Abstract
Background
There is limited research on the combined use of propofol and esketamine for anesthesia induction during flexible laryngeal mask airway (FLMA) in pediatric patients, and the effective dosage of propofol for FLMA smooth insertion remains unclear. We explored the effective dose of propofol combined with intravenous esketamine for the smooth insertion of FLMA in two distinct age groups of preschool children.
Methods
This is a prospective, observer-blind, interventional clinical study. Based on age, preschool children scheduled for elective surgery were divided into group A (aged 1–3 years) and group B (aged 3–6 years). Anesthesia induction was started with intravenous administration of esketamine (1.0 mg.kg− 1) followed by propofol administration. The FLMA was inserted 2 min after propofol administration at the target dose. The initial dose of propofol in group A and group B was 3.0 mg.kg− 1 and 2.5 mg.kg− 1, respectively. The target dose of propofol was determined with Dixon’s up-and-down method, and the dosing interval of propofol was 0.5 mg.kg− 1. If there was smooth insertion of FLMA in the previous patient, the target dose of propofol for the next patient was reduced by 0.5 mg.kg− 1; otherwise, it was increased by 0.5 mg.kg− 1. The median 50% effective dose (ED50) for propofol was estimated using Dixon’s up-and-down method and Probit analysis, while the 95% effective dose (ED95) was estimated through Probit analysis. Vital signs and adverse events during induction were recorded.
Results
Each group included 24 pediatric patients. Using Dixon’s up-and-down method, the ED50 of propofol combined with esketamine for smooth insertion of FLMA in group A was 2.67 mg.kg− 1 (95%CI: 1.63–3.72), which was higher than that in group B (2.10 mg. kg− 1, 95%CI: 1.36–2.84) (p = 0.04). Using Probit analysis, the ED50 of propofol was calculated as 2.44 (95% CI: 1.02–3.15) mg.kg− 1 in group A and 1.93 (95% CI: 1.39–2.32) mg.kg− 1 in group B. The ED95 of propofol was 3.72 (95%CI: 3.07–15.18) mg.kg− 1 in group A and 2.74 (95%CI: 2.34–5.54) mg.kg− 1 in group B. In Group B, one pediatric patient experienced laryngospasm.
Conclusion
The effective dose of propofol when combined with intravenous esketamine for smooth insertion of FLMA in children aged 1–3 years is 2.67 mg.kg− 1, which is higher than that in children aged 3–6 years (2.10 mg. kg− 1).
Trial registration
Chinese Clinical Trial Registry Center (Registration Number: ChiCTR2100044317; Registration Date: 2021/03/16)
Similar content being viewed by others
Background
Laryngeal mask airway (LMA) is commonly used for airway management during pediatric anesthesia, and compared with tracheal intubation, it has the advantages of low risk of anesthesia complications, short anesthesia time, and relatively small airway stimulation. LMA is especially suitable for relatively short surgery and can avoid the administration of muscle relaxants during anesthesia [1, 2]. The flexible laryngeal mask airway (FLMA) has reliable ventilation and low airway adverse reactions and can be easily inserted into the pharynx, even without muscle relaxants [3]. In comparison to the classic LMA, the FLMA tube is more flexible and longer, enabling increased movement without the need for cuff rotation or compromising the seal against the larynx [4]. However, airway reflex caused by pharyngeal irritation during FLMA insertion may lead to FLMA insertion failure or displacement, which is associated with insufficient anesthetic depth. Therefore, appropriate anesthesia depth, which also reduces airway reflexes resulting from pharyngeal irritation, is a determining factor for improving the success rate of FLMA insertion [5, 6].
