by moderate evidence (Table 2).
Time to onset of successful induction
All studies [18,19,20,21,22,23] evaluated the time to onset of successful induction between ciprofol and propofol groups. As shown in Fig. 3b, significant statistical heterogeneity was detected (p = 0.01, I2 = 66.0%), therefore the random-effects model was used for meta-analysis. The merged result showed that there was no significant difference between ciprofol and propofol in this outcome (MD: 3.08, 95% CI: -0.93 to 7.09, z = 1.51, p = 0.13), which was supported by the moderate evidence (Table 2).
Time to disappearance of eyelash reflex
Three studies [18, 21, 22] evaluated the time to disappearance of eyelash reflex between ciprofol and propofol groups. As shown in Fig. 3c, no statistical heterogeneity was detected (p = 0.15, I2 = 47.0%), therefore the fixed-effects model was used for meta-analysis. The merged result showed that ciprofol was comparable to propofol in this outcome (MD: 0.55, 95% CI: -1.50 to 2.60, z = 0.52, p = 0.60), which was supported by the moderate evidence (Table 2).
Overall estimate means in BIS
All studies [18,19,20,21,22,23] evaluated the overall estimated means in BIS between ciprofol and propofol. As shown in Fig. 3d, statistical heterogeneity was insignificant (p = 0.79, I2 = 0.0%), therefore the fixed-effects model was used for meta-analysis. The merged result showed that ciprofol was better than propofol in terms of overall estimated means in BIS (MD: -3.79, 95% CI: -4.57 to -3.01, z = 9.54, p < 0.001), which was supported by the high evidence (Table 2).
Meta-analysis of safety
Time to full alertness
Three studies [19, 20, 23] evaluated to time to full alertness between ciprofol and propofol. As shown in Fig. 4, no significant statistical heterogeneity was detected (p = 0.77, I2 = 0.0%), therefore the fixed-effects model was used for meta-analysis. The merged result showed that there was no difference in this outcome between ciprofol and propofol groups (MD: 0.67, 95% CI: -0.03 to 1.36, z = 1.89, p = 0.06), which was supported by the high evidence (Table 2).
Incidence of hypotension
All studies [18,19,20,21,22,23] evaluated the incidence of hypotension between ciprofol and propofol. As shown in Fig. 5a, significant statistical heterogeneity was detected (p = 0.01, I2 = 66.0%), therefore the random-effects model was used for meta-analysis. The merged result showed that, compared with propofol, ciprofol was associated with lower incidence of hypotension (RR: 0.63, 95% CI: 0.42 to 0.94, z = 2.29, p = 0.02), which was supported by the moderate evidence (Table 2).
Incidence of arrhythmia
All studies [18,19,20,21,22,23] evaluated the incidence of arrhythmia between ciprofol and propofol. As shown in Fig. 5b, no significant statistical heterogeneity was detected (p = 0.65, I2 = 0.0%), therefore the fixed-effects model was used for meta-analysis. The merged result showed that there was no statistical difference in the incidence of arrhythmia between ciprofol and propofol (RR: 0.81, 95% CI: 0.55 to 1.21, z = 1.02, p = 0.31), which was supported by very the high evidence (Table 2).
Incidence of injection-site pain
All studies [18,19,20,21,22,23] evaluated the incidence of injection-site pain between ciprofol and propofol, but one study [23] was excluded from data analysis because it reported zero event in the both groups. As shown in Fig. 5c, significant statistical heterogeneity was detected (p = 0.04, I2 = 60.0%), therefore the random-effects model was used for meta-analysis. In addition, because previous studies have demonstrated the correlation between injection speed and the incidence of injection-site pain, therefore subgroup analysis was also introduced according to the method of injection (manual intravenous vs. pump intravenous). The merged result showed that, compared with propofol, ciprofol was associated with significantly lower incidence of injection-site pain (RR: 0.26, 95% CI: 0.14 to 0.47, z = 4.46, p < 0.001), which was supported by the moderate evidence (Table 2). Subgroup analysis showed that patients who received ciprofol with manual intravenous (RR: 0.31, 95% CI: 0.21 to 0.44, z = 6.55, p < 0.001) or pump intravenous (RR: 0.02, 95% CI: 0.00 to 0.14, z = 3.91, p < 0.001) experience significantly lower incidence of injection-site pain, while pump intravenous might be better than manual intravenous (p = 0.007, I2 = 86.1%).
