Abstract
Background
Cesarean section often requires an urgent transfusion load due to decreased blood pressure after spinal anesthesia. This prospective randomized study aimed to investigate whether a preoperative oral rehydration solution (ORS) stabilized perioperative circulatory dynamics.
Methods
Sixty-three parturients scheduled for cesarean section under combined spinal epidural anesthesia (CSEA) were randomly allocated to one of three groups: Group O received 500 mL ORS before bedtime and 500 mL 2 h before CSEA; Group M received mineral water instead of ORS; and Group C had no fluid intake (controls). After entering the operating room, stomach size was measured using ultrasound. Blood samples were obtained, and CSEA was induced. Vasopressors were administered when systolic blood pressure was < 90 mmHg or decreased by > 20%. As a vasopressor, phenylephrine (0.1 mg) was administered at ≥ 60 beats/min heart rate or ephedrine (5 mg) at < 60 beats/min heart rate. The primary outcome was the total number of vasopressor boluses administered. Secondary outcomes were the cross-sectional area of the stomach antrum, maternal plasma glucose levels, serum sodium levels, total intravenous fluid, bleeding volume, urine volume, operative time, and cord blood gas values after delivery.
Results
The total number of vasopressor boluses was lower in Group O than in Group C (P < 0.05). Group O had lower total dose of phenylephrine than Group C (P < 0.05). There were no significant differences between Group M and other groups. No differences were detected regarding secondary outcomes.
Conclusions
In women scheduled for cesarean section, preoperative ORS stabilized perioperative circulatory dynamics. Neither ORS nor mineral water consumption increased the stomach content volume.
Trial Registration
This trial is registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000019825: Date of registration 17/11/2015).
Similar content being viewed by others
Background
Regional anesthesia (spinal, epidural, and combined spinal epidural [CSEA]) is the recommended standard practice for elective cesarean section because it offers a rapid onset and reliable surgical condition. In addition, it avoids the most common risks associated with general anesthesia, such as difficulty with airway management, pulmonary aspiration of gastric contents, and the negative effects of general anesthetics on the fetus. However, maternal arterial hypotension during regional anesthesia for cesarean section remains the main disadvantage, particularly for spinal anesthesia, and can result in fetal distress and maternal discomfort [1, 2]. The pathophysiology of hypotension following spinal anesthesia is believed to be caused by sympathetic vasomotor blockade, which causes arterial and arteriolar vasodilation and decreases systemic vascular resistance, resulting in hypotension. Venodilation also occurs, resulting in decreased cardiac preload, reduced cardiac output, and maternal hypotension. In addition, preoperative dehydration is a risk factor for hypotension during spinal anesthesia [3]. To attenuate spinal hypotension, many approaches have been investigated, [4,5,6] notably fluid loading, vasopressors, or a combination of both. Preoperative oral rehydration therapy (PORT) has been reported to prevent hemodynamic changes during anesthesia [7, 8].
Human and animal studies have found that carbohydrate loading before surgery leads to an improved response to surgical stress and postoperative conditions compared with traditional fasting guidelines. From such positive findings, PORT before elective surgeries has been recommended as an essential element of the enhanced recovery after surgery protocol. However, in general, late pregnancy causes delayed gastric emptying. The Practice Guidelines for Obstetric Anesthesia approved by the American Society of Anesthesiologists (ASA) suggests that women with uncomplicated pregnancies undergoing elective cesarean delivery should adhere to the same presurgical fasting guidelines as non-pregnant women, such as 6–8 h of no solid food and 2 h of no liquids before the scheduled surgical procedure [9, 10]; therefore, the safety of using oral rehydration solution (ORS) in women undergoing cesarean section remains unclear.
Our primary hypothesis with this prospective, randomized, open-label (but assessors are blinded), blinded-endpoint controlled clinical trial was to determine if preoperative intake of ORS (OS-1®) stabilized perioperative circulatory dynamics during cesarean section. We also measured stomach size using ultrasound to investigate the safety of ORS.
Methods
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Kushiro Red Cross Hospital (Hokkaido, Japan) and is registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000019825). The trial was conducted from February 2014 to July 2017 at Kushiro Red Cross Hospital, Japan, which is affiliated with the Department of Anesthesiology and Critical Care Medicine of Asahikawa Medical University. Written informed consent was obtained from all participants at least 24 h before the operation.
Study participants
Parturients with no complications who were scheduled for elective cesarean section were recruited for this study. The inclusion criteria were healthy parturients aged > 18 years and with ASA physical status class II with term singleton pregnancies undergoing elective cesarean section under CSEA. All parturients were scheduled to enter the operating room at 9:00 am.
The exclusion criteria were emergency cesarean section, being scheduled for cesarean section under general anesthesia, multiple fetuses, abnormal pregnancy (such as placenta previa and placenta accrete), pregnancy complications (such as pregnancy-induced hypertension, gestational diabetes, bleeding disorders, and coagulopathy), fetal anomalies, and inability to undergo epidural anesthesia. In addition, failure to assess gastric measurement resulted in the exclusion of the participants from the data analysis.
Randomization and masking
Eligible parturients were randomized into one of three groups using a web-based tool (http://www.graphpad.com/quickcalcs/index.cfm): ORS (Group O), mineral water (Group M), or fasted controls (Group C). Group O received 500 mL of ORS (2.5% carbohydrates, 10 kcal/100 mL; OS-1® Otsuka Pharmaceutical Factory, Japan) before bedtime the day before surgery and 500 mL 2 h before anesthesia induction. Group M received an equal volume of mineral water at the stated times. Group C was instructed not to have oral fluid intake for > 8 h before surgery. All parturients were allowed to eat and drink freely before bedtime. After bedtime, oral eating and drinking was forbidden, except for 500 mL of the appropriate drink in Groups O and M.
OS-1® contains water, glucose, and electrolytes and is packaged in a 500-mL plastic bottle; its composition is presented in Table 1.
Blinding of parturients was not feasible because of the taste of the drinks; instead, well-trained anesthesiologists were made unaware of patient allocation, and other study investigators analyzed the data.
Gastric emptying assessment before anesthesia induction
On arrival to the operating room, the parturients were placed in the right lateral decubitus position. Before anesthesia induction, the gastric antral cross-sectional area (CSA) was measured using a Venue 40 (GE Healthcare, Tokyo, Japan) ultrasound system with a 2 to 5 MHz curvilinear array low-frequency 4 C-RS probe to measure gastric emptying [11,12,13,14,15].
The gastric antrum was generally imaged in the parasagittal plane just right of the midline of the epigastric area in parturients, surrounded by the left lobe of the liver anteriorly and pancreas posteriorly. The CSA of the antrum was calculated according to the formula described by Bolondi et al. [16] using two maximum perpendicular diameters representing the surface area of an ellipse, as follows: CSA = AP × CC × π/4 (AP, anteroposterior diameter; CC, craniocaudal diameter) (Fig. 1).
Anesthesia methods
After the ultrasound examination, routine monitoring, including heart rate (HR), blood pressure, electrocardiogram, and peripheral oxygen saturation, was implemented, and baseline values were recorded. Blood samples were collected after inserting a peripheral venous catheter, and a rapid infusion of 6% hydroxyethyl starch (HES) 130/0.4 (Voluven®, Fresenius Kabi Japan, Tokyo, Japan) was started; a total of 1000 mL was administered during surgery. Subsequently, we placed the parturients in the lateral decubitus position and attempted CSEA. All parturients underwent epidural catheterization before spinal anesthesia. An 18-gauge Tuohy needle was introduced using loss of resistance to air to confirm the epidural space. A 19-gauge epidural catheter was inserted through the epidural needle, 3–4 cm into the Th12/L1 epidural space. Instead of an epidural test dose, we injected 2 mL saline through the epidural catheter to prevent obstruction of the catheter by a blood clot. During skin closure, the parturient received continuous epidural infusion of 0.2% ropivacaine for postoperative analgesia.
All parturients received spinal anesthesia at the L3–4 interspace using a 25-gauge Quincke spinal needle (TOP Corp, Tokyo, Japan). Next, 0.5% hyperbaric bupivacaine (8 mg) mixed with fentanyl (20 µg) was administered intrathecally after free flow of cerebrospinal fluid was observed. According to the results of the pinprick tests, the surgeon commenced the operation once a sensory blockade above the T4 level was achieved. Vasopressors were administered when the systolic blood pressure was < 90 mmHg or decreased by > 20% of the baseline value. As a vasopressor, 1 mL phenylephrine (1 mg diluted to 10 mL [0.1 mg/mL] with normal saline) was administered for hypotension with HR ≥ 60 beats/min or 1 mL ephedrine (40 mg diluted to 8 mL [5 mg/mL] with normal saline) for hypotension with HR < 60 beats/min. We counted the administration of 0.1 mg of phenylephrine or 5 mg ephedrine as one dose.
The primary outcome of this study was the total number of vasopressor boluses and dose of vasopressors among the three groups. The secondary outcomes were the CSA of the stomach antrum, maternal plasma glucose levels, serum sodium levels, total intravenous fluid, bleeding volume, urine volume, operative time, and cord blood gas values after delivery.
Data processing and analysis
A power analysis showed that 16 parturients per group would provide an α value of 0.05 and a β value of 0.1, based on the total dose of vasopressors during cesarean section in a pilot study of 10 parturients. The Kruskal–Wallis test was used for comparison between groups, followed by Dunn’s post hoc test for pairwise comparisons. Data are presented as median and interquartile range. All statistical analyses were performed using GraphPad Prism® version 6.01 (GraphPad Software, Inc., La Jolla, CA), and P < 0.05 was considered to indicate a statistically significant difference.
Results
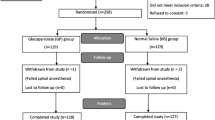
Sixty-one parturients were enrolled in this study and randomized into one of the three groups. The CONSORT diagram is shown in Fig. 2. We excluded five parturients in Group O, two parturients in Group C, and three parturients in Group M, as shown in the diagram. Finally, 17 parturients from each group were analyzed. The baseline characteristics of our study population and intraoperative data were compared between groups (Table 2).
BMI, body mass index
The Kruskal–Wallis test indicated a statistically significant difference in the total number of vasopressor boluses used (P = 0.009). Post hoc comparisons using Dunn’s test showed that Group O required significantly fewer vasopressor boluses than Group C. There were no significant differences between Groups O and M or between Groups C and M (Fig. 3; Table 3). The difference in the total ephedrine dose was not significant between the groups. However, there was a statistically significant difference in the total phenylephrine dose (P = 0.017). Post hoc comparisons using Dunn’s test showed significant differences between Group O and Group C, with a lower dose used in Group O, whereas the other groups showed no significant differences (Fig. 4).
There was no significant difference in secondary outcome among the three groups (Table 3).
Furthermore, no side effects or serious complications were observed in any of the groups.
Discussion
In this prospective randomized study of 51 parturients undergoing scheduled cesarean section, we found that PORT with OS-1® reduced the number of vasopressor boluses and phenylephrine doses during surgery compared with the fasting group. In addition, gastric emptying in healthy parturients was not delayed after drinking 500 mL of clear fluid 2 h before cesarean section compared to that in the fasting group. Similarly, a previous study concluded that PORT prevents hypotension after spinal anesthesia induction [7]. Another study reported that PORT increased the circulating blood volume and maintained a high cardiac index during the induction of general anesthesia [8]. To the best of our knowledge, this is the first study to evaluate the effect of preoperative oral rehydration on the incidence of spinal anesthesia-induced hemodynamic changes during scheduled elective cesarean section.
In this study, we used OS-1®, which meets the oral rehydration therapy guidelines recommended by the World Health Organization and is a balanced mixture of water, glucose, and electrolytes [17, 18]. Its composition was based on the guidelines of the American Academy of Pediatrics [19]. There are reports that preoperative intake of ORS prevents dehydration, [20, 21] and administration of ORS is believed to restore the circulatory volume. Three possible explanations can be considered. First, because ORS contains sodium and glucose, the active absorption of sodium and glucose by sodium-coupled glucose transporter-1 promotes the absorption of water in the small intestine [22,23,24]. Second, a solution with 45–60 mmol/L sodium and 80–110 mmol/L glucose resulted in effective fluid absorption, [25] and the composition of OS-1® was similar to this ratio. Finally, hypotonic solutions may promote increased water and solute absorption in the jejunum, and the osmolality of OS-1® is hypotonic, 270 mOsm/L.
Generally, parturients are at an increased risk of anesthesia aspiration. An increase in the intragastric pressure due to the gravid uterus, a relaxed gastroesophageal sphincter due to the increased progesterone level, and delayed gastric emptying during pregnancy contribute to the risk. For this reason, although recent guidelines recommend the intake of clear liquids at least 2 h before elective surgery, [9, 10] traditional prolonged preoperative fasting remains common, especially among the parturients, and we set the duration of fasting time of fasted controls (group C). Several previous studies have demonstrated the usefulness of the gastric content volume assessment by ultrasonography in parturients, [26,27,28,29,30] which is accurate despite being a simple, noninvasive technique.
Regarding the safety of PORT, we found that as the ORS is highly absorbable and has a short stagnation time in the stomach, ultrasound assessment of the gastric antral CSA in parturients did not increase. There was no apparent or potential risk of aspiration, vomiting, or other drink-related complications before, during, or after surgery. Therefore, we confirmed, by ultrasound, that 500 mL of ORS 2 h before surgery could be acceptable in parturients undergoing cesarean section under spinal anesthesia.
Preoperative infusion is said to be equally effective for the prevention of dehydration during the induction of anesthesia [31]. However, there are reports that excessive infusions cause intestinal edema, prolong the recovery of intestinal function, [32] and increase postoperative complications [33]; therefore, oral rehydration is preferable to reduce the perioperative infusion volume, including preoperative infusions. Oral rehydration is also superior to transfusion for optimizing fluid balance, such as supplying electrolytes and maintaining urine output. Furthermore, 6% HES 130/0.4 was used as an intraoperative infusion in this study, as it has been shown to be effective in preventing hypotension following spinal anesthesia for cesarean Sects [34, 35]. Generally, colloid fluids have some side effects, especially on the hemostatic system. However, Voluven® is a new HES with fewer side effects because of its low molecular weight [36].
In the present study, the ORS group showed significant differences from the fasting group in regards to the suppression of circulatory changes; however, ORS and mineral water did not show significant differences. Regarding excretion from the stomach, water and ORS were excreted at rates similar to those in previous studies on healthy adults. Thus, ORS is well excreted from the stomach; in addition, previous research has shown that ORS can correct electrolytes and reduce insulin resistance [37, 38]. Considering all these factors, ORS may be more beneficial than water.
Nonetheless, our study has some potential limitations. First, as it was impossible to obtain informed consent for emergent cesarean section because of the study protocol; all participants had scheduled elective cesarean sections. We also did not include parturients with complications or ASA physical status class ≥ III. This may limit the generalizability of our conclusions to more severe cases. Accordingly, further studies are needed to confirm whether the results would differ in such cases. Second, it was not a blinded clinical trial because of the taste of the drink, which may have increased the bias. However, the data were analyzed by a study investigator who was not involved in providing anesthesia. Third, although preoperative drinking could cause perioperative nausea or vomiting, this effect was not examined in the present study and is an issue for future research. Finally, sonographic gastric examination is often difficult, especially because pregnancy increases the technical difficulty.
Conclusions
Our study showed that in women scheduled for cesarean section, preoperative oral rehydration with OS-1® stabilized perioperative circulatory dynamics and neither ORS nor mineral water consumed preoperatively increased the stomach content volume.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on request.
Abbreviations
- AP:
-
anteroposterior diameter
- ASA:
-
American Society of Anesthesiologists
- CSA:
-
cross-sectional area
- CSEA:
-
combined spinal epidural anesthesia
- CC:
-
craniocaudal diameter
- ORS:
-
oral rehydration solution
- PORT:
-
preoperative oral hydration therapