Abstract
Background
The influence of anesthesia techniques on cancer recurrence and metastasis following oncological surgery is a topic of growing interest. This meta-analysis investigates the potential effects of regional anesthesia (RA), either independently or combined with general anesthesia (GA), on these outcomes.
Methods
We performed an extensive search across PubMed, Embase, and the Cochrane Library databases. The primary outcome was cancer recurrence, while the secondary outcomes were local recurrence and distant metastasis. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated by utilizing random-effects models. The Newcastle-Ottawa Scale (NOS) was used for quality assessment of observational studies, the Cochrane Risk of Bias Tool for Randomized Trials (Rob 2.0) was used for randomized controlled trials, and all the outcomes were assessed by using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE).
Results
This study included 32 studies comprising 24,724 cancer patients. RA, either alone or in combination with GA, was significantly associated with reduced cancer recurrence compared to GA alone (OR = 0.82; 95% CI = 0.72 to 0.94; p < 0.01). This association remained significant for prostate cancer patients in subgroup analyses (OR = 0.71; 95% CI = 0.51 to 0.98; p = 0.04) and in the context of epidural anesthesia combined with GA. However, there were no significant associations noted for local recurrence or distant metastasis.
Conclusions
This meta-analysis provides evidence that RA, used alone or adjunctively with GA, is associated with a lower risk of cancer recurrence, particularly in patients with prostate cancer. However, no significant effects were observed on local recurrence or distant metastasis. Further prospective studies should be conducted to clarify this important issue.
Similar content being viewed by others
Background
Cancer ranks as a leading cause of mortality for diseases worldwide, with 10 million cancer deaths worldwide in 2020 [1]. Surgical resection is a mainstay therapy for cancer. Surgery can’t be conducted without anesthesia, but different anesthesia techniques could affect the recurrence and metastasis of cancer after surgery [2,3,4]. For example, the addition of regional anesthesia (RA) to general anesthesia (GA) is proven to be beneficial to postoperative oncological outcomes compared with GA alone among patients with prostate cancer [2] or breast cancer [3]. Besides, RA alone (spinal anesthesia) was associated with a lower 5-year tumor recurrence rate compared with GA after transurethral resection of bladder tumors [5].
RA includes spinal anesthesia, epidural anesthesia, local anesthesia infiltration, and nerve block. RA can largely attenuate the neuroendocrine stress response to surgery by reducing the catecholamine levels and minimizing immunosuppression [6], which can not only provide effective pain control but also reduce exposure to opioids; in return, it reduces the potential effects of the latter on postoperative prognosis [7, 8]. Furthermore, similar findings have been shown in animal models [9, 10].
However, studies on the impact of RA on cancer recurrence and metastasis yielded negative and positive results. For example, some studies have reported that RA with or without GA was not significantly associated with a lower incidence of cancer recurrence and metastasis rate than GA in cancer resection surgery [11,12,13,14]. Some meta-analyses [15,16,17] investigated the impact of RA with or without GA on cancer recurrence and metastasis, and the results indicated that RA with or without GA did not reduce cancer recurrence and metastasis rate after surgery. These results of meta-analysis should be interpreted with caution due to the low level of evidence, such as a limited number of studies (N ≤ 10), no adjusted for different types of cancer (breast cancer, colorectal cancer, and prostate cancer), and cancer recurrence (local recurrence and distant metastasis).
Given existing individual studies [2,3,4], and contrasting evidence from previous meta-analyses [15,16,17], the present study aimed to conduct a comprehensive meta-analysis to investigate the impact of RA on the incidence of cancer recurrence and metastasis rate after surgery. To provide more detailed insights, we also conducted subgroup analyses based on cancer types [2,3,4]and cancer recurrence types [15,16,17]. Based on results presented in existing literature, we hypothesize that regional anesthesia (RA) may have an impact on cancer recurrence and metastasis rate after surgery, and this impact may vary depending on the type of cancer or the type of cancer recurrence.
Methods
The meta-analysis was performed according to the Preferred Reporting Item for Systematic Reviews and Meta-analysis (PRIMA) [18]. This study is registered with the PROSPERO registry, number CRD42022370267.
Search strategy
Literature was retrieved through PubMed, Embase, and the Cochrane Library (updated to August 29, 2022) using the following keywords: neoplasms, cancer, tumor, local anesthesia, regional anesthesia, epidural anesthesia, recurrence, metastasis, prognosis, and survival. Besides, we searched the reference list of relevant reviews and eligible studies to identify additional studies.
Inclusion and exclusion criteria
Inclusion and exclusion criteria in the present study were based on the Population, Intervention, Comparator, Outcomes, and Study designs (PICOS) structure.
-
1.
Population: patients who underwent any type of cancer resection surgery. Adults only.
-
2.
Intervention: a comparison of the use of regional anesthesia, regardless of types of RA.
-
3.
Comparator: versus general anesthesia, regardless of volatile anesthesia or total intravenous anesthetic agents.
-
4.
Outcome: studies reported rates of cancer recurrence or metastasis after surgery.
-
5.
Study design: any prospective or retrospective cohort, case-control observational studies, and randomized controlled trials (RCTs).
Besides, Reviews, meta-analyses, conference abstracts, animal trials, and studies that did not provide sufficient data were excluded.
Data extraction
Two independent reviewers extracted the essential data. We extracted the following data from each eligible study: the first author, publication year, study design, cancer type, sample size and the number of patients assigned in each group, RA techniques, median follow-up time, whether propensity score matching or not and the information of methodological quality. Whenever discrepancies in data extraction occurred, the consensus was achieved through discussion or consulting a third reviewer.
Outcomes
The primary outcome was defined as post-operative cancer recurrence or metastasis rate as reported by the study authors. Cancer recurrence is defined as the emergence of a new tumor at or near the original tumor site after treatment. Depending on the location, it is classified into local recurrence and distant metastasis. Local recurrence refers to the reappearance of cancer at or near the original site. Distant metastasis refers to the spread of cancer cells from the original site to other parts of the body. The secondary outcomes included subgroup analyses based on cancer types, cancer recurrence types, anesthetic technique, and study design [19].
Quality assessment
Quality assessment of the included studies will be carried out independently by two reviewers and any disagreements will be resolved through discussion or consultation with a third reviewer.
The Newcastle-Ottawa Scale (NOS) was used to assess the methodological quality of the observational studies [20]. NOS contains three dimensions, including patient selection (three items), comparability of the two study arms, and assessment of the outcomes (two items). The total points ranged from 0 to 9 stars. Generally, 0–4 points were considered poor quality, 5–6 points as moderate quality, and 7–9 points as high quality.
The Cochrane Collaboration’s tool for assessing the risk of bias (ROB-2) [21]. The following five dimensions are included: bias arising from the randomization process, bias due to deviation from the intended intervention, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of reported results. Each of these aspects will be labeled as high risk, some concern, and low risk, depending on the degree of match between the facts presented in the eligible studies and the assessment criteria. The overall level will be labeled as low risk, some concern, or high risk, depending on the results of the assessment in each of the five categories. Any disagreement between the two authors on the risk of bias assessment will be resolved through discussion to reach an agreement.
The certainty of evidence for each study was graded according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group method [22]. This method considers the study design, risk of bias, inconsistency, indirectness, imprecision, and other factors to grade the level of certainty as high, moderate, low, or very low.
Statistical analysis
All analyses were performed using the STATA SE 14.0 software (StataCorp, College Station, Texas, USA). The odds ratio (OR) and corresponding 95% confidence interval (CI) were used to summarize the results. The Q-test and I2 statistic were used to describe heterogeneity among studies. If the I2 value was over 50%, indicating significant heterogeneity, a random-effects model was used. Conversely, a fixed-effects model was utilized when the I2 value was 50% or less. I2 values of > 75%, 25-75% and < 25% were defined as high, moderate, and low heterogeneity, respectively. Subgroup analysis was used to explore possible sources of heterogeneity. Sensitivity analysis by leave-one-out method was used to test the robustness of the results. Publication bias was assessed using funnel plots and Egger’s test, and if significant bias was present, trim-and-fill analysis was used to account for any potential missing studies. P < 0.05 indicated statistical significance.
Results
Study selection
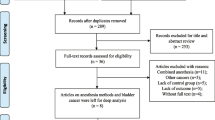
A total of 7370 studies were retrieved as potentially relevant literature reports through the initial searches in PubMed, Embase, and the Cochrane Library databases, and 2093 duplicate studies were deleted. Then, 5233 kinds of literature were excluded after reviewing the title or abstract. After retrieving 44 full-length articles, ultimately, 27 studies [2, 3, 5, 11,12,13,14, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] were eligible for data extraction and meta-analysis. Besides, five studies [4, 43,44,45,46] were in our meta-analysis by manual search. The study selection process is presented in Fig. 1.
Study characteristics
The characteristics of the eight included studies are summarized in Table 1. In our study, 32 articles were included, involving 24,724 cancer patients. The sample size of each included study ranged from 91 to 5960. Of all the included studies, 24 were retrospective cohort studies, five were RCTs, two were prospective cohort studies, and one was a cross-sectional study. Cancer types included bladder cancer, breast cancer, colorectal cancer, esophageal cancer, gastric cancer, hepatocellular carcinoma, ovarian cancer, and prostate cancer. A total of 12 studies investigated the association between only RA and GA on cancer recurrence and metastasis rate, and 20 studies examined the association between RA + GA and GA on cancer recurrence and metastasis rate.