Sensitivity analysis
Detailed results of the sensitivity analysis are shown in supplementary Fig. 1 to 3. The results showed that the merged results of individual meta-analyses did not change significantly after excluding one study a time, meaning that all pooled results were robust.
Publication bias
Funnel plots of all outcomes are displayed in supplementary Fig. 4 to 6. Visual inspection for these funnel plots showed symmetric outlines; however, the results of Egger’s test showed that the successful induction rate (p = 0.024) and incidence of hypotension (p = 0.012) were at risk of publication bias. For the other 6 outcomes, Egger’s test showed evidence supporting the absence of publication bias, with p-values of 0.372, 0.602, 0.615, 0.692, 0.184, and 0.162 for the time to onset of successful induction, the time to disappearance of eyelash reflex, time to full alertness, overall estimated mean in BIS, incidence of arrhythmia, and incidence of Injection-site pain, respectively.
Discussion
Ciprofol has recently emerged as a potential alternative to propofol due to its better GABAA receptor affinity. However, no definitive conclusion has been drawn as to whether ciprofol is better than propofol in patients undergoing elective surgeries under general anesthesia. In this meta-analysis, we accumulated a total of 712 patients to further evaluate the comparative efficacy and safety of ciprofol versus propofol in patients underwent elective surgeries under general anesthesia. This present meta-analysis indicated that ciprofol was more effective in providing deeper anesthesia (as shown by overall higher estimated mean in BIS) and led to lower incidences of hypotension and injection-site pain compared with propofol. When compared to propofol, ciprofol had a similar rate of induction rate, time to onset of successful induction, time to disappearance of eyelash reflex, time to full alertness, and incidence of arrhythmia. As a newly developed intravenous anesthetic drug, ciprofol exhibits good pharmacodynamic properties, including rapid onset of action and rapid recovery [14]. Furthermore, it binds more tightly to the GABA type A (GABAA) receptor than propofol and exhibits lower lipophilicity and a more appropriate steric bulk [18]. Therefore, ciprofol has been regarded as a promising alternative to propofol [16]. In this meta-analysis, we found that ciprofol was comparable to propofol in terms of successful induction rate, time to onset of successful induction, time to disappearance of eyelash reflex, time to full alertness, and incidence of arrhythmias. These results provide evidence that ciprofol has similar sedative and anesthetic efficacy to propofol in general anesthesia.
Intraoperative accidental awareness is a very serious consequence of general anesthesia that can cause patients to experience recurring anxiety, nightmares and psychological repercussion, and can also lead to posttraumatic stress disorder in more severe cases [33]. Anesthesia depth is one of the major contributors to the occurrence of intraoperative accidental awareness [34]. So, an appropriate anesthesia depth should be achieved and maintained during intraoperative maintenance. BIS is an electroencephalogram-derived parameter used to monitor the depth of anesthesia during operation [35], with BIS < 60 indicating sedation status [19]. However, deep anesthesia (BIS < 40) must also be avoided as it has been found to be associated with increased risk of electroencephalogram burst suppression and cardiovascular dysfunctions [36]. This meta-analysis showed that the overall estimated BIS in patients received ciprofol was less than that in patients received propofol, suggesting that ciprofol achieved a better anesthesia depth than propofol.
Hypotension is also one of the known common adverse effect of the administration of propofol for general anesthesia [20]. Growing evidence suggests that intraoperative hypotension is linked to increased rates of damage to vital organs (e.g., heart, kidneys and brain) and mortality in high-risk patients [37,38,39]. In this meta-analysis, we found that the administration of ciprofol was associated with a significantly lower incidence of hypotension than propofol, which was consistent with the results of some previous studies [19,20,21].
