Abstract
Background
Anemia, characterized by low hemoglobin levels, is a global public health concern. Anemia is an independent factor worsening outcomes in various patient groups. Blood transfusion has been the traditional treatment for anemia; its triggers, primarily based on hemoglobin levels; however, hemoglobin level is not always an ideal trigger for blood transfusion. Additionally, blood transfusion worsens clinical outcomes in certain patient groups. This narrative review explores alternative triggers for red blood cell transfusion and their physiological basis.
Main Text
The review delves into the physiology of oxygen transport and highlights the limitations of using hemoglobin levels alone as transfusion trigger. The main aim of blood transfusion is to optimize oxygen delivery, necessitating an individualized approach based on clinical signs of anemia and the balance between oxygen delivery and consumption, reflected by the oxygen extraction rate. The narrative review covers different alternative triggers. It presents insights into their diagnostic value and clinical applications, emphasizing the need for personalized transfusion strategies.
Conclusion
Anemia and blood transfusion are significant factors affecting patient outcomes. While restrictive transfusion strategies are widely recommended, they may not account for the nuances of specific patient populations. The search for alternative transfusion triggers is essential to tailor transfusion therapy effectively, especially in patients with comorbidities or unique clinical profiles. Investigating alternative triggers not only enhances patient care by identifying more precise indicators but also minimizes transfusion-related risks, optimizes blood product utilization, and ensures availability when needed. Personalized transfusion strategies based on alternative triggers hold the potential to improve outcomes in various clinical scenarios, addressing anemia’s complex challenges in healthcare. Further research and evidence are needed to refine these alternative triggers and guide their implementation in clinical practice.
Similar content being viewed by others
Introduction
Anemia is a clinical hematological syndrome which is characterized by low levels of hemoglobin and, in most cases, red blood cells (RBCs) [1]. The World Health Organization (WHO) identifies anemia as a global problem for public health and according to an estimation using prevalence data from 1993–2005, 24.8% of the global population was affected by this pathology. In particular, anemia is concentrated in children, pregnant and non-pregnant women [2]. The global anemia prevalence in 2010 was 32.9% (more than 2.2 billion people) and the most common cause of anemia was iron-deficiency [3].Thus, anemia is considered to be the most common hematological syndrome in clinical practice.
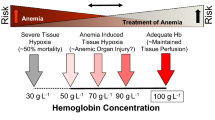
According to the WHO classification, anemia is defined as a hemoglobin concentration below 129 g/l in men and 119 g/l in women at sea level (Table 1) [4].
Anemia in the perioperative period and in critically ill patients is a particular problem. The rate of preoperative anemia depends on age, sex, comorbidity and surgical pathology and can reach up to 75% [5, 6]. Despite such a high prevalence in clinical practice, preoperative anemia has long been perceived as a relatively minor problem that can be easily eliminated by blood transfusion [7]. Meanwhile, numerous clinical trials suggest that preoperative anemia is associated with poor outcomes and increased mortality in both cardiac and non-cardiac surgery patients [8,9,10,11,12]. Transfusion of RBCs remains the most common approach for perioperative anemia treatment. Thus, the American Red Cross reports that nearly 16 million blood components units are transfused annually in the USA, with approximately 29,000 units of RBCs needed daily [13].
As shown in Table 2, blood transfusion has varying effects on clinical outcomes—both improvement outcomes and an increase in the risks of adverse outcomes. A similar situation arises in studies comparing restrictive and liberal transfusion strategies. For example, in the randomized ‘Transfusion Requirements in Critical Care (TRICC)’trial, a restrictive strategy showed a similar 30-day mortality compared to a liberal strategy in critically ill patients. However, mortality in the restrictive group was significantly lower among the subgroups- patients with Acute Physiology and Chronic Health Evaluation II score of ≤ 20 and younger patients (less than 55 years) and was also lower during hospitalization. Nevertheless, there was no difference in mortality among patients with cardiac pathology [23]. In accordance, another multicenter randomized trial demonstrated an identical mortality rate in septic shock patients between a liberal and restrictive transfusion strategy. The number of patients experiencing ischemic events (including cerebral ischemia and acute myocardial ischemia), adverse transfusion reactions and requiring life support were comparable in both groups [24]. Additionally, more recently, a systematic Cochrane database review analysed 48 trials and showed that a restrictive blood transfusion strategy led to a 41% reduction in the proportion of individuals subjected to red blood cell transfusions [25]. Authors suggested that there is no evidence of influence of the restrictive strategy on mortality and morbidity (such as stroke and myocardial infarction). Nevertheless, the authors emphasized that in some patient groups, higher hemoglobin levels may be preferable, but there was insufficient evidence to recommend applying a specific strategy to certain patient subgroups (traumatic brain injury, myocardial infarction, etc). Importantly, the authors were of the conviction that hemoglobin levels do not always reliably reflect the need for transfusion in some patients. A further large meta-analysis also demonstrated a reduction in in-hospital mortality among critically ill patients treated with a restrictive transfusion strategy [26]. On the other hand, a liberal blood transfusion strategy improved survival and decreased complications specifically in elderly patients and patients with cardiovascular diseases [27,28,29]. This issue is well described by Patel and co-authors, who, in a systematic review and meta-analysis, compared both cardiac and non-cardiac randomized clinical trials, as well as observational studies, investigating restrictive and liberal strategies [30]. The authors demonstrated that the results of randomized studies in cardiac surgery were not in accordance with the a general assumption that a liberal strategy significantly increases mortality and morbidity. Thus available evidence supports the inconsistency of the hemoglobin level as a transfusion trigger, especially in specific cohorts of patients. However, several modern guidelines suggest using the hemoglobin level as the only blood transfusion trigger and in most cases recommend a restrictive strategy [31,32,33].
The main aim of blood transfusion administration is an optimization of oxygen delivery rather than to approach a certain level of hemoglobin. Consequently, many authors and guidelines suggest an individualized approach to blood transfusions, based not only on the level of hemoglobin (which often cannot clearly reflect the need for oxygen delivery), but also on the clinical signs of anemia and the balance between oxygen delivery and oxygen consumption [32, 34,35,36].
One of the indications for blood transfusion may also be one of its physiological effects - increasing intravascular volume and, consequently, increasing blood pressure [37]. Thus, sustained perioperative hypotension and increased vasopressor support might also represent a trigger for a more liberal administration of blood components. The concept of physiological triggers for blood transfusion is based on the principles of anemia correction in terms of the oxygen delivery/consumption ratio.
The aim of our narrative review is to summarize the current role of alternative triggers for RBCs transfusion, such as the oxygen extraction ratio, blood lactate level, central venous/mixed venous oxygen saturation, oxygen content arterial-venous difference and ischemic signs on the electrocardiogram. Thus, we provide insights into the physiology of oxygen transport and emphasize the importance of oxygen delivery optimization regarding decision making in transfusion therapy. We give an overview of studies that which explore alternative triggers and their limitations in clinical practice and current blood transfusion guidelines.
Main text
Physiological basis of oxygen transport
Oxygen, discovered by Joseph Priestley in 1772, is the eighth element according to the serial number in the periodic system [38]. Also among the pioneers and researchers of oxygen were renowned scientists Carl Wilhelm Scheele and Antoine-Laurent Lavoisier. Oxygen plays an integral role in cellular respiration and is, therefore, essential for the life of all aerobic organisms. Oxygen partial pressure (tension) (PO2) in the atmospheric air at sea level fluctuates within 150–160 mmHg, while the transport chain of oxygen to the target cells is characterized by a physiological stepwise decrease in oxygen tension from inhaled air to the mitochondria. This phenomenon is called the “oxygen cascade” (Fig. 1) [39]. At the onset of inspiration the progress of declining oxygen tension occurs due to tracheal air humidification and alveolar carbon dioxide admixture and subsequently due to diffusion of oxygen through the alveolocapillary membrane and venous admixture and shunting [40, 41]. The partial pressure of oxygen continues to decrease in accordance with significant diffusion gradients between the capillaries and the cytoplasm of cells and finally the mitochondria. Current measurements demonstrate that tissue PO2 of most organs is in the range of 20–40 mmHg under resting conditions [42]. At rest and under physiologic conditions, oxygen delivery to the tissue still exceeds oxygen consumption by far with an oxygen extraction ratio near 25% [43]. However, the physiologic decrease of cellular oxygen levels can be further aggravated by pathophysiological states, as hypoventilation or ventilation/perfusion mismatch, that will finally result in tissue hypoxia.
Oxygen cascade (Explanation in text. With the permission from: Springer Nature, License Number 5,666,460,857,294) [39]
Most of the oxygen (98–99%) binds to hemoglobin, while only 1–2% of the total amount of oxygen is dissolved in the plasma and does not play a significant role in the global oxygen delivery.
The term “total oxygen content” (СtO2) indicates the sum of the volumetric concentrations of both bound and dissolved oxygen in plasma [43]:
1,34- Huffner’s constant - describes the volume of O2(ml) that binds 1 gram of hemoglobin at full saturation, 0,031- the Bunsen coefficient reflects the volume of oxygen that dissolves in 1 L of plasma for every 1 mmHg of partial pressure, Hb- hemoglobin concentration, g/l, PO2- partial pressure of oxygen, SO2- hemoglobin oxygen saturation. To calculate this indicator in arterial or venous blood, it is necessary to use the corresponding SO2and PO2values.
As formula (1) shows, PO2 equal to100 mmHg can only provide 3 ml of dissolved oxygen in 1 L of blood, while a saturation of 99% means that 134 ml of oxygen is bound at a hemoglobin level of 100 g/L. This clearly demonstrates the low possibility of increasing oxygen delivery in patients with anemia.
Oxygen delivery is determined by the following formula [43]:
DO2 - oxygen delivery, ml/min, CаO2 – total oxygen content in arterial blood, ml/l, CO- cardiac output, liters per minute.
Oxygen consumption is calculated using the following formula [43]:
VO2– oxygen consumption, ml/min, CvO2 – total oxygen content in mixed venous blood, ml/dl.
Oxygen consumption is also expressed as an oxygen extraction ratio (O2ER) or an oxygen extraction index [43, 44]:
O2ER- oxygen extraction ratio, %, CаO2– total oxygen content in arterial blood, ml/dl; CvO2- total oxygen content in mixed venous blood, ml/dl.
Normal values of these indicators are 20–30%.
Concepts of tissue oxygen delivery
Anemia and oxygen delivery
According to the classification proposed by Joseph Barcroft in 1920, anemia leads to anemic hypoxia associated with decreased blood oxygen content. According to this classification other types are hypoxemic hypoxia due to lack of oxygen in the inhaled air or ventilation disorders and circulatory hypoxia - associated with acute and/or chronic heart failure [45]. A few years later this classification was supplemented with the fourth type named histotoxic (tissue) hypoxia by J.P. Peters and D.D. Van Slyke, which is based on the blockade of respiratory enzymes [46]. Hemoglobin in red blood cells has been traditionally viewed as a passive transport system for oxygen and the main determinant of oxygen deliverly. Nevertheless, adequate local blood flow and thus tissue oxygen delivery are largely defined by the ability of the cell to generate adenosine 5′-triphosphate ATP from oxygen. One important fact of oxygen transport physiology is the independence of oxygen consumption upon oxygen delivery:oxygen consumption is constant over a wide range of DO2 under resting conditions, and decreases only when DO2 falls below a critical level (DO2crit) [47,48,49].
In this context overall oxygen consumption remained stable in healthy resting humans during severe acute limitation of oxygen delivery due to isovolemic reduction of hemoglobin concentration to various low levels (Table 3).
However, globally maintained oxygen consumption does not reflect the heterogenous difference inorgan specific oxygen supply and demand, and various organ systems might have different tolerances for anemia [50].
Adaptive responses to anemia
The first line of compensation for reduced oxygen delivery is increased blood flow through the tissues i.e. increased cardiac output [51]. During anemia an increase in cardiac index and heart rate associated with a sharp increase in myocardial oxygen consumption maintains global overall systemic oxygen delivery [52]. Given a constant systemic oxygen use, this fact requires that other organs, such as the gut or the kidney, must actually reduce oxygen consumption [51, 53].Thus organs of a higher metabolic demand namely the heart and the brain receive a greater proportion of blood flow and oxygen delivery [54]. Due to a limited blood flow response to anemia of the renal tissue, renal hypoxia is proportional to the degree of anemia and at comparable hemoglobin levels, more severe levels of tissue hypoxia are observed in the kidney, relative to the brain. In this context the kidney serves as an early central oxygen sensor activating local responses including the increased production of serum erythropoietin as well as adaptive cardiovascular responses in order to protect vital organ perfusion [55, 56].
In contrast, the organism cannot compensate for a reduced oxygen delivery due to a decreased cardiac output, as there are no physiological mechanisms available to increase the hemoglobin level or the oxygen saturation above a certain given level. Even an increase of red blood cells might than not be sufficient to increase oxyden delivery adequately [57]. It is also important to remember that elevating cardiac output may be unsafe for patients with decreased cardiac functional reserve as commonly observed in (patients with cardiovascular diseases or of advanced age). Moreover the majority to these specific patients receive ß-blocker treatment, which has been shown to attenuate the cardiovascular response to acute anemia and to accentuate the risk of brain and heart ischemia [58, 59].
In anemia, arterial oxygen tension remains almost unchanged. However, anemia leads to a decrease in blood oxygen content, which is compensated by an increase in cardiac output and an increase in oxygen extraction by tissues associated with a shift in the oxyhemoglobin dissociation curve to the right by increasing the concentration of 2,3-diphosphoglycerate [60, 61]. It is important to note that an increase in the level of 2,3- diphosphoglycerate and, accordingly, a decrease in the affinity of hemoglobin for oxygen is a reaction that is typical for all types of anemia including chronic anemia [61, 62]. In healthy volunteers a decreased tissue oxygen availability with a concomitant increased oxygen extraction and a reduced mixed venous oxygen saturation was present at hemoglobin levels below 6 g/dL. Nevertheless, this hemoglobin concentration did not result in inadequate oxygen transport asassessed by plasma lactate concentration [52].