Intravenous anesthesia induction is the most commonly used induction method. Compared with inhalation induction, intravenous induction can reduce the incidence of perioperative adverse events in high-risk pediatric patients with respiratory distress [7], while avoiding the fear caused by the mask during inhalation induction and the impact of the pungent smell of inhaled anesthetics on pediatric patients [8]. Propofol, a widely used intravenous anesthetic for induction, has a rapid onset, can effectively inhibit airway reflex, and is often used for LMA insertion. However, high doses of propofol can lead to respiratory and cardiovascular depression [6]. Although the combination of propofol and opioids can reduce the dosage of propofol during LMA placement, it also increases the incidence of apnea [9]. Esketamine, an S-enantiomer of ketamine, is twice as potent as racemic ketamine, which can achieve more reliable sedation and analgesia with a relatively low risk of psychotomimetic and cognitive adverse effects than racemic ketamine [10]. It can maintain airway tension and hemodynamic stability and is an ideal anesthetic induction agent [11, 12]. In addition, low-dose esketamine may reduce the incidence of anesthesia-related respiratory depression by increasing ventilatory CO2 sensitivity [13]. In pediatric studies, esketamine has been shown to reduce postoperative pain in children [14] and decrease the occurrence of emergency agitation [15]. Currently, the safety and efficacy of propofol in combination with esketamine for sedation and gastroscopy in children have been reported [16, 17]. However, there is no clear evidence of the effective dose of propofol in combination with esketamine for FLMA insertion during anesthesia induction in children.
Many studies use the age of 3 as the criteria for grouping and selecting study participants [18,19,20]. Additionally, these studies have verified the variations in effective doses of intravenous anesthetics among pediatric patients in different age groups [18,19,20]. Propofol, being lipophilic, has its distribution volume associated with the body’s fat content. The fat content in children decreases progressively with age, potentially impacting the effective dose of propofol [12]. Consequently, the present study narrowed down the age range and specifically enlisted preschool children as study participants. Moreover, we conducted a comparison between preschool children under 3 years old and those over 3 years old, without the utilization of muscle relaxants, to ascertain the effective propofol dose required for the smooth insertion of FLMA in combination with esketamine. We hypothesize that the effective dose of propofol for smooth insertion of FLMA may vary in preschool children of different ages, and the effective dose of propofol for children younger than 3 years old may be higher than that for preschool children older than 3 years old.
Methods
Study design and ethics
This is a prospective, observer-blind, interventional clinical study. It was registered before patient enrollment at the Chinese Clinical Trial Registry Center (Registration Number: ChiCTR2100044317; Registration date: 16/03/2021). This study was approved by the Ethics Committee of the Qilu Children’s Hospital of Shandong University (IRB: QLET-ITB/P-2,021,031). All methods were performed following the Declaration of Helsinki. Written informed consent was obtained from the parents or other legal guardians of all enrolled children participating in the trial.
Participants
Pediatric patients who were scheduled for elective surgery at the Qilu Children’s Hospital of Shandong University from March 2021 to July 2022 were recruited. Inclusion criteria: (1) preschool children aged from 1 to 6 years old; (2) patients with American Society of Anesthesiologists (ASA) physical status of I or II; (3) patients who received minor surgeries (such as skin lumpectomy, debridement, and suturing, and injection therapy for skin hemangioma) and had spontaneous breathing under FLMA ventilation and general anesthesia. Exclusion criteria: (1) patients with body weight less than 10 Kg; (2) obese patients with body mass index > 35 kg. m− 2; (3) patients with reactive airway disease; (4) patients with evident difficult airway (Mallampati III or IV, micro mandible, limited mouth opening, limited neck movement, etc.); (5) patients with obstructive sleep apnea-hypopnea syndrome; (6) patients with risk of gastroesophageal reflux; (7) patients with major organ diseases (such as kidney, liver, and heart diseases); (8) patients with abnormal laboratory examination results; (9) patients who were allergic to either propofol or esketamine. Based on the inclusion and exclusion criteria, pediatric patients were randomly selected. Based on the cut-off age of 3 years, the enrolled children were grouped into Group A (age older than 1 year old and younger than or equal to 3 years old) and Group B (age older than 3 years and younger than 6 years).