Injection pain is among the most frequently reported propofol-related adverse effects, with an estimated incidence of 50–80% [40,41,42]. This meta-analysis showed an accumulated incidence of 45.1% for pain on injection, while the incidence of injection-site pain was only 8.8% in ciprofol group. Many factors may contribute to the occurrence of injection-site pain, such as the concentration of the drug and injection speed. Ciprofol is an isomer of propofol, and a cyclopropyl group is inserted into the chemical structure of propofol, which improves its pharmacological and physicochemical properties and therefore reduces pain during injection [14, 17]. In addition, the lower plasma concentration of ciprofol may also be associated with a lower incidence of injection-site pain relative to propofol [17]. Furthermore, the results of subgroup analysis also prove that injection speed is closely related to pain on injection.
We must admit that our meta-analysis encounters four major limitations. First and foremost, only limited eligible studies with limited sample size were accumulated to evaluate the difference in efficacy and safety between ciprofol and propofol, therefore it was inevitably to compromise the robustness of the merged results. Although inclusion of both RCT and non-RCT may be beneficial for including more eligible studies, we must realize that this strategy will inevitably introduce bias to impair the reliability of findings [43]. Second, this meta-analysis only included studies in which 0.4–0.5 mg/kg ciprofol were used; however, we need to interpret that other doses have also been available for ciprofol, such as 0.2 and 0.3 mg/kg, and all these doses showed promising potential [44, 45]. However, these available doses were not directly compared with propofol, thus resulting in impossibility to evaluate the differences between these doses of ciprofol and propofol. So, future studies need to determine the optimal dose of ciprofol after the advantages of ciprofol compared to propofol has been confirmed. Third, all studies were conducted in China, there was no study conducted in other countries to evaluate the comparative efficacy and safety of ciprofol versus propofol. Therefore, our findings should be interpreted cautiously into other countries. Fourth, publication bias is detected for successful induction rate and the incidence of hypotension, thus inevitably compromising the certainty of the evidence. So, interpretation about these two outcomes should be made with cautious.
Conclusions
Based on the available data, we conclude that ciprofol may be a promising alternative to propofol for patients undergoing elective surgeries under general anesthesia because of its better anesthesia depth and lower incidence of hypotension and injection-site pain. However, future multicenter studies with large-scale are warranted to validate our findings because only limited eligible studies were cumulated in this meta-analysis.
Data availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- CNKI:
-
Chinese national knowledge infrastructure
- RCT:
-
Randomized controlled trials
- MD:
-
Mean difference
- CI:
-
Confidence interval
- RR:
-
Risk ratio
- GRADE:
-
Grading of recommendations, assessment, development and evaluation
- ASA:
-
American society of anesthesiologists
- BIS:
-
Bispectral index
- MESH:
-
Medical subject heading
- SBP:
-
Systolic blood pressure
References
Zhang W, Zhu Z, Zheng Y. Effect and safety of propofol for sedation during colonoscopy: a meta-analysis. J Clin Anesth. 2018;51:10–8.
Barr J, Egan TD, Sandoval NF, Zomorodi K, Cohane C, Gambus PL, et al. Propofol dosing regimens for ICU sedation based upon an integrated pharmacokinetic–pharmacodynamic model. J Am Soc Anesthesiologists. 2001;95:324–33.
Sridharan K, Sivaramakrishnan G. Comparison of fentanyl, remifentanil, sufentanil and alfentanil in combination with propofol for general anesthesia: a systematic review and meta-analysis of randomized controlled trials. Curr Clin Pharmacol. 2019;14:116–24.
Marana E, Colicci S, Meo F, Marana R, Proietti R. Neuroendocrine stress response in gynecological laparoscopy: TIVA with propofol versus sevoflurane anesthesia. J Clin Anesth. 2010;22:250–5.
McNeir DA, Mainous EG, Trieger N. Propofol as an intravenous agent in general anesthesia and conscious sedation. Anesth Prog. 1988;35:147.
Nishizawa T, Suzuki H. Propofol for gastrointestinal endoscopy. United Eur Gastroenterol J. 2018;6:801–5.