Critical level of oxygen delivery
When the level of oxygen delivery decreases, oxygen consumption is provided by an increase in oxygen extraction [57, 63]. The development of oxygen debt is indicated by an increase in the level of blood lactate associated with a further increase in oxygen extraction and a decrease in the saturation of hemoglobin in the venous blood (Fig. 2) [64].
This level of critical oxygen delivery can vary under anemic conditions, as indicated by both clinical and experimental investigations. A clinical case that gave impetus to this concept was presented already some decades ago [65]. The report refers to an 84-year-old male patient with stomach cancer who underwent gastrectomy complicated by excessive bleeding with a blood loss of 4500 mL. The patient refused blood transfusions for religious reasons and died 12 h after surgery. The patient was invasively monitored with a pulmonary artery catheter and under conditions of hypervolemic hemodilution, a critical level of oxygen delivery-4.9 ml/kg per minute at a hemoglobin level of 40 g/l was identified. In this case oxygen consumption dependence upon delivery did not develop before at a very low level of hemoglobin has been reached. However, experimental studies showed various levels of hemoglobin that led to critical oxygen delivery in anemia (Table 3). The presented data demonstrate the imperfection of hemoglobin as a trigger for transfusion in specific clinical situations.
As seen in Table 3, the hemoglobin level at which critical oxygen delivery is achieved varies and depends on age and comorbidities. It is important to understand that reaching the critical level of oxygen delivery in anemia is associated with a significantly increase in mortality. This is well demonstrated in a study of patients with anemia who did not undergo blood transfusion (patient refusals for religious and other reasons) [70]. Moreover, it has also been shown that reaching the critical level of oxygen delivery in anemia leads to a reduction in time to death and a limited time for intervention [70, 71].
Nevertheless, a strong association between decreased hemoglobin values and adverse events and mortality has also been established even before a critical level of oxygen delivery has been reached. Specifically, mortality rises sharply as hemoglobin decreases below 5-6 g/dl [72], while oxygen consumption may become dependent on delivery at even lower hemoglobin levels. Thus in clinical practice anemia treatment usually is initiated already well above this critical threshold and anemia induced organ injury due to a reduction of organ specific tissue oxygenation might be a more relevant mechanism for unfavourable outcomes.
Relationship between delivery and consumption of oxygen. There is an increase in the level of lactate and a further increase in oxygen extraction with a decrease in the saturation of hemoglobin in venous blood when reaching the critical oxygen delivery (DO2- oxygen delivery, VO2- oxygen consumption, SvO2-saturation of hemoglobin in mixed/central venous blood, OER-oxygen extraction ratio, DO2crit- critical level of oxygen delivery)(With the permission from Elsevier Publisher, License Number 5,633,500,709,980) [64]
Blood transfusion triggers
As noted above, the main and only purpose of blood transfusion is to increase the oxygen– binding capacity of the blood and, as a result, oxygen delivery to organs and tissues. From a physiological point of view, the isolated hemoglobin level does not always reflect adequate oxygen delivery, which became the basis for the search for alternative blood transfusion triggers.
Modern requirements for an “ideal trigger” include not only high sensitivity and specificity and the possibility of its continuous monitoring, but also the ability to reflect the degree of anemia compensation by the body, contributing to timely and reasonable blood transfusions [73].
In the 1980s, an expert consensus, published in the Journal of the American Medical Association with relatively liberal approaches to blood transfusion, clearly states the need for an individualized approach in prescribing blood transfusion – assessment of clinical parameter, mixed venous blood oxygen tension, oxygen extraction level, and cardiac output [74].
Further studies in search of an alternative blood transfusion trigger suggested using the saturation of the level of oxygen extraction, central venous/mixed venous oxygen saturation, oxygen content arterial-venous difference, electrocardiogram, serum lactate and others.
Oxygen extraction ratio
The oxygen extraction ratio reflects oxygen consumption by the body, while, as mentioned above, its extraction increases with a decrease in the oxygen delivery level, which ensures oxygen consumption at a constant rate. The level of oxygen extraction is one of the laboratory indicators of anemic hypoxia [75]. This indicator has demonstrated diagnostic value primarily in reducing the number of blood transfusions and served as a trigger for blood transfusions in a number of studies [75,76,77,78,79]. Furthermore, the significance of this indicator as a criterion for blood transfusion prescriptions was demonstrated in earlier and experimental studies [80, 81]. It should be noted that no large randomized trials of a blood transfusion strategy based on oxygen extraction level assessment have been conducted.
Central venous/mixed venous oxygen saturation
Saturation of hemoglobin in mixed venous blood (SvO2) is one of the indicators reflecting the balance between oxygen delivery and consumption [82]. From a physiological point of view, SvO2 is considered an indicator that reflects the extraction of oxygen and disturbances in the ratio between its delivery and consumption [83]. The concept of using SvO2 as a blood transfusion trigger was mostly developed in the works of B. Vallet et al. Studies based on the physiological rationale demonstrated high sensitivity and specificity of SvO2 as a blood transfusion trigger [84,85,86].
However, the use of mixed venous blood hemoglobin saturation is associated with an invasive method (pulmonary artery catheter insertion) and high cost. In this regard, a possible alternative is the use of the hemoglobin saturation of the central venous blood (blood obtained from the mouths of the vena cava, ScvO2) [87]; normal SvO2 values are 68–77%, and ScvO2 is approximately 5% higher [88]. In a recent study, N. Themelin and coauthors assessed changes in systemic oxygen delivery and consumption after transfusions of donor RBCs and the diagnostic value of ScvO2 in patients in the ICU [89]. The authors have evaluated central venous blood hemoglobin saturation, blood lactate level, venous-to-arterial carbon dioxide (CO2) tension difference and cardiac index. Only hemoglobin saturation of the central venous blood with a baseline value of less than 70% showed positive changes after blood transfusion, while the effect on clinical outcomes remains unclear. It is necessary to remember that a decrease in SvO2(ScvO2) can develop with a decrease in arterial oxygenation (hypoxic hypoxia), a decrease in cardiac output (circulatory hypoxia), and in conditions accompanied by an increase in oxygen demand (fever, pain, convulsions) [84, 90].
Arterial-venous oxygen difference
This indicator was also described as an alternative trigger, but is less common in the literature. The arterio-venous oxygen difference is the difference between the oxygen content in arterial and venous blood:
In a prospective observational study, A. Fogagnolo et al. showed the benefits of using the arterio-venous difference as an additional blood transfusion trigger: in the group with high A-VO2 diff, blood transfusion was associated with a decrease in 90-day mortality [91]. However, another study devoted to this blood transfusion trigger has not been found.