Anesthesia protocol
A standardized anesthetic regimen was used in all participants. No premedication was administered. Peripheral venous access was made in the ward. All patients fasted for at least 6 h for solids and 2 h for clear fluids and were transferred to the operating room. The electrocardiography, noninvasive blood pressure, and peripheral oxygen saturation (SpO2) of each patient were monitored. Children were placed in a supine position with a thin pillow under the shoulder, and the head was kept slightly backward to maintain airway patency. Pure oxygen was inhaled and the oxygen flow rate was 2 L/min by mask ventilation to ensure oxygen supply during anesthesia induction. Glycopyrrolate (5 μg.kg− 1) and ondansetron (0.1 mg.kg− 1) were intravenously administrated to reduce oral secretions and the risk of postoperative vomiting, respectively. Then, anesthesia induction was initiated by intravenous administration of esketamine 1.0 mg.kg− 1 (lasting more than 30 s), followed by slow administration (more than 1 min) of the target dose of propofol. At 2 min after the propofol injection, the mask was gently removed and mask ventilation was terminated. The FLMA of corresponding sizes was inserted using the standard method. The size of FLMA was selected according to children’s body weight: size 2.0 for 10–20 Kg and 20 Kg; and, size 2.5 for 20-30Kg. The FLMA cuff was deflated and lubricated dorsally with water-based jelly. During FLMA insertion, the children were kept in a neck-flexed-and-head-extended position. The tip of the FLMA was pressed against the hard palate, and the FLMA was slowly pushed back along the midline of the palate. The insertion of FLMA was stopped when resistance was felt, and then the anesthesia machine was connected with a semi-open anesthesia circuit. The FLMA cuff was inflated until the cuff pressure reached 40 cmH2O according to a manometer. The respiratory rhythm of the children and the waveform of end-tidal CO2 on the anesthesia machine were immediately monitored for 1 min. When stable spontaneous breathing was achieved, the FLMA was fixed. All FLMA insertion was performed by the same anesthesiologist with experience in FLMA insertion more than 500 times.
During and after the insertion of FLMA, if there were minimal physical movements or weak hemodynamic fluctuations, additional propofol (2 mg.kg− 1) was slowly injected. If there were significant physical movements or hemodynamic fluctuations, the FLMA was immediately removed. Subsequently, anesthesia was deepened by administering inhaled sevoflurane through a mask before reinserting the FLMA.
Anesthesia was maintained with a combination of inhaled sevoflurane and fentanyl. Spontaneous breathing was maintained during surgery. The body temperature of all pediatric patients was continuously monitored during surgery, and blankets were used to maintain body temperature in non-surgical areas. After the surgery, the FLMA was removed and the children were transferred to the post-anesthesia care unit for monitoring.
Data records
Heart rate (HR) and mean arterial pressure (MAP) before induction (baseline) were recorded by the anesthesiologist performing the FLMA insertion. HR and MAP before FLMA insertion, and at 1 min after FLMA insertion were recorded by a different anesthesiologist who was blinded to study grouping and drug administration. The blinded anesthesiologist was not present in the operating room before induction and was called into the operating room by a nurse immediately after the administration of propofol, to monitor the anesthesia induction. The blinded anesthesiologist also observed the response of children during FLMA insertion, the respiratory condition at 1 min after insertion, and any adverse events that occurred during induction.
Based on previous studies [21, 22], smooth insertion of FLMA was defined as follows: the FLMA was easily inserted without significant resistance; there was no physical movement, coughing, swallowing, breath holding, apnea, and laryngospasm; there was stable spontaneous breathing at 1 min after connecting the anesthesia machine; and the fluctuation of HR and MAP during and at 1 min after FLMA insertion was less than 20% of those before insertion. Otherwise, it was considered an unsmooth FLMA insertion.
Adverse events during induction were recorded: hypoxemia (SpO2 < 90% for more than 1 min), hypotension (MAP below 20% of baseline), tachycardia (HR more than 180 beats/min), bradycardia (for children aged 1–3 years: HR less than 80 beats per min; for children aged 3–6 years: HR less than 65 beats per min) [23]. The adverse events during and after surgery were also recorded.
Dixon’s up-and-down method
Following intravenous administration of esketamine, the target dose of propofol was determined by the response of the previous patient to the insertion of FLMA using Dixon’s up-and-down method [24]. The initial dose of propofol administered to the first child aged 1–3 years and 3–6 years was 3.0 and 2.5 mg.kg− 1, respectively, and the step size of propofol was 0.5 mg.kg− 1. If FLMA was inserted smoothly in the previous child, the target dose of propofol in the subsequent child was set at 0.5 mg.kg− 1 lower than the previous child. If there was unsmooth FLMA insertion in the previous child, the target dose of propofol in the subsequent child was set at 0.5 mg.kg− 1 higher than the previous child. A single dose was obtained from each patient, and the sequence was continued until seven crossover pairs (unsmooth FLMA insertion to smooth FLMA insertion) were reached in each group.