Quality assessment
Observational studies were assessed using the Newcastle-Ottawa Scale, and all included studies were of acceptable quality.20 studies were considered to be of high quality and 7 were considered to be of moderate quality (Supplementary Table 2). For randomized controlled studies, methodological quality was assessed using the Cochrane Collaboration’s Risk of Bias Assessment Tool (ROB 2.0). All RCTs were considered to be at low risk except one [23] (Supplementary Table 3). Each study was assessed using GRADE and the results are shown in Table 1.
Cancer recurrence
Twenty-nine studies provided suitable data for cancer recurrence. The pooled OR of cancer recurrence showed a significant difference between RA with or without GA and GA groups (OR = 0.82; 95%CI = 0.72 to 0.94; I2 = 58.9%) (Fig. 2).
Subgroup analysis reported that significant associations were also observed in prostate cancer (OR = 0.71; 95%CI = 0.51 to 0.98; I2 = 70.2%) (Table 2). Furthermore, significant associations were also observed in subgroup analysis based on anesthesia technique (epidural anesthesia with GA: OR = 0.87; 95%CI = 0.79 to 0.97; I2 = 0.0%) (Table 2), and study design (retrospective cohort: OR = 0.82; 95%CI = 0.69 to 0.98; I2 = 56.8%) (Table 2). Also, all the subgroup differences were not statistically significant (P > 0.05) (Table 2).
Local recurrence
A total of seven studies provided suitable data for local recurrence. No significant positive association between RA with or without GA and GA groups in local recurrence (OR = 0.82; 95%CI = 0.47 to 1.45; I2 = 47.3%) (Fig. 3). Subgroup analysis reported that no significant associations were also observed in breast cancer, epidural anesthesia with GA, and retrospective cohort studies (Table 2).
Distant metastasis
Twelve studies provided suitable data for distant metastasis. The pooled OR of distant metastasis showed no significant difference between RA with or without GA and GA groups (OR = 0.87; 95%CI = 0.71 to 1.08; I2 = 14.5%) (Fig. 4). Subgroup analysis reported that no significant associations were also observed based on cancer type, anesthesia technique, and study design (Table 2). Again, all the subgroup differences were not statistically significant (P > 0.05) (Table 2).
Publication bias and sensitivity analysis
Sensitivity analyses showed that the pooled effect size results were robust (Supplementary Figs. 4–6). Funnel plot and Egger’s test were used to evaluate the publication bias of the included studies in the meta-analysis. The funnel plot did not reveal any evidence of asymmetry (Supplementary Figs. 1–3). Egger’s tests were not significant, indicating the absence of publication bias among the included studies (Table 3).
Discussion
Our meta-analysis, which included a comprehensive collection of 32 studies, revealed a significant difference in cancer recurrence between groups receiving RA with or without GA and groups receiving GA alone. Specifically, the pooled OR for cancer recurrence was found to be 0.82 (95% confidence interval (CI) = 0.72 to 0.94), suggesting a lower risk of cancer recurrence in patients receiving RA alone or concurrent GA. Furthermore, subgroup analyses underscored the significance of this finding in prostate cancer, epidural anesthesia with GA, and retrospective cohort studies.
A growing number of studies have found that anesthesia techniques could affect the recurrence and metastasis of cancer after surgery [2,3,4]. However, a previous meta-analysis [16, 17] suggested that RA with or without GA did not reduce cancer recurrence and metastasis, which was inconsistent with individuals’ studies [2,3,4]. Therefore, our study conducted a large-scale meta-analysis to investigate the impact of RA on postoperative cancer recurrence and metastasis.
The present meta-analysis indicated that compared with GA, the use of RA alone or in combination with GA was significantly associated with cancer recurrence, but specifically, no significant association was found in cancer metastasis and local recurrence. Anesthetics are commonly used in the operative treatment of tumors. The choice of different anesthetics and anesthesia techniques can affect cancer proliferation, metastasis, recurrence, and prognosis. It is hypothesized that one of the mechanisms by which RA reduces cancer recurrence is through anti-inflammatory effects and reduction of surgical stress response [47]. For example, some studies found a small-modest reduction in inflammatory biomarkers (i.e., interleukin 1 [IL-1], IL-6, MMP-3, and MMP-9) and markers of the stress response (i.e., serum cortisol, serum glucose, and C-reactive protein) in patients who received a paravertebral block (PVB) [48,49,50], which supported the hypothesis. Studies found that RA can not only reduce the number of opioids [51] but also inhibit tumor recurrence by blocking sodium channels of cancer cells [52], decreasing inflammation [53], and improving immune function [54]. Studies suggested that opioids can be beneficial to tumor growth by inducing immune suppression and stimulating the proliferation of cancer metastasis [51]. Therefore, the American Society of Anesthesiologists (ASA) advocated minimizing the use of opioids in cancer patients.