ECG signs of ischemia
One of the alternative blood transfusion triggers is ischemic signs on the electrocardiogram (ECG). The physiological rationale for this trigger is a decrease in oxygen delivery to the myocardium and consequently the development of myocardial ischemia [92]. Changes in the ECG are often functional and reversible and may be associated with tachycardia, but a number of authors indicate the possibility of using this feature as an additional physiological criterion for prescribing blood transfusion [93, 94]. Additionally, it is worth highlighting the low specificity of this trigger [73].
Serum lactate
Another alternative trigger for blood transfusion might be the serum lactate level, which has been widely recognized as a reliable indicator of tissue hypoxia. A systematic review by A. Tran et al. showed the predictive value of lactate levels for transfusion and haemostatic interventions during traumatic hemorrhage [95]. On the other hand, there area large number of reasons for the rise in blood lactate levels, such as shock, sepsis, severe liver disease, which sharply reduce its specificity as a blood transfusion trigger [96, 97]. There are also no large randomized trials evaluating lactate as an indicator for blood transfusion.
It is important to note that changes in blood lactate levels and ECG during anemia often manifest late, which may lead to a delay in anemia treatment. Moreover, it has been shown that blood transfusion has no impact on reducing lactate levels, which may also limit its use as a trigger for blood transfusion [98].
Near Infrared Spectroscopy
Near Infrared Spectroscopy is a non-invasive method for monitoring tissue oxygenation and can also be used as a physiological trigger for blood transfusion. In a systematic review published by Crispin and Forwood, 69 studies were included where Near Infrared Spectroscopy was employed to measure tissue oxygenation and guide decisions regarding blood transfusion. For certain patient groups (trauma patients), tissue oxygenation levels demonstrated low sensitivity and higher specificity for transfusion decisions. In other patient groups (cardiac surgery, neurosurgery, and neonatal cases), the use of Near Infrared Spectroscopy reduced the number of transfused units of red blood cells but did not impact outcomes [99]. The benefits of Near Infrared Spectroscopy in blood transfusion decision-making lie in its non-invasiveness; however, the available clinical data is insufficient to recommend it as a physiological trigger for blood transfusion.
Exercise capacity
As mentioned above, many authors recommend considering not only the hemoglobin level but also clinical data, including a decrease in physical exercise tolerance, for blood transfusion decisions [32]. Cardiopulmonary exercises are typically used to assess exercise tolerance and are employed to stratify the risk of patients undergoing elective surgery. An increase in mortality and complications risk after surgery has been demonstrated in patients with reduced exercise capacity [100]. Some patients with anemia also exhibit decreased exercise capacity, which may be considered an additional trigger for blood transfusion. In a prospective clinical study, Wright et al. showed a significant improvement in exercise capacity after blood transfusion [101]. However, there is also insufficient data to recommend this method, and we suggest that it should be assessed in conjunction with other clinical data.
Biomarkers of tissue hypoxia
A special interest in the study of anemia lies in markers of tissue hypoxia. Indicators such as methemoglobin and erythropoietin can serve as markers of anemia-induced tissue hypoxia. It is suggested that an increase in methemoglobin may be associated with an elevated risk of stroke, and assessing its level could be valuable in the management of anemia [102]. Erythropoietin has also shown an increase in response to tissue hypoxia and may be considered a marker of anemia-induced renal hypoxia [103]. However, further research on markers of anemia-induced tissue hypoxia and their role in the decision-making regarding blood transfusion is needed.
As seen from Table 4, current mainstream recommendations from professional medical societies for blood transfusion generally rely primarily on hemoglobin levels. At the same time, several alternative triggers for making decisions about blood transfusion have not gained widespread acknowledgment within these recommendations. However, many experts acknowledge the imperfections of hemoglobin levels as an isolated trigger for blood transfusion and the limitations in its application.
The recognition of the imperfections and limitations associated with relying solely on hemoglobin levels for blood transfusion decisions underscores the need for substantial clinical research into alternative transfusion triggers. Large clinical studies are crucial to comprehensively evaluate the effectiveness and safety of these alternative triggers, taking into account diverse patient populations and clinical scenarios.
A personalized strategy could consider not only hemoglobin levels but also other relevant clinical parameters, patient-specific factors, and physiological considerations. This personalized approach aims to optimize transfusion practices, ensuring a more tailored and effective intervention while minimizing unnecessary transfusions and associated risks.
In essence, the call for large clinical studies and the exploration of personalized approaches in the context of blood transfusion decisions stem from the recognition that a more nuanced understanding of patient needs and responses is essential to enhance the precision and appropriateness of transfusion practices.
Conclusion
Anemia is a fairly common clinical and hematological syndrome with a decrease in hemoglobin level as the main diagnostic criterion. Existing conservative drug treatments cannot always be used in patients in the perioperative period. The main treatment for anemia is blood transfusion, and the main trigger is the hemoglobin level. Sometimes additional triggers are used such as clinical signs of anemic syndrome. However, the clinical signs of anemia are not specific and cannot always be used in the clinical practice. Numerous studies have shown that both anemia and blood transfusion are independent factors that worsen outcomes.
The generally accepted consensus between these two problems is currently being solved by a restrictive transfusion strategy with reference points to a lower threshold hemoglobin level. However this strategy does not take into account some features of patients with comorbidities. As a result, studies on the threshold level of hemoglobin in patients with various acute diseases remain controversial. This fact has recently been emphasized by the results from the Myocardial Ischemia and Transfusion (MINT) trial, which evaluated whether a restrictive transfusion strategy (hemoglobin trigger, 7–8 g/dL) differed from a liberal transfusion strategy (hemoglobin trigger, < 10 g/dL) among patiens with acute myocardial infarction. Although the groups did not differ significantly regarding the primary composite outcome recurrent myocardial infarction or death at 30 days, the liberal treatment showed advantages in specific subgruoups [108]. This underlines again the importance of a multifactorial approach which addresses multiple patient specific risk factors and confirms the need for alternative blood transfusion triggers.
Despite the availability and interest in physiologic blood transfusion triggers, the level of evidence for their use is extremely low, which is primarily due to the lack of large randomized trials. At the same time, the need for personification of blood transfusion therapy is necessary. Vincent J.L., in one of his recent articles, gives a simple example: a hemoglobin level of 80 g/l is safe in a young healthy patient with an injury and the same hemoglobin level in an elderly patient with severe tachycardia and a history of myocardial infarction can play a critical role [36]. Current recommendations do not provide a clear solution to resolve this dilemma.
The application and study of alternative triggers for blood transfusion are crucial for several reasons. First, traditional triggers for transfusion, such as hemoglobin levels, may not fully capture an individual patient’s oxygen-carrying capacity and overall physiological status. By exploring alternative triggers, we can potentially identify more accurate and tailored indicators of when a patient truly requires a blood transfusion.
Second, the use of blood products comes with inherent risks, including transfusion reactions, infections, and immunological complications. By investigating alternative triggers, we can aim to reduce unnecessary transfusions, thus minimizing these potential adverse events and improving patient safety.
Moreover, studying alternative triggers can lead to more effective and efficient blood utilization, addressing the issue of blood shortages and ensuring the availability of blood products for patients who genuinely need them. This becomes especially critical in high-demand situations, such as during critical care or perioperative periods.
Furthermore, different patient populations may have distinct responses to anemia, making it essential to explore alternative triggers for blood transfusion in specific groups, such as critically ill patients or those undergoing surgical procedures. Tailoring transfusion practices based on individualized triggers may optimize patient outcomes and resource allocation.
In summary, the application and study of alternative triggers for blood transfusion are necessary to enhance patient care, minimize risks associated with transfusions, improve blood product utilization, and ultimately promote better outcomes in diverse clinical scenarios.
Data availability
Not applicable.
References
Addo OY, Yu EX, Williams AM, et al. Evaluation of hemoglobin cutoff levels to define Anemia among healthy individuals. JAMA Netw Open. 2021;4(8):e2119123. https://doi.org/10.1001/jamanetworkopen.2021.19123.
McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. PublicHealthNutr. 2009;12(4):444–54. https://doi.org/10.1017/S1368980008002401.
Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–24. https://doi.org/10.1182/blood-2013-06-508325.
WHO: Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization., 2011 http://www.who.int/vmnis/indicators/haemoglobin.pdf, Accessed 20 Dec 2022.
Gómez-Ramirez S, Jericó C, Muñoz M. Perioperative anemia: prevalence, consequences and pathophysiology. TransfusApherSci. 2019;58(4):369–74. https://doi.org/10.1016/j.transci.2019.06.011.
Muñoz M, Laso-Morales MJ, Gómez-Ramírez S, Cadellas M, Núñez-Matas MJ, García-Erce JA. Pre-operative haemoglobin levels and iron status in a large multicentre cohort of patients undergoing major elective surgery. Anaesthesia. 2017;72(7):826–34. https://doi.org/10.1111/anae.13840.
Lin Y. Preoperative anemia-screening clinics. Hematol Am Soc Hematol Educ Program. 2019;2019(1):570–6. https://doi.org/10.1182/hematology.2019000061.
Fowler AJ, Ahmad T, Phull MK, Allard S, Gillies MA, Pearse RM. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg. 2015;102(11):1314–24. https://doi.org/10.1002/bjs.9861.
Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378(9800):1396–407. https://doi.org/10.1016/S0140-6736(11)61381-0.
Baron DM, Hochrieser H, Posch M, et al. Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br J Anaesth. 2014;113(3):416–23. https://doi.org/10.1093/bja/aeu098.
Padmanabhan H, Siau K, Curtis J, et al. Preoperative Anemia and outcomes in Cardiovascular surgery: systematic review and Meta-analysis. Ann Thorac Surg. 2019;108(6):1840–8. https://doi.org/10.1016/j.athoracsur.2019.04.108.
Mueller MM, Van Remoortel H, Meybohm P et al. Patient Blood Management: Recommendations From the 2018 Frankfurt Consensus Conference. JAMA. 2019;321(10):983–997. https://doi.org/10.1001/jama.2019.0554.
American Red Cross. : US Blood Supply Facts. https://www.redcrossblood.org/donate-blood/how-to-donate/how-blood-donations-help/blood-needs-blood-supply.html, Accessed 20 Dec 2022.
Smilowitz NR, Oberweis BS, Nukala S, et al. Association between Anemia, bleeding, and transfusion with long-term mortality following noncardiac surgery. Am J Med. 2016;129(3):315–23e2. https://doi.org/10.1016/j.amjmed.2015.10.012.
Glance LG, Dick AW, Mukamel DB, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114(2):283–92. https://doi.org/10.1097/ALN.0b013e3182054d06.
Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J AmCollSurg. 2009;208(5):931–9. https://doi.org/10.1016/j.jamcollsurg.2008.11.019.
Woldendorp K, Manuel L, Srivastava A, Doane M, Bassin L, Marshman D. Perioperative transfusion and long-term mortality after cardiac surgery: a meta-analysis. GenThoracCardiovascSurg. 2023;71(6):323–30. https://doi.org/10.1007/s11748-023-01923-w.
Li Y, Cheang I, Zhang Z, Zuo X, Cao Q, Li J. Prognostic Association between Perioperative Red Blood Cell Transfusion and postoperative cardiac surgery outcomes. FrontCardiovascMed. 2021;8:730492. https://doi.org/10.3389/fcvm.2021.730492. Published 2021 Sep 24.
Park DW, Chun BC, Kwon SS, et al. Red blood cell transfusions are associated with lower mortality in patients with severe sepsis and septic shock: a propensity-matched analysis*. CritCareMed. 2012;40(12):3140–5. https://doi.org/10.1097/CCM.0b013e3182657b75.
Jang SY, Cha YH, Yoo JI, et al. Blood transfusion for Elderly patients with hip fracture: a Nationwide Cohort Study. J KoreanMedSci. 2020;35(37):e313. https://doi.org/10.3346/jkms.2020.35.e313. Published 2020 Sep 21.
Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345(17):1230–6. https://doi.org/10.1056/NEJMoa010615.
Zheng Y, Lu C, Wei S, Li Y, Long L, Yin P. Association of red blood cell transfusion and in-hospital mortality in patients admitted to the intensive care unit: a systematic review and meta-analysis. Crit Care. 2014;18(6):515. https://doi.org/10.1186/s13054-014-0515-z. Published 2014 Nov 14.
Hébert PC, Wells G, Blajchman MA et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group [published correction appears in N Engl J Med. 1999;340(13):1056]. N Engl J Med. 1999;340(6):409–417. https://doi.org/10.1056/NEJM199902113400601.
Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371(15):1381–91. https://doi.org/10.1056/NEJMoa1406617.
Carson JL, Stanworth SJ, Dennis JA, et al. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst Rev. 2021;12(12):CD002042. https://doi.org/10.1002/14651858.CD002042.pub5. Published 2021 Dec 21.
Zhang W, Zheng Y, Yu K, Gu J. Liberal Transfusion versus restrictive transfusion and outcomes in critically ill adults: a Meta-analysis. TransfusMedHemother. 2021;48(1):60–8. https://doi.org/10.1159/000506751.
Docherty AB, O’Donnell R, Brunskill S, et al. Effect of restrictive versus liberal transfusion strategies on outcomes in patients with cardiovascular disease in a non-cardiac surgery setting: systematic review and meta-analysis. BMJ. 2016;352:i1351. https://doi.org/10.1136/bmj.i1351. Published 2016 Mar 29.
Simon GI, Craswell A, Thom O, Fung YL. Outcomes of restrictive versus liberal transfusion strategies in older adults from nine randomised controlled trials: a systematic review and meta-analysis. LancetHaematol. 2017;4(10):e465–74. https://doi.org/10.1016/S2352-3026(17)30141-2.
Cortés-Puch I, Wiley BM, Sun J, et al. Risks of restrictive red blood cell transfusion strategies in patients with cardiovascular disease (CVD): a meta-analysis. Transfus Med. 2018;28(5):335–45. https://doi.org/10.1111/tme.12535.
Patel NN, Avlonitis VS, Jones HE, Reeves BC, Sterne JA, Murphy GJ. Indications for red blood cell transfusion in cardiac surgery: a systematic review and meta-analysis. LancetHaematol. 2015;2(12):e543–53. https://doi.org/10.1016/S2352-3026(15)00198-2.
Mueller MM, Van Remoortel H, Meybohm P, et al. Patient blood management: recommendations from the 2018 Frankfurt Consensus Conference. JAMA. 2019;321(10):983–97. https://doi.org/10.1001/jama.2019.0554.
Carson JL, Stanworth SJ, Guyatt G, et al. Red blood cell transfusion: 2023 AABB International guidelines. JAMA. 2023;330(19):1892–902. https://doi.org/10.1001/jama.2023.12914.
Vlaar AP, Oczkowski S, de Bruin S, et al. Transfusion strategies in non-bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. 2020;46(4):673–96. https://doi.org/10.1007/s00134-019-05884-8.
Pagano D, Milojevic M, Meesters MI, et al. 2017 EACTS/EACTA guidelines on patient blood management for adult cardiac surgery. Eur J CardiothoracSurg. 2018;53(1):79–111. https://doi.org/10.1093/ejcts/ezx325.
Sakr Y, Vincent JL. Should red cell transfusion be individualized? Yes. IntensiveCareMed. 2015;41(11):1973–6. https://doi.org/10.1007/s00134-015-3950-7.
Vincent JL. Transfusion thresholds: the dangers of guidelines based on randomized controlled trials. IntensiveCareMed. 2020;46(4):714–6. https://doi.org/10.1007/s00134-019-05889-3.
Laine L, Barkun AN, Saltzman JR, Martel M, Leontiadis GI. ACG Clinical Guideline: Upper Gastrointestinal and Ulcer Bleeding [published correction appears in Am J Gastroenterol. 2021;116(11):2309]. Am J Gastroenterol. 2021;116(5):899–917. https://doi.org/10.14309/ajg.0000000000001245.
Courtière A. Biochemistry of oxygen. In: Mathieu D. Handbook on Hyperbaric Medicine.Springer.2006: 25–30. https://doi.org/10.1007/1-4020-4448-8.
Neuhaeuser C. CO2, and acid-base balance. In: Klauwer D, Neuhaeuser C, Thul J, Zimmermann R, editors. A practical handbook on Pediatric Cardiac Intensive Care Therapy. Cham: Springer; 2019. Klauwer, D. O2Supply.
Dominelli PB, Wiggins CC, Roy TK, Secomb TW, Curry TB, Joyner MJ. The Oxygen Cascade During Exercise in Health and Disease. Mayo Clin Proc. 2021;96(4):1017–1032. https://doi.org/10.1016/j.mayocp.2020.06.063.
Xuefei Y, Xinyi Z, Qing C, et al. Effects of Hyperoxia on mitochondrial homeostasis: are Mitochondria the hub for bronchopulmonary dysplasia? Front Cell Dev Biol. 2021;9:642717. https://doi.org/10.3389/fcell.2021.642717. Published 2021 Apr 30.
Ortiz-Prado E, Dunn JF, Vasconez J, Castillo D, Viscor G. Partial pressure of oxygen in the human body: a general review. Am J Blood Res. 2019;9(1):1–14. Published 2019 Feb 15.
Rosen IM, Manaker S. Oxygen delivery and consumption In: UpToDate, Post TW, editor, UpToDate, Waltham, MA: UpToDate Inc. http://www.uptodate.com. Accessed Feb 2023.
Dunn J-OC, Mythen MG, Grocott MP. October, Physiology of oxygen transport, BJA Education, 16, issue 10, 2016, Pages 341–8, https://doi.org/10.1093/bjaed/mkw012.
Barcroft J. Anoxemia Lancet. 1920;2:485–9.
Wilson WC, Shapiro B. Perioperative hypoxia. The clinical spectrum and current oxygen monitoring methodology. AnesthesiolClinNorthAm. 2001;19(4):769–812.
Schumacker PT, Samsel RW. Analysis of oxygen delivery and uptake relationships in the Krogh tissue model. J ApplPhysiol (1985). 1989;67(3):1234–1244. https://doi.org/10.1152/jappl.1989.67.3.1234.
Cain SM. Oxygen delivery and uptake in dogs during anemic and hypoxic hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1977;42(2):228–34. https://doi.org/10.1152/jappl.1977.42.2.228.
Ronco JJ, Fenwick JC, Tweeddale MG, et al. Identification of the critical oxygen delivery for anaerobic metabolism in critically ill septic and nonseptic humans. JAMA. 1993;270(14):1724–30.
van Bommel J, Siegemund M, Henny CP, Ince C. Heart, kidney, and intestine have different tolerances for anemia. Transl Res. 2008;151(2):110–7. https://doi.org/10.1016/j.trsl.2007.11.001.
Hare GM, Tsui AK, Ozawa S, Shander A. Anaemia: can we define haemoglobin thresholds for impaired oxygen homeostasis and suggest new strategies for treatment? Best Pract Res Clin Anaesthesiol. 2013;27(1):85–98. https://doi.org/10.1016/j.bpa.2012.12.002.
Weiskopf RB, Viele MK, Feiner J, et al. Human Cardiovascular and metabolic response to Acute, severe isovolemic Anemia. JAMA. 1998;279(3):217–21. https://doi.org/10.1001/jama.279.3.217.
Mathru M, Solanki DR, Woodson LC, et al. Splanchnic oxygen consumption is impaired during severe acute normovolemic anemia in anesthetized humans. Anesthesiology. 2006;105(1):37–44. https://doi.org/10.1097/00000542-200607000-00010.
Shander A, Javidroozi M, Ozawa S, Hare GM. What is really dangerous: anaemia or transfusion? Br J Anaesth. 2011;107(Suppl1):i41–i59. https://doi.org/10.1093/bja/aer350.
Abrahamson JR, Read A, Chin K, et al. Renal tissue Po2 sensing during acute hemodilution is dependent on the diluent. Am J PhysiolRegulIntegr Comp Physiol. 2020;318(4):R799–R812. https://doi.org/10.1152/ajpregu.00323.2019.
Chin K, Joo H, Jiang H, et al. Importance of assessing biomarkers and physiological parameters of anemia-induced tissue hypoxia in the perioperative period. Braz J Anesthesiol. 2023;73(2):186–97. https://doi.org/10.1016/j.bjane.2022.10.004.
Vincent JL. DO2/VO2 relationships. In: Pinsky MR, Payen D. Functional Hemodynamic Monitoring. Update in Intensive Care and Emergency Medicine, vol 42. Springer, Berlin, 2005: 251–258, https://doi.org/10.1007/3-540-26900-2_20.
POISE Study Group, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–47. https://doi.org/10.1016/S0140-6736(08)60601-7.
van Klei WA, Bryson GL, Yang H, Forster AJ. Effect of beta-blocker prescription on the incidence of postoperative myocardial infarction after hip and knee arthroplasty. Anesthesiology. 2009;111(4):717–24. https://doi.org/10.1097/ALN.0b013e3181b6a761.
Anaemia. In: Lumb AB, Thomas SR, Nunn and Lumb’s Applied respiratory physiology, ninth edition, Elsevier, 2021: 279–285.
Bunn HF. Oxygen delivery in the treatment of Anemia. N Engl J Med. 2022;387(25):2362–5. https://doi.org/10.1056/NEJMra2212266.
Mulhausen R, Astrup P, Kjeldsen K. Oxygen affinity of hemoglobin in patients with cardiovascular diseases, anemia, and cirrhosis of the liver. Scand J ClinLabInvest. 1967;19(3):291–7. https://doi.org/10.3109/00365516709090641.
Zhang H, Spapen H, Benlabed M, Vincent JL. Systemic oxygen extraction can be improved during repeated episodes of cardiac tamponade. J CritCare. 1993;8(2):93–9. https://doi.org/10.1016/0883-9441(93)90013-b.
Smithline HA, Ward KR, Chiulli DA, Blake HC, Rivers EP. Whole body oxygen consumption and critical oxygen delivery in response to prolonged and severe carbon monoxide poisoning. Resuscitation. 2003;56(1):97–104. https://doi.org/10.1016/s0300-9572(02)00272-1.
van Woerkens EC, Trouwborst A, van Lanschot JJ. Profound hemodilution: what is the critical level of hemodilution at which oxygen delivery-dependent oxygen consumption starts in an anesthetized human? AnesthAnalg. 1992;75(5):818–21. https://doi.org/10.1213/00000539-199211000-00029.
Lieberman JA, Weiskopf RB, Kelley SD, et al. Critical oxygen delivery in conscious humans is less than 7.3 ml O2 x kg(-1) x min(-1). Anesthesiology. 2000;92(2):407–13. https://doi.org/10.1097/00000542-200002000-00022.
Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology. 2000;93(4):1004–10. https://doi.org/10.1097/00000542-200010000-00023.
Spahn DR, Zollinger A, Schlumpf RB, et al. Hemodilution tolerance in elderly patients without known cardiac disease. AnesthAnalg. 1996;82(4):681–6. https://doi.org/10.1097/00000539-199604000-00002.
Spahn DR, Schmid ER, Seifert B, Pasch T. Hemodilution tolerance in patients with coronary artery disease who are receiving chronic beta-adrenergic blocker therapy. AnesthAnalg. 1996;82(4):687–94. https://doi.org/10.1097/00000539-199604000-00003.
Guinn NR, Cooter ML, Weiskopf RB. Lower hemoglobin concentration decreases time to death in severely anemic patients for whom blood transfusion is not an option. J TraumaAcuteCareSurg. 2020;88(6):803–8. https://doi.org/10.1097/TA.0000000000002632.
Tobian AA, Ness PM, Noveck H, Carson JL. Time course and etiology of death in patients with severe anemia. Transfusion. 2009;49(7):1395–9. https://doi.org/10.1111/j.1537-2995.2009.02134.x.
Shander A, Javidroozi M, Naqvi S, et al. An update on mortality and morbidity in patients with very low postoperative hemoglobin levels who decline blood transfusion (CME). Transfusion. 2014;54(10 Pt 2):2688–2687. https://doi.org/10.1111/trf.12565.
Tomic Mahecic T, Dünser M, Meier J. RBC Transfusion Triggers: is there anything New? TransfusMedHemother. 2020;47(5):361–8. https://doi.org/10.1159/000511229.
Perioperative Red Blood Cell Transfusion. JAMA. 1988;260(18):2700–3. https://doi.org/10.1001/jama.1988.03410180108040.
Vincent JL, De Backer D. Oxygen transport-the oxygen delivery controversy. IntensiveCareMed. 2004;30(11):1990–6. https://doi.org/10.1007/s00134-004-2384-4.
Sehgal LR, Zebala LP, Takagi I, Curran RD, Votapka TV, Caprini JA. Evaluation of oxygen extraction ratio as a physiologic transfusion trigger in coronary artery bypass graft surgery patients. Transfusion. 2001;41(5):591–5. https://doi.org/10.1046/j.1537-2995.2001.41050591.x.
Orlov D, O’Farrell R, McCluskey SA, et al. The clinical utility of an index of global oxygenation for guiding red blood cell transfusion in cardiac surgery. Transfusion. 2009;49(4):682–8. https://doi.org/10.1111/j.1537-2995.2008.02022.x.
O’Farrell R, Ghannam M, McCluskey M, Beattie S, Karkouti K. Oxygen extraction ratio (OER) and blood transfusion in cardiac surgery. Can J Anesth. 2006;53(1):26342–2. https://doi.org/10.1007/bf03016969.
Nasser B, Tageldein M, AlMesned A, Kabbani M. Effects of blood transfusion on oxygen extraction ratio and central venous saturation in children after cardiac surgery. AnnSaudiMed. 2017;37(1):31–7. https://doi.org/10.5144/0256-4947.2017.31.
Levy PS, Chavez RP, Crystal GJ, et al. Oxygen extraction ratio: a valid indicator of transfusion need in limited coronary vascular reserve? J Trauma. 1992;32(6):769–74. https://doi.org/10.1097/00005373-199206000-00018.
Wilkerson DK, Rosen AL, Gould SA, Sehgal LR, Sehgal HL, Moss GS. Oxygen extraction ratio: a valid indicator of myocardial metabolism in anemia. J SurgRes. 1987;42(6):629–34. https://doi.org/10.1016/0022-4804(87)90006-0.
Dunn JO, Mythen MG, Grocott MP. Physiology of oxygen transport. BJA Educ. 2016;16(10):341–8. https://doi.org/10.1093/bjaed/mkw012.
Bloos F, Reinhart K. Venous oximetry. Intensive Care Med. 2005;31(7):911–3. https://doi.org/10.1007/s00134-005-2670-9.
Vallet B, Robin E, Lebuffe G. Venous oxygen saturation as a physiologic transfusion trigger. CritCare. 2010;14(2):213. https://doi.org/10.1186/cc8854.
Vallet B, Adamczyk S, Barreau O, Lebuffe G. Physiologic transfusion triggers. BestPractResClinAnaesthesiol. 2007;21(2):173–81. https://doi.org/10.1016/j.bpa.2007.02.003.
Adamczyk S, Robin E, Barreau O, et al. Apport De La saturation veineuse centrale enoxygène dans la décisiontransfusionnellepostopératoire [Contribution of central venous oxygen saturation in postoperative blood transfusion decision]. Ann Fr AnesthReanim. 2009;28(6):522–30. https://doi.org/10.1016/j.annfar.2009.03.013.
Dueck MH, Klimek M, Appenrodt S, Weigand C, Boerner U. Trends but not individual values of central venous oxygen saturation agree with mixed venous oxygen saturation during varying hemodynamic conditions. Anesthesiology. 2005;103(2):249–57. https://doi.org/10.1097/00000542-200508000-00007.
Reinhart K, Kuhn HJ, Hartog C, Bredle DL. Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. IntensiveCareMed. 2004;30(8):1572–8. https://doi.org/10.1007/s00134-004-2337-y.
Themelin N, Biston P, Massart J, Lelubre C, Piagnerelli M. Effects of red blood cell transfusion on global oxygenation in anemic critically ill patients. Transfusion. 2021;61(4):1071–9. https://doi.org/10.1111/trf.16284.
Squara P. Central venous oxygenation: when physiology explains apparent discrepancies. CritCare. 2014;18(6):579. Published 2014 Nov 10. https://doi.org/10.1186/s13054-014-0579-9.
Fogagnolo A, Taccone FS, Vincent JL, et al. Using arterial-venous oxygen difference to guide red blood cell transfusion strategy [published correction appears in Crit Care. 2022;26(1):254]. Crit Care. 2020;24(1):160. https://doi.org/10.1186/s13054-020-2827-5. Published 2020 Apr 20.
Szekely P. Electrocardiographic findings in anaemia. Br Heart J. 1940;2(1):1–8. https://doi.org/10.1136/hrt.2.1.1.
Pappachan LG, Williams A, Sebastian T, Korula G, Singh G. Changes in central venous oxygen saturation, lactates, and ST segment changes in a V lead ECG with changes in hemoglobin in neurosurgical patients undergoing craniotomy and tumor excision: a prospective observational study. J AnaesthesiolClinPharmacol. 2019;35(1):99–105. https://doi.org/10.4103/joacp.JOACP_304_17.
Stanojević M, Stankov S. Elektrokardiografskepromenekodbolesnika s hronicnomanemijom [Electrocardiographic changes in patients with chronic anemia]. SrpArhCelokLek. 1998;126(11–12):461–6.
Tran A, Matar M, Lampron J, Steyerberg E, Taljaard M, Vaillancourt C. Early identification of patients requiring massive transfusion, embolization or hemostatic surgery for traumatic hemorrhage: a systematic review and meta-analysis. J TraumaAcuteCareSurg. 2018;84(3):505–16. https://doi.org/10.1097/TA.0000000000001760.
Suetrong B, Walley KR. Lactic acidosis in Sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest. 2016;149(1):252–61. https://doi.org/10.1378/chest.15-1703.
Seheult J, Fitzpatrick G, Boran G. Lactic acidosis: an update. Clin Chem Lab Med. 2017;55(3):322–33. https://doi.org/10.1515/cclm-2016-0438.
Czempik PF, Gierczak D, Wilczek D, Krzych ŁJ. The impact of Red Blood Cell Transfusion on Blood Lactate in Non-bleeding critically Ill Patients-A Retrospective Cohort Study. J ClinMed. 2022;11(4):1037. https://doi.org/10.3390/jcm11041037. Published 2022 Feb 17.
Crispin P, Forwood K. Near Infrared Spectroscopy in Anemia Detection and Management: a systematic review. TransfusMedRev. 2021;35(1):22–8. https://doi.org/10.1016/j.tmrv.2020.07.003.
Wilson RJ, Davies S, Yates D, Redman J, Stone M. Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br J Anaesth. 2010;105(3):297–303. https://doi.org/10.1093/bja/aeq128.
Wright SE, Pearce B, Snowden CP, Anderson H, Wallis JP. Cardiopulmonary exercise testing before and after blood transfusion: a prospective clinical study. Br J Anaesth. 2014;113(1):91–6. https://doi.org/10.1093/bja/aeu050.
Mistry N, Hare GMT, Shehata N et al. Methemoglobin as a marker of acute anemic stress in cardiac surgery. iScience. 2023;26(8):107429. Published 2023 Jul 20. https://doi.org/10.1016/j.isci.2023.107429.
Hare GMT, Han K, Leshchyshyn Y, et al. Potential biomarkers of tissue hypoxia during acute hemodilutional anemia in cardiac surgery: a prospective study to assess tissue hypoxia as a mechanism of organ injury. Can J Anaesth. 2018;65(8):901–13. https://doi.org/10.1007/s12630-018-1140-0. Biomarqueurspotentiels de l’hypoxietissulaire au cours de l’anémie par hémodilution de la chirurgiecardiaque: étude prospective évaluantl’hypoxietissulairecommemécanisme de lésiond’organes.
Executive Committee of the German Medical Association on the Recommendation of the Scientific Advisory Board. Cross-Sectional Guidelines for Therapy with Blood Components and Plasma Derivatives, 4th revised and updated edition, 2014.
Akselrod BA, Balashova EN, Bautin AE, Bakhovadinov BB, et al. Clinical guidelines for red blood cell transfusion. Russian J Hematol Transfusiology. 2018;63(4):372–435. https://doi.org/10.25837/HAT.2019.62.39.006.
Klein AA, Arnold P, Bingham RM, et al. AAGBI guidelines: the use of blood components and their alternatives 2016. Anaesthesia. 2016;71(7):829–42. https://doi.org/10.1111/anae.13489.
American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology. 2015;122(2):241–75. https://doi.org/10.1097/ALN.0000000000000463.
Carson JL, Brooks MM, Hébert PC, et al. Restrictive or Liberal Transfusion Strategy in myocardial infarction and Anemia. N Engl J Med. 2023;389(26):2446–56. https://doi.org/10.1056/NEJMoa2307983.
Acknowledgements
Not applicable.
Funding
No applicable.
Author information
Authors and Affiliations
Contributions
A.A. played a pivotal role in conducting an extensive literature search and review. He meticulously collected and organized relevant research papers, books, and other scholarly sources. He identified key themes and trends, which formed the foundation for the article’s comprehensive literature review section.D.K. brought a critical analytical perspective to the review article. She was responsible for synthesizing the gathered literature, identifying gaps in existing research, and providing valuable insights into the current state of the field. D.K. also played a central role in structuring the article and ensuring that it flowed logically from one topic to the next.B.K. contributed significantly to the writing and editorial aspects of the review article. B.K. drafted all article sections, weaving together the insights from the literature to create a coherent narrative. She also played a crucial role in revising and refining the article for clarity, coherence, and overall quality.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Arynov, A., Kaidarova, D. & Kabon, B. Alternative blood transfusion triggers: a narrative review. BMC Anesthesiol 24, 71 (2024). https://doi.org/10.1186/s12871-024-02447-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02447-3