Sample size
Dixon’s methodology recommends continuing the experiment until a minimum of four crossovers are reached [24, 25]. Paul et al. revealed that the inaccuracy of Dixon’s up-and-down method was minimized as the number of crossover pairs increased, but the improvement diminished as the number of crossover pairs exceeded six [26]. According to a similar study [25], our study enrolled patients until seven crossover pairs were obtained.
Statistical analysis
All data were processed by IBM SPSS Statistics 26.0 (IBM SPSS Statistics for Windows, Version 26.0, Armonk, NY, IBM Corp; 2017). A 2-sided p-value less than 0.05 was considered statistically significant. Continuous variables with normal or non-normal distribution were presented as the mean ± standard deviation (SD) or median (interquartile range), respectively. For analysis of the HR and MAP recorded at various time points within the group, repeated-measures ANOVA with Bonferroni correction was used. According to Dixon’s up-and-down method [24], the ED50 of propofol enabling smooth FLMA insertion was determined by calculating the mean of the midpoint doses of seven independent pairs of children who experienced a crossover from unsmooth insertion to smooth insertion. The ED50 values between groups were compared using Student’s t-test. The data were also assessed by probit regression analysis to obtain the ED50 and 95% effective dose (ED95) of propofol for smooth FLMA insertion.
Results
Baseline characteristics of participants
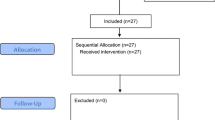
The flowchart for patient enrollment is shown in Fig. 1. A total of 54 children were initially recruited. After screening, 6 children were excluded, and 48 children were included in the final analysis, including 24 cases in Group A and 24 in Group B. The baseline characteristics are shown in Table 1.
The primary outcome: ED50 and ED95 of propofol for smooth FLMA insertion
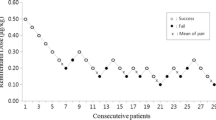
Using Dixon’s up-and-down method, the ED50 of propofol for smooth insertion of FLMA in group A was 2.67 (95% CI: 1.63–3.72) mg.kg− 1, which was significantly higher than that in group B (2.10, 95% CI: 1.36–2.84) mg.kg− 1 (P = 0.04) (Table 2). The sequences of smooth and unsmooth FLMA insertion in group A and group B are shown in Fig. 2. Probit regression analysis showed that the ED50 and ED95 of propofol for smooth insertion of FLMA in group A were 2.44 (95% CI: 1.02–3.15) mg.kg− 1and 3.72 (95% CI: 3.07–15.18) mg.kg− 1, respectively (Table 2). Meanwhile, the ED50 and ED95 of propofol in group B were 1.93 (95% CI: 1.39–2.32) mg.kg− 1 and 2.74 (95% CI: 2.34–5.54) mg.kg− 1, respectively (Table 2). The dose-response curve of propofol in each group is shown in Fig. 3.
The secondary outcomes
Hemodynamic parameters during induction at each time point are shown in Table 3. In group A, the HR at 1 min after FLMA insertion was significantly higher than that before FLMA insertion (P = 0.011). In group B, the HR at 1 min after FLMA insertion was significantly higher than the baseline value (P = 0.005) and that before FLMA insertion (P < 0.001). During anesthesia induction, there were no adverse events (such as tachycardia, bradycardia, and hypotension) in each group. One child in group A experienced transient hypoxemia (less than 1 min, minimum SpO2: 84%) resulting from weakened respiratory activity. After mandibular lifting and increased oxygen flow, hypoxemia was rapidly relieved, and spontaneous respiration was restored. One child in group B suffered laryngospasm after insertion of FLMA, which was relieved by the injection of propofol (2 mg.kg− 1) and mask ventilation without other additional intervention. Physical movement and increase of HR and MAP were the main manifestations of unsmooth FLMA insertion in both groups (Table 4).
All children remained stable during surgery. In the post-anesthesia care unit, one child in group A and one child in group B developed agitation. One child in group A had excessive oral secretions. After the adverse reactions disappeared, the patients were transferred to the ward. One child in group B had a sore throat. No other adverse reactions were observed.
Discussion
In this study, we used Dixon’s up-and-down method to determine the effective dose of propofol combined with intravenous administration of esketamine 1 mg.kg− 1 for smooth insertion of FLMA in two distinct age groups of preschool children. We found that the effective dose of propofol in children aged 1–3 years was higher than that in children aged 3–6 years.
Several studies have shown that the effective dose of anesthetics in children is age-related, and the younger the child, the greater the effective dose required [19, 27]. A previous study focusing on pediatric sedation in emergency departments showed that children under 6 years old required increased doses of ketamine for sedation than children over 6 years old, and children under 3 years old required more ketamine than children over 3 years old [19]. Khalila et al. showed that the older the children, the greater the amount of propofol needed for satisfactory sedation during gastroscopy [27]. Consistently, in this study, when combined with the same dose of esketamine, the effective dose of propofol for smooth insertion of FLMA was higher in children aged 1–3 years compared to those aged 3–6 years. This result was consistent with our hypothesis.
The distribution volume of drugs is an important determinant of the loading dose. Propofol is fat-soluble and its pharmacokinetics are affected by the fat content [28]. The fat content of children gradually decreases with the increase of age [18, 29]. Therefore, the higher distribution volume of propofol in children is the reason for higher loading doses of propofol in children than in adults [18]. The study by Lifshitz et al. [30] showed that the fat mass percentage of 2-6-year-old preschool children gradually decreased with age, although there were no significant statistical differences. Perhaps even slight changes in fat content in preschool children may lead to significant differences in the distribution volume, resulting in different effective doses of propofol for smooth insertion of FLMA in children aged 3–6 years compared to those aged 1–3 years. This could be the reason for the different effective doses of propofol in the two age groups observed in this study.
The ED50 is a common way to measure drug effects, while ED95 is more clinically relevant. Although Dixon’s up-and-down method is a commonly used approach to assess the ED50 of drugs, it is unable to obtain the ED95 [16]. Therefore, in this study, we used Probit regression analysis to determine the ED95 of propofol, which showed that the ED95 of propofol for smooth insertion of FLMA in children aged 1–3 years was also higher than that in those aged 3–6 years.
Although the administration of propofol (2.0-2.5 mg.kg− 1) alone can provide satisfactory conditions for LMA insertion in adults, there are also clinical manifestations caused by inadequate anesthesia depth, such as choking, limb movement, and swallowing [31]. In this study, considering the synergistic effect of propofol and esketamine on anesthesia depth during FLMA insertion, the initial dose of propofol in children aged 3–6 years old was set at 2.5 mg.kg− 1. Considering relatively higher fat content in children aged 1–3 years old and based on the results of our pilot study, the initial dose of propofol was set at 3.0 mg.kg− 1, which was higher than that in children aged 3–6 years old. We found that although the ED95 and initial dosage of propofol varied among different groups, the differences were not significant, especially for children aged 3–6 years, which further verified the effectiveness of the initial dose of propofol. In addition, the ED50 obtained by Probit regression analysis was close to but not the same as the ED50 obtained by Dixon’s up-and-down method, which has also been reported in other similar studies [32, 33].
Abedini et al. suggested that the appropriate time for LMA insertion with lower complications and rapid placement was 15 s after propofol administration [5]. In this study, FLMA insertion was performed at 2 min after the administration of propofol, but not 15 s. This difference may be explained by the following two reasons. Firstly, the drug regimen for anesthesia induction was different between our study and the study by Abedini et al. Abedini et al. used a combination of propofol, midazolam, and fentanyl for induction, which may induce anesthesia more rapidly and in a shorter time. Secondly, propofol takes effect in 40 s, and in this study, the FLMA was inserted 2 min after propofol administration to ensure the full effect of propofol. Meanwhile, the potential risks of esketamine combined with propofol on respiration and circulation could be observed during this time. In this study, there was no reported hypoxemia caused by prolonged asphyxia and no cardiorespiratory depression such as bradycardia and hypotension. The HR after FLMA insertion was higher than that before FLMA insertion, which was related to the design of this study. Although the stimulation of FLMA insertion resulted in increased HR, there was no tachycardia in both groups during anesthesia induction.
Esketamine can maintain hemodynamic stability during anesthesia induction [34, 35]. This study also showed that esketamine combined with propofol was a safe and effective regimen for anesthesia induction in children. Esketamine stimulates the sympathetic nerve system, which balances the cardiorespiratory depression caused by propofol. Although esketamine has less effect on the central respiratory drive, it can also produce respiratory depression when administered at high doses or rapidly [11]. In this study, we conducted slow injections of esketamine and propofol, which may also decrease the risk of respiratory depression.
In addition, we found that pretreatment with esketamine might alleviate the injection pain of propofol (data not shown). After the injection of propofol, children in each group did not show any reactions similar to pain stimuli such as involuntary limb movement of the injection site and rapid increase of HR. Fu et al. have also confirmed that the intravenous injection of low-dose esketamine (0.15 mg.kg− 1) in adults could reduce the incidence of propofol injection pain [36]. They considered that the main reason was the peripheral local anesthetic action of esketamine in the vascular endothelium, rather than the central analgesic effect.
There were several limitations in this study. Firstly, the anesthetic dose or depth of anesthesia for the insertion of different types of LMA is different [37, 38]. This study only focused on FLMA and may not be completely applicable to other types of LMA. Secondly, ketamine or esketamine can increase the bispectral index value of patients and thus interfere with the reliability of BIS [39]. Therefore, we did not use BIS to assess the depth of anesthesia when FLMA was inserted. To ensure an effective anesthesia depth, we used the maximum induction dose of esketamine. Thirdly, we did not use the target-controlled infusion to guide the propofol dose during induction. Although target-controlled infusion is becoming more and more popular among children, the accuracy of pharmacokinetic models in children still needs to be improved [18]. Finally, each group of children received identical doses of esketamine, which may have varying efficacy in children of different ages, thus impacting the effective propofol dosage. Therefore, in this study, the effective dose of propofol for each group of children was the effective dose when intravenously injected with 1.0 mg.kg− 1 of esketamine.
Conclusion
The effective dose of propofol combined with intravenous esketamine for smooth insertion of FLMA was different in preschool children of different age groups. In detail, the effective dose of propofol in children aged 1–3 years was 2.67 mg.kg− 1, which was higher than that in those aged 3–6 years (2.10 mg. kg− 1). Propofol combined with esketamine is a safe and effective medication regimen for the smooth insertion of FLMA and can maintain hemodynamic and respiratory stability during FLMA insertion in preschool children.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- ED50 :
-
50% effective dose
- ED95 :
-
95% effective dose
- FLMA:
-
Flexible laryngeal mask airway
- HR:
-
Heart rate
- LMA:
-
Laryngeal mask airway
- MAP:
-
Mean arterial pressure
- SD:
-
Standard deviation
- SpO2 :
-
Peripheral oxygen saturation
References
Nevescanin A, Vickov J, Elezovic Baloevic S, Pogorelic Z. Laryngeal mask airway versus tracheal intubation for laparoscopic hernia repair in children: analysis of respiratory complications. J Laparoendoscopic Adv Surg Techniques Part A. 2020;30(1):76–80.
Dumas GA, Bryant AS, Ibey J, Long JA, Vicinanzo MG, Boyd GL. Safety comparison of laryngeal mask use with endotracheal intubation in patients undergoing dacryocystorhinostomy surgery. Ophthal Plast Reconstr Surg. 2018;34(4):324–8.
Liu F, Xi C, Cui X, Wang G. Efficacy and safety of flexible laryngeal mask ventilation in otologic surgery: a retrospective analysis. Risk Manage Healthc Policy. 2022;15:945–54.
van Zundert TC, Cattano D. The LMA-flexible: time to celebrate a unique extraglottic airway device. Minerva Anestesiol. 2017;83(9):895–8.
Abedini N, Parish M, Farzin H, Pourfathi H, Akhsham M. The determination of an appropriate time for placement of the classic laryngeal mask airway in patients undergoing general anesthesia. Anesthesiology and pain Medicine. 2018;8(2):e64427.
Yoo JY, Kwak HJ, Kim YB, Park CK, Lee SY, Kim JY. The effect of dexmedetomidine pretreatment on the median effective bolus dose of propofol for facilitating laryngeal mask airway insertion. J Anesth. 2017;31(1):11–7.
Ramgolam A, Hall GL, Zhang G, Hegarty M, von Ungern-Sternberg BS. Inhalational versus IV induction of anesthesia in children with a high risk of perioperative respiratory adverse events. Anesthesiology. 2018;128(6):1065–74.
Zielinska M, Holtby H, Wolf A. Pro-con debate: intravenous vs inhalation induction of anesthesia in children. Paediatr Anaesth. 2011;21(2):159–68.
Zaballos M, Bastida E, Agusti S, Portas M, Jimenez C, Lopez-Gil M. Effect-site concentration of propofol required for LMA-supreme insertion with and without remifentanil: a randomized controlled trial. BMC Anesthesiol. 2015;15:131.
Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet. 2016;55(9):1059–77.
Trimmel H, Helbok R, Staudinger T, Jaksch W, Messerer B, Schochl H, Likar R. S(+)-ketamine: current trends in emergency and intensive care medicine. Wiener Klinische Wochenschrift. 2018;130(9–10):356–66.
Barrett W, Buxhoeveden M, Dhillon S. Ketamine: a versatile tool for anesthesia and analgesia. Curr Opin Anaesthesiol. 2020;33(5):633–8.
Jonkman K, van Rijnsoever E, Olofsen E, Aarts L, Sarton E, van Velzen M, Niesters M, Dahan A. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117–27.
Liu F, Kong F, Zhong L, Wang Y, Xia Z, Wu J. Preoperative esketamine alleviates postoperative pain after endoscopic plasma adenotonsillectomy in children. Clin Med Res. 2023;21(2):79–86.
Li Q, Fan J, Zhang W. Low-dose esketamine for the prevention of emergency agitation in children after tonsillectomy: a randomized controlled study. Front Pharmacol. 2022;13:991581.
Zheng XS, Shen Y, Yang YY, He P, Wang YT, Tao YY, Zheng JJ, Sun Y. ED(50) and ED(95) of propofol combined with different doses of esketamine for children undergoing upper gastrointestinal endoscopy: a prospective dose-finding study using up-and-down sequential allocation method. J Clin Pharm Ther. 2022;47(7):1002–1009.
Eich C, Verhagen-Henning S, Roessler M, Cremer F, Cremer S, Strack M, Russo SG. Low-dose S-ketamine added to propofol anesthesia for magnetic resonance imaging in children is safe and ensures faster recovery–a prospective evaluation. Paediatr Anaesth. 2011;21(2):176–8.
Anderson BJ, Bagshaw O. Practicalities of total intravenous anesthesia and target-controlled infusion in children. Anesthesiology. 2019;131(1):164–85.
Forrester KR, Thomas SM, Gupta NK, Karumuri M, Gerard JM. Repeat intravenous ketamine dosing in children undergoing emergency department procedural sedation. J Emerg Med. 2019;56(1):1–6.
Zhang W, Fan Y, Zhao T, Chen J, Zhang G, Song X. Median effective dose of intranasal dexmedetomidine for rescue sedation in pediatric patients undergoing magnetic resonance imaging. Anesthesiology. 2016;125(6):1130–5.
Rahmat Ameen Noorazyze NAN, Nor NM, Zain JM, Mohamad Yusof A, Yong LC. Intravenous fentanyl vs. topical lignocaine for ProSeal™ laryngeal mask airway insertion with propofol induction. Front Med. 2022;9:979275.
Joshi GP, Kamali A, Meng J, Rosero E, Gasanova I. Effects of fentanyl administration before induction of anesthesia and placement of the laryngeal mask airway: a randomized, placebo-controlled trial. J Clin Anesth. 2014;26(2):136–42.
Baruteau AE, Perry JC, Sanatani S, Horie M, Dubin AM. Evaluation and management of bradycardia in neonates and children. Eur J Pediatrics. 2016;175(2):151–61.
Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;15(1):47–50.
Zaballos M, Bastida E, Agustí S, Portas M, Jiménez C, López-Gil M. Effect-site concentration of propofol required for LMA-supreme™ insertion with and without remifentanil: a randomized controlled trial. BMC Anesthesiol. 2015;15:131.
Paul M, Fisher DM. Are estimates of MAC reliable? Anesthesiology. 2001;95(6):1362–70.
Khalila A, Shavit I, Shaoul R. Propofol sedation by pediatric gastroenterologists for endoscopic procedures: a retrospective analysis. Front Pead. 2019;7:98.
Chidambaran V, Costandi A, D’Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015;29(7):543–63.
Leskinen T, Eloranta AM, Tompuri T, Saari A, Ollila H, Makela J, Niinikoski H, Lagstrom H. Changes in body composition by age and obesity status in preschool-aged children: the STEPS study. Eur J Clin Nutr. 2021;75(1):57–65.
Lifshitz F, Hecht JP, Bermudez EF, Gamba CA, Reinoso JM, Casavalle PL, Friedman SM, Rodriguez PN. Body composition analysis by dual-energy X-ray absorptiometry in young preschool children. Eur J Clin Nutr. 2016;70(10):1203–9.
Siddik-Sayyid SM, Aouad MT, Taha SK, Daaboul DG, Deeb PG, Massouh FM, Muallem MA, Baraka AS. A comparison of sevoflurane-propofol versus sevoflurane or propofol for laryngeal mask airway insertion in adults. Anesth Analg. 2005;100(4):1204–9.
He L, Wang X, Zhang XF, Tang SR. Effects of different doses of remifentanil on the end-tidal concentration of sevoflurane required for tracheal intubation in children. Anaesthesia. 2009;64(8):850–5.
Zhang B, Wang J, Li M, Qi F. Minimum alveolar concentration of sevoflurane with cisatracurium for endotracheal intubation in neonates. Med Sci Monitor: Int Med J Experimental Clin Res. 2019;25:7982–8.
Tu W, Yuan H, Zhang S, Lu F, Yin L, Chen C, Li J. Influence of anesthetic induction of propofol combined with esketamine on perioperative stress and inflammatory responses and postoperative cognition of elderly surgical patients. Am J Translational Res. 2021;13(3):1701–9.
Li J, Wang Z, Wang A, Wang Z. Clinical effects of low-dose esketamine for anaesthesia induction in the elderly: a randomized controlled trial. J Clin Pharm Ther. 2022;47(6):759–66.
Fu D, Wang D, Li W, Han Y, Jia J. Pretreatment with low-dose esketamine for reduction of propofol injection pain: a randomized controlled trial. Pain Res Manag. 2022;2022:4289905.
Choi JB, Kwak HJ, Lee KC, Lee SR, Lee SY, Kim JY. Comparison of remifentanil EC50 for facilitating i-gel and laryngeal mask airway insertion with propofol anesthesia. J Anesth. 2016;30(3):377–83.
Ghai B, Sethi S, Bansal D, Ram J. Optimum sevoflurane concentration for I-gel insertion in unpremedicated children. J Clin Anesth. 2015;27(8):627–31.
Wang J, Hu W, Zhao X, Ren W, Huang X, Zhang B. Sedative effect and safety of different doses of S-ketamine in combination with propofol during gastro-duodenoscopy in school-aged children: a prospective, randomized study. BMC Anesthesiol. 2022;22(1):346.
Acknowledgements
Not applicable.
Funding
This study was funded by the Special Fund for Comfort Medical Research of the Shandong Medical Association.
Author information
Authors and Affiliations
Contributions
Bin Zhang designed the study, recorded, analyzed, and interpreted the data, and wrote the paper. Mingzhuo Li helped record and analyze the data and write the paper. Yuejiao Han and Xianliang Zhao helped record the data and searched the literature. Chunhong Duan and Junxia Wang designed the study, collected the fund, and revised the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was registered before patient enrollment at the Chinese Clinical Trial Registry Center (Registration Number: ChiCTR2100044317). This study was approved by the Ethics Committee of the Qilu Children’s Hospital of Shandong University (IRB: QLET-ITB/P-2,021,031). All methods were performed following the Declaration of Helsinki. Written informed consent was obtained from the parents or other legal guardians of all enrolled children participating in the trial.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, B., Li, M., Han, Y. et al. Effective dose of propofol combined with intravenous esketamine for smooth flexible laryngeal mask airway insertion in two distinct age groups of preschool children. BMC Anesthesiol 24, 50 (2024). https://doi.org/10.1186/s12871-024-02421-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02421-z