Lee W-K, Kim M-S, Kang S-W, Kim S, Lee J-R. Type of anaesthesia and patient quality of recovery: a randomized trial comparing propofol–remifentanil total iv anaesthesia with desflurane anaesthesia. Br J Anaesth. 2015;114:663–8.
Dubey PK, Kumar A. Pain on injection of lipid-free propofol and propofol emulsion containing medium-chain triglyceride: a comparative study. Anesth Analgesia. 2005;101:1060–2.
Singh A, Anjankar AP, Anjankar A. Propofol-related infusion syndrome: a clinical review. Cureus. 2022;14.
Kotani Y, Pruna A, Landoni G. Mechanisms of action of the detrimental effects of Propofol on Survival. J Cardiothorac Vasc Anesth. 2023;37:2176–80.
Zhu Q, Luo Z, Wang X, Wang D, Li J, Wei X et al. Efficacy and safety of ciprofol versus propofol for the induction of anesthesia in adult patients: a multicenter phase 2a clinical trial. Int J Clin Pharm. 2023:1–10.
Liao J, Li M, Huang C, Yu Y, Chen Y, Gan J, et al. Pharmacodynamics and pharmacokinetics of HSK3486, a novel 2, 6-disubstituted phenol derivative as a general anesthetic. Front Pharmacol. 2022;13:830791.
Li J, Wang X, Liu J, Wang X, Li X, Wang Y, et al. Comparison of ciprofol (HSK3486) versus propofol for the induction of deep sedation during gastroscopy and colonoscopy procedures: a multi-centre, non-inferiority, randomized, controlled phase 3 clinical trial. Basic Clin Pharmacol Toxicol. 2022;131:138–48.
Qin L, Ren L, Wan S, Liu G, Luo X, Liu Z, et al. Design, synthesis, and evaluation of novel 2, 6-disubstituted phenol derivatives as general anesthetics. J Med Chem. 2017;60:3606–17.
Bian Y, Zhang H, Ma S, Jiao Y, Yan P, Liu X, et al. Mass balance, pharmacokinetics and pharmacodynamics of intravenous HSK3486, a novel anaesthetic, administered to healthy subjects. Br J Clin Pharmacol. 2021;87:93–105.
Li X, Yang D, Li Q, Wang H, Wang M, Yan P, et al. Safety, pharmacokinetics, and pharmacodynamics of a single bolus of the γ-aminobutyric acid (GABA) receptor potentiator HSK3486 in healthy Chinese elderly and non-elderly. Front Pharmacol. 2021;12:735700.
Teng Y, Ou M, Wang X, Zhang W, Liu X, Liang Y, et al. Efficacy and safety of ciprofol for the sedation/anesthesia in patients undergoing colonoscopy: phase IIa and IIb multi-center clinical trials. Eur J Pharm Sci. 2021;164:105904.
Chen BZ, Yin XY, Jiang LH, Liu JH, Shi YY, Yuan BY. The efficacy and safety of ciprofol use for the induction of general anesthesia in patients undergoing gynecological surgery: a prospective randomized controlled study. BMC Anesthesiol. 2022;22:245.
Liang P, Dai M, Wang X, Wang D, Yang M, Lin X, et al. Efficacy and safety of ciprofol vs. propofol for the induction and maintenance of general anaesthesia: a multicentre, single-blind, randomised, parallel-group, phase 3 clinical trial. Eur J Anaesthesiol. 2023;40:399–406.
Man Y, Xiao H, Zhu T, Ji F. Study on the effectiveness and safety of ciprofol in anesthesia in gynecological day surgery: a randomized double-blind controlled study. BMC Anesthesiol. 2023;23:92.
Wang X, Wang X, Liu J, Zuo YX, Zhu QM, Wei XC, et al. Effects of ciprofol for the induction of general anesthesia in patients scheduled for elective surgery compared to propofol: a phase 3, multicenter, randomized, double-blind, comparative study. Eur Rev Med Pharmacol Sci. 2022;26:1607–17.
Yin XY, Liu JH, Yuan BY, Shi YY, Liu T, Chen BZ. Effectiveness and safety of ciprofol for general anesthesia iduction of gynecological day surgery. China Pharmaceuticals. 2023;32:101–4.
Zeng Y, Wang DX, Lin ZM, Liu J, Wei XC, Deng J, et al. Efficacy and safety of HSK3486 for the induction and maintenance of general anesthesia in elective surgical patients: a multicenter, randomized, open-label, propofol-controlled phase 2 clinical trial. Eur Rev Med Pharmacol Sci. 2022;26:1114–24.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Brady KM, Hudson A, Hood R, DeCaria B, Lewis C, Hogue CW. Personalizing the definition of hypotension to protect the brain. Anesthesiology. 2020;132:170–9.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Spineli LM, Pandis N. Meta-analysis: Random-effects model. Am J Orthod Dentofac Orthop. 2020;157:280–2.
Page MJ, McKenzie JE, Higgins JPT. Tools for assessing risk of reporting biases in studies and syntheses of studies: a systematic review. BMJ Open. 2018;8:e019703.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630.
Whitlock EL, Rodebaugh TL, Hassett AL, Shanks AM, Kolarik E, Houghtby J, et al. Psychological sequelae of surgery in a prospective cohort of patients from three intraoperative awareness prevention trials. Anesth Analg. 2015;120:87.
Pandit J, Andrade J, Bogod D, Hitchman J, Jonker W, Lucas N, et al. 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia: summary of main findings and risk factors. Br J Anaesth. 2014;113:549–59.
Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane database of systematic reviews; 2014.
Chan MT, Cheng BC, Lee TM, Gin T, Group CT. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42.
Saugel B, Bebert E-J, Briesenick L, Hoppe P, Greiwe G, Yang D et al. Mechanisms contributing to hypotension after anesthetic induction with sufentanil, propofol, and rocuronium: a prospective observational study. J Clin Monit Comput. 2021:1–7.
Wesselink E, Kappen T, Torn H, Slooter A, Van Klei W. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121:706–21.
Xu S, Shen X, Liu S, Yang J, Wang X. Efficacy and safety of norepinephrine versus phenylephrine for the management of maternal hypotension during cesarean delivery with spinal anesthesia: a systematic review and meta-analysis. Medicine. 2019;98.
Mangar D, Holak EJ. Tourniquet at 50 mm hg followed by intravenous lidocaine diminishes hand pain associated with propofol injection. Anesth Analg. 1992;74:250–2.
Tan C, Onsiong M. Pain on injection of propofol. Anaesthesia. 1998;53:468–76.
Valtonen M, Iisalo E, Kanto J, Rosenberg P. Propofol as an induction agent in children: pain on injection and pharmacokinetics. Acta Anaesthesiol Scand. 1989;33:152–5.
Sarri G, Patorno E, Yuan H, Guo JJ, Bennett D, Wen X et al. Framework for the synthesis of non-randomised studies and randomised controlled trials: a guidance on conducting a systematic review and meta-analysis for healthcare decision making. BMJ Evid Based Med. 2020.
Ding YY, Long YQ, Yang HT, Zhuang K, Ji FH, Peng K. Efficacy and safety of ciprofol for general anaesthesia induction in elderly patients undergoing major noncardiac surgery: a randomised controlled pilot trial. Eur J Anaesthesiol. 2022;39:960–3.
Duan G, Lan H, Shan W, Wu Y, Xu Q, Dong X, et al. Clinical effect of different doses of ciprofol for induction of general anesthesia in elderly patients: a randomized, controlled trial. Pharmacol Res Perspect. 2023;11:e01066.
Acknowledgments
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: DA(II) Administrative support: DA(III) Provision of study materials or patients: DA(IV) Collection and assembly of data: DA(V) Data analysis and interpretation: DA(VI) Manuscript writing: DA(VII) Final approval of manuscript: WWJ, DA.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests..
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ainiwaer, D., Jiang, W. Efficacy and safety of ciprofol versus propofol for anesthesia induction in adult patients received elective surgeries: a meta‑analysis. BMC Anesthesiol 24, 93 (2024). https://doi.org/10.1186/s12871-024-02479-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02479-9