A second mechanism by which regional anesthesia reduces postoperative cancer recurrence is decreasing the concentration of growth factors with proliferative or angiogenic effects. For example, Jaura et al. [55] and Deegan et al. [56] found that serum from breast cancer women treated with sevoflurane/opioids was antiapoptotic, whereas serum from women treated with PVB/propofol drugs was inhibitory to cell proliferation. Besides, it has been hypothesized that RA attenuates the inhibitory effects of surgery itself, volatile anesthetics, and opioids on these cells. Furthermore, inhaled anesthetics and intravenous opioids may inhibit the activity of natural killer (NK) and functional T cells for several days [57,58,59]. However, RA can maintain NK cell function in tumor patients [60]. However, prior some meta-analyses have indicated that RA with or without GA did not reduce cancer recurrence and metastasis rate after surgery [16, 17], which did not follow our results. These mate analyses are based on a few original studies (N ≤ 10), which may cause unstable results. For example, Lee et al. [17] only recruited three studies to calculate the pooled OR of cancer recurrence between RA and GA. Ang et al. [17] also only included six studies in the meta-analysis.
Furthermore, within the subgroup of prostate cancer patients, RA with or without GA was revealed to be associated with lower cancer recurrence, but the same result was not found in a subgroup analysis of cancer type. The previous meta-analysis was in agreement with our findings. For example, Pei et al. [16] found that general-epidural anesthesia (EGA) might be associated with cancer-free survival benefits among patients with operable prostate cancer; however, no significant benefits were detected in colorectal cancer. Besides, Lee et al. demonstrated that the use of regional analgesia contributed to improving overall survival in patients after prostatectomy [61]. The incidence of postoperative cancer recurrence may depend on the nature and different types of cancer. Biochemical recurrence rates for prostate cancer range from 20 to 40%, which is significantly lower than more aggressive cancer types such as hepatocellular carcinoma (HCC) [62,63,64]. In the present study, our results also indicated that EA could decrease the cancer recurrence rate in cancer resection surgery, compared with GA, which was consistent with previous studies [16]. Animal models have reported that EA could improve perioperative immune suppression and enhance immune surveillance among cancer patients, thereby decreasing cancer recurrence [9]. We note that the larger RCTs related to breast cancer in the included studies did not show a difference. Zhang and Du raised a similar issue [11, 42, 65,66,67,68,69,70], that regional anesthesia has a beneficial effect on breast cancer recurrence compared with general anesthesia, but this effect has only been reported in some observational studies and research (in vitro), not in RCTs (including this review). The reasons for this may be the huge differences in the duration between anesthesia experiments (in vitro) and clinical application of anesthesia [71], as well as the biological characteristics of different cancers [72]. In addition, there were RCTs believed that regional anesthesia was effective for the recurrence of the surgery whose wound is large, while breast cancer surgery is less invasive [23, 73]. In addition, the weights of these five RCTs included in this study, Li (5.25%), Tsui (1.75%), Sessler (5.7%), Christo (3.04%), and Karmakar (3.21%), were not overwhelming, which may be one of the reasons why the larger RCTs included did not show differences. Finally, all sensitivity analyses showed that the pooled effect size results were robust.
Although the impact of RA on cancer recurrence was inconclusive, our study supported that the use of RA was associated with a lower incidence of cancer recurrence rate than GA in cancer resection surgery. However, our findings should be interpreted with caution due to some limitations. First, there were only five RCTs although 32 studies were included. Therefore, our meta-analysis was limited by the nature of the nonrandomized and retrospective studies with significant heterogeneity and low-quality evidence. Second, our study did not control some other confounding variables, such as changes in the definition of recurrence, and different lengths of follow-up, which hampers our conclusions. Third, 31 studies in the English language were included in the present meta-analysis, which introduces “English language bias” and reduces the accuracy of our results. Fourth, the title of this study was adjusted according to the results of the studies compared to the registration, and the original title was “Anesthesia type may impact on cancer recurrence and metastasis after cancer surgery: a meta-analysis”. In addition, the RCT quality assessment method was adjusted from NOS to ROB2.0. Given the limited and heterogeneous evidence, it may be too early to change the anesthesia practice in surgeries for cancer. However, we believe our findings provided a reference for future studies in this area.
Recently, the incidence of cancer has gradually increased, and although the mortality rate has decreased with the increasing maturity of treatment, the mortality rate is still at a high level, so inhibiting tumor recurrence and metastasis and increasing the survival rate of patients with tumors have become the focus of people’s research. Although we found a slight apparent advantage of regional anesthesia in some subgroups, these findings should be interpreted cautiously when formulating hypotheses because the combined effects in subgroups were derived from a small number of original studies and were not corrected for multiple comparisons. Given the study limitations and various findings, it may be too early to change anesthetic practices in cancer surgery. Still, we believe that our findings provide recommendations for future research in this field.
Conclusions
In conclusion, our meta-analysis indicated that RA may be associated with lower cancer recurrence in cancer patients after surgery, especially for these prostate cancer patients. Furthermore, our results suggested a significant positive association between EGA and cancer recurrence. However, no significant findings were found in cancer metastasis and local recurrence. Further prospective studies should be conducted to clarify this important issue.
Data Availability
All data generated or analysed during this study are included in this published article.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. An anesthetic technique for radical prostatectomy Surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109(2):180–7.
Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary Breast cancer Surgery affect recurrence or Metastasis? Anesthesiology. 2006;105(4):660–4.
Holler JP, Ahlbrandt J, Burkhardt E, Gruss M, Röhrig R, Knapheide J, Hecker A, Padberg W, Weigand MA. Peridural analgesia may affect long-term survival in patients with Colorectal cancer after Surgery (PACO-RAS-Study): an analysis of a cancer registry. Ann Surg. 2013;258(6):989–93.
Choi WJ, Baek S, Joo EY, Yoon SH, Kim E, Hong B, Hwang JH, Kim YK. Comparison of the effect of spinal anesthesia and general anesthesia on 5-year Tumor recurrence rates after transurethral resection of bladder tumors. Oncotarget. 2017;8(50):87667–74.
Peach G, Kim C, Zacharakis E, Purkayastha S, Ziprin P. Prognostic significance of circulating Tumor cells following surgical resection of colorectal cancers: a systematic review. Br J Cancer. 2010;102(9):1327–34.
Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anesthesia improve outcome after total hip arthroplasty? A systematic review. Br J Anaesth. 2009;103(3):335–45.
Wall T, Sherwin A, Ma D, Buggy DJ. Influence of perioperative anesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth. 2019;123(2):135–50.
Snyder GL, Greenberg S. Effect of anesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105(2):106–15.
Bar-Yosef S, Melamed R, Page GG, Shakhar G, Shakhar K, Ben-Eliyahu S. Attenuation of the tumor-promoting effect of Surgery by spinal blockade in rats. Anesthesiology. 2001;94(6):1066–73.
Karmakar MK, Samy W, Lee A, Li JW, Chan WC, Chen PP, Tsui BCH. Survival analysis of patients with Breast Cancer undergoing a modified radical mastectomy with or without a thoracic paravertebral block: a 5-Year follow-up of a Randomized Controlled Trial. Anticancer Res. 2017;37(10):5813–20.
Sessler DI, Pei L, Huang Y, Fleischmann E, Marhofer P, Kurz A, Mayers DB, Meyer-Treschan TA, Grady M, Tan EY, et al. Recurrence of Breast cancer after regional or general anesthesia: a randomized controlled trial. Lancet (London England). 2019;394(10211):1807–15.
Li M, Zhang Y, Pei L, Zhang Z, Tan G, Huang Y. Potential influence of anesthetic interventions on Breast Cancer early recurrence according to Estrogen receptor expression: a Sub-study of a Randomized Trial. Front Oncol. 2022;12:837959.
Wang X, Xie W, Gan S, Wang T, Chen X, Su D, Sun J, Lin J, Wu F, Xu P, et al. Effects of general anesthesia versus local anesthesia in primary hepatocellular carcinoma patients presenting for thermal ablation Surgery: a multiple center retrospective cohort study with propensity score matching. Ann Transl Med. 2020;8(6):277.
Chang XL, Zhu D, Ren XL, Lv HW. Influence of Combined General and Epidural Anesthesia on Cancer Prognosis: a Meta-analysis. Chin J Evid-based Med. 2011;11(8):954–9.
Pei L, Tan G, Wang L, Guo W, Xiao B, Gao X, Wang L, Li H, Xu Z, Zhang X, et al. Comparison of combined general-epidural anesthesia with general anesthesia effects on survival and cancer recurrence: a meta-analysis of retrospective and prospective studies. PLoS ONE. 2014;9(12):e114667.
Lee ZX, Ng KT, Ang E, Wang CY, Binti S. Effect of perioperative regional anesthesia on cancer recurrence: a meta-analysis of randomized controlled trials. Int J Surg. 2020;82:192–9.
Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583.
Wanat M, Boulton M, Watson E. Patients’ experience with cancer recurrence: a meta-ethnography. Psychooncology. 2016;25(3):242–52.
GA Wells BS, D O’Connell J, Peterson V, Welch M, Losos P, Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane collaboration’s tool for assessing the risk of bias in randomized trials. BMJ. 2011;343:d5928.
Tornøe AS, Pind AH, Laursen CCW, Andersen C, Maagaard M, Mathiesen O. Ketamine for postoperative pain treatment in spinal Surgery: systematic review with meta-analysis and trial sequential analysis. Acta Anaesthesiol Scand 2023.
Christopherson R, James KE, Tableman M, Marshall P, Johnson FE. Long-term survival after colon Cancer Surgery: a variation associated with choice of anesthesia. Anesth Analg. 2008;107(1):325–32.
De Oliveira GS Jr, Ahmad S, Schink JC, Singh DK, Fitzgerald PC, McCarthy RJ. Intraoperative neuraxial anesthesia but not postoperative neuraxial analgesia is associated with increased relapse-free survival in Ovarian cancer patients after primary cytoreductive Surgery. Reg Anesth Pain Med. 2011;36(3):271–7.
Gottschalk A, Ford JG, Regelin CC, You J, Mascha EJ, Sessler DI, Durieux ME, Nemergut EC. Association between epidural analgesia and cancer recurrence after Colorectal cancer Surgery. Anesthesiology. 2010;113(1):27–34.
Hasselager RP, Hallas J, Gögenur I. Epidural analgesia and recurrence after Colorectal Cancer Surgery: a Danish Retrospective Registry-based Cohort Study. Anesthesiology. 2022;136(3):459–71.
Heinrich S, Janitz K, Merkel S, Klein P, Schmidt J. Short- and long-term effects of epidural analgesia on morbidity and mortality of Esophageal cancer Surgery. Langenbecks Arch Surg. 2015;400(1):19–26.
Karanlik H, Klllç B, Ylldlrlm I, Bademler S, Ozgur I, Ilhan B, Onder S. Breast-conserving Surgery under local Anesthesia in Elderly patients with severe Cardiorespiratory comorbidities: a hospital-based case-control study. Breast Care. 2017;12(1):29–33.
Koumpan Y, Jaeger M, Mizubuti GB, Tanzola R, Jain K, Hosier G, Hopman W, Siemens DR. Spinal anesthesia is Associated with Lower Recurrence Rates after Resection of Nonmuscle invasive Bladder Cancer. J Urol. 2018;199(4):940–6.
Kuo YH, Chung KC, Hung CH, Lu SN, Wang JH. The impact of general anesthesia on radiofrequency ablation of hepatocellular carcinoma. Kaohsiung J Med Sci. 2014;30(11):559–65.
Lai R, Peng Z, Chen D, Wang X, Xing W, Zeng W, Chen M. The effects of anesthetic technique on cancer recurrence in percutaneous radiofrequency ablation of small hepatocellular carcinoma. Anesth Analg. 2012;114(2):290–6.
Lee SW, Tae BS, Choi YJ, Yoon SM, Lee YS, Kim JH, Shin HW, Park JY, Bae JH. A comparison of the anesthetic methods for recurrence rates of Bladder Cancer after Transurethral Resection of Bladder Tumors Using National Health Insurance Claims Data of South Korea. J Clin Med 2022, 11(4).
Lu Y, Liu T, Wang P, Chen Y, Ji F, Hernanz F, Zucca-Matthes G, Youssef S, Peng S, Xu D. Can anesthetic effects and pain treatment influence the long-term prognosis of early-stage lymph node-negative Breast cancer after breast-conserving Surgery? Ann Transl Med. 2021;9(18):1467.
Macleod LC, Turner RM 2nd, Lopa S, Hugar LA, Davies BJ, Ben-David B, Chelly JE, Jacobs BL, Nelson JB. Effect of multimodal analgesia with paravertebral blocks on biochemical recurrence in men undergoing open radical prostatectomy. Urol Oncol 2018, 36(8):364.e369-364.e314.
Mu DL, Xue C, An B, Wang DX. [Epidural block associated with improved long-term survival after Surgery for Colorectal cancer: a retrospective cohort study with propensity score matching]. Beijing Da Xue Xue Bao Yi Xue Ban. 2021;53(6):1152–8.
Pei JP, Zhang CD, Liang Y, Zhang C, Wu KZ, Zhao ZM, Dai DQ. Effects of epidural combined with general anesthesia versus general anesthesia alone in gastric cancer Surgery: a propensity score matching analysis. Ann Transl Med. 2020;8(7):473.
Sprung J, Scavonetto F, Yeoh TY, Kramer JM, Jeffrey Karnes R, Eisenach JH, Schroeder DR, Weingarten TN. Outcomes after radical prostatectomy for cancer: a comparison between general anesthesia and epidural anesthesia with fentanyl analgesia: a matched cohort study. Anesth Analg. 2014;119(4):859–66.
Tseng KS, Kulkarni S, Humphreys EB, Carter HB, Mostwin JL, Partin AW, Han M, Wu CL. Spinal anesthesia does not impact Prostate cancer recurrence in a cohort of men undergoing radical prostatectomy: an observational study. Reg Anesth Pain Med. 2014;39(4):284–8.
Tsui BC, Rashiq S, Schopflocher D, Murtha A, Broemling S, Pillay J, Finucane BT. Epidural anesthesia and cancer recurrence rates after radical prostatectomy. Can J Anaesth. 2010;57(2):107–12.
Wuethrich PY, Hsu Schmitz SF, Kessler TM, Thalmann GN, Studer UE, Stueber F, Burkhard FC. Potential influence of the anesthetic technique used during open radical prostatectomy on prostate cancer-related outcome: a retrospective study. Anesthesiology. 2010;113(3):570–6.
Wuethrich PY, Thalmann GN, Studer UE, Burkhard FC. Epidural analgesia during open radical prostatectomy does not improve long-term cancer-related outcome: a retrospective study in patients with advanced Prostate cancer. PLoS ONE. 2013;8(8):e72873.
Zhang J, Chang CL, Lu CY, Chen HM, Wu SY. Paravertebral block in regional anesthesia with propofol sedation reduces locoregional recurrence in patients with Breast cancer receiving breast Conservative Surgery compared with volatile inhalational without propofol in general anesthesia. Biomed Pharmacother. 2021;142:111991.
Lin L, Liu C, Tan H, Ouyang H, Zhang Y, Zeng W. Anaesthetic technique may affect prognosis for ovarian serous adenocarcinoma: a retrospective analysis. Br J Anaesth. 2011;106(6):814–22.
Capmas P, Billard V, Gouy S, Lhommé C, Pautier P, Morice P, Uzan C. Impact of epidural analgesia on survival in patients undergoing complete cytoreductive Surgery for Ovarian cancer. Anticancer Res. 2012;32(4):1537–42.
Hiller JG, Hacking MB, Link EK, Wessels KL, Riedel BJ. Perioperative epidural analgesia reduces cancer recurrence after gastro-oesophageal Surgery. Acta Anaesthesiol Scand. 2014;58(3):281–90.
Gupta A, Björnsson A, Fredriksson M, Hallböök O, Eintrei C. Reduction in mortality after epidural anesthesia and analgesia in patients undergoing rectal but not colonic cancer Surgery: a retrospective analysis of data from 655 patients in central Sweden. Br J Anaesth. 2011;107(2):164–70.
Cata JP, Gottumukkala V, Sessler DI. How regional anesthesia might reduce postoperative cancer recurrence. Eur J Pain Suppl. 2012;5:345–55.
O’Riain SC, Buggy DJ, Kerin MJ, Watson RWG, Moriarty DC. Inhibition of the stress response to Breast cancer Surgery by regional anesthesia and analgesia does not affect vascular endothelial growth factor and prostaglandin E2. Anesth Analg. 2005;100(1):244–9.
Deegan CA, Murray D, Doran P, Moriarty DC, Sessler DI, Mascha E, Kavanagh BP, Buggy DJ. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary Breast cancer Surgery. Reg Anesth Pain Med. 2010;35(6):490–5.
Sultan SS. Paravertebral block can attenuate cytokine response when it replaces general anesthesia for cancer breast surgeries. Saudi J Anaesth. 2013;7(4):373–7.
MacFater WS, Xia W, Barazanchi A, Su’a B, Svirskis D, Hill AG. Intravenous local Anaesthetic compared with intraperitoneal local anaesthetic in abdominal Surgery: a systematic review. World J Surg. 2018;42(10):3112–9.
Djamgoz MBA, Fraser SP, Brackenbury WJ. In vivo evidence for Voltage-gated Sodium Channel Expression in Carcinomas and Potentiation of Metastasis. Cancers (Basel) 2019, 11(11).
Hayden JM, Oras J, Block L, Thörn SE, Palmqvist C, Salehi S, Nordstrom JL, Gupta A. Intraperitoneal ropivacaine reduces time interval to initiation of chemotherapy after Surgery for advanced Ovarian cancer: randomized controlled double-blind pilot study. Br J Anaesth. 2020;124(5):562–70.
Wang L, Liang S, Chen H, Xu Y, Wang Y. The effects of epidural anesthesia and analgesia on T lymphocytes differentiation markers and cytokines in patients after gastric cancer resection. BMC Anesthesiol. 2019;19(1):102.
Jaura AI, Flood G, Gallagher HC, Buggy DJ. Differential effects of serum from patients administered distinct anesthetic techniques on apoptosis in Breast cancer cells in vitro: a pilot study. Br J Anaesth. 2014;113(Suppl 1):i63–67.
Deegan CA, Murray D, Doran P, Ecimovic P, Moriarty DC, Buggy DJ. Effect of anesthetic technique on estrogen receptor-negative Breast cancer cell function in vitro. Br J Anaesth. 2009;103(5):685–90.
Lewis JW, Shavit Y, Terman GW, Gale RP, Liebeskind JC. Stress and morphine affect the survival of rats challenged with a mammary Ascites Tumor (MAT 13762B). Nat Immun Cell Growth Regul. 1983;3(1):43–50.
Beilin B, Martin FC, Shavit Y, Gale RP, Liebeskind JC. Suppression of natural killer cell activity by high-dose narcotic anesthesia in rats. Brain Behav Immun. 1989;3(2):129–37.
Markovic SN, Knight PR, Murasko DM. Inhibition of interferon stimulation of natural killer cell activity in mice anesthetized with halothane or isoflurane. Anesthesiology. 1993;78(4):700–6.
Cata JP, Ramirez MF, Velasquez JF, Di AI, Popat KU, Gottumukkala V, Black DM, Lewis VO, Vauthey JN. Lidocaine stimulates the function of natural killer cells in different experimental settings. Anticancer Res. 2017;37(9):4727–32.
Lee BM, Singh Ghotra V, Karam JA, Hernandez M, Pratt G, Cata JP. Regional anesthesia/analgesia and the risk of cancer recurrence and mortality after prostatectomy: a meta-analysis. Pain Manag. 2015;5(5):387–95.
Kupelian P, Katcher J, Levin H, Zippe C, Klein E. Correlation of clinical and pathologic factors with rising prostate-specific antigen profiles after radical prostatectomy alone for clinically localized Prostate cancer. Urology. 1996;48(2):249–60.
Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167(2 Pt 1):528–34.
Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ Jr., Dotan ZA, Fearn PA, Kattan MW. Preoperative nomogram predicting the 10-year probability of Prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98(10):715–7.
Xu ZZ, Li HJ, Li MH, Huang SM, Li X, Liu QH, Li J, Li XY, Wang DX, Sessler DI. Epidural anesthesia-analgesia and recurrence-free survival after Lung Cancer Surgery: a Randomized Trial. Anesthesiology. 2021;135(3):419–32.
Du YT, Li YW, Zhao BJ, Guo XY, Feng Y, Zuo MZ, Fu C, Zhou WJ, Li HJ, Liu YF, et al. Long-term survival after combined epidural-general anesthesia or general anesthesia alone: follow-up of a Randomized Trial. Anesthesiology. 2021;135(2):233–45.
Lusty AJ, Hosier GW, Koti M, Chenard S, Mizubuti GB, Jaeger M, Siemens DR. Anesthetic technique and oncological outcomes in urology: a clinical practice review. Urol Oncol. 2019;37(12):845–52.
Zhang YL, Pei LJ, Sun C, Zhao MY, Che L, Huang YG. Regional anesthesia and cancer recurrence in patients with late-stage cancer: a systematic review and meta-analysis. Chin Med J (Engl). 2021;134(20):2403–11.
Huang YH, Lee MS, Lou YS, Lai HC, Yu JC, Lu CH, Wong CS, Wu ZF. Propofol-based total intravenous anesthesia did not improve survival compared to desflurane anesthesia in Breast cancer Surgery. PLoS ONE. 2019;14(11):e0224728.
Makito K, Matsui H, Fushimi K, Yasunaga H. Volatile versus total intravenous anesthesia for Cancer Prognosis in patients having Digestive Cancer Surgery. Anesthesiology. 2020;133(4):764–73.
Lirk P, Hollmann MW, Fleischer M, Weber NC, Fiegl H. Lidocaine and ropivacaine, but not bupivacaine, demethylate deoxyribonucleic acid in Breast cancer cells in vitro. Br J Anaesth. 2014;113(Suppl 1):i32–38.
Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, Buzdar AU, Smith IE, Symmans WF, Singh B, et al. Preoperative therapy in invasive Breast cancer: pathologic assessment and systemic therapy issues in operable Disease. J Clin Oncol. 2008;26(5):814–9.
Binczak M, Tournay E, Billard V, Rey A, Jayr C. Major abdominal Surgery for cancer: does epidural analgesia have a long-term effect on recurrence-free and overall survival? Ann Fr Anesth Reanim. 2013;32(5):e81–88.
Acknowledgements
Not applicable.
Funding
This work was supported by the project supported by the Hainan Province Clinical Medical Center, a project supported by the Health Commission of Hainan Province (22A200074 to WH), and the funding for talent introduction by the Second Affiliated Hospital of Hainan Medical University (TP2022002 to WH). This study was supported by grants from the Jinan Science and Technology Plan (202134071 to FM).
Author information
Authors and Affiliations
Contributions
SX, LL, and FQM carried out the studies, participated in collecting data, and drafted the manuscript. SX and HLW performed the statistical analysis and participated in its design. LL and HLW participated in the acquisition, analysis, or interpretation of data and draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xie, S., Li, L., Meng, F. et al. Regional anesthesia might reduce recurrence and metastasis rates in adult patients with cancers after surgery: a meta-analysis. BMC Anesthesiol 24, 19 (2024). https://doi.org/10.1186/s12871-023-02400-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-023-02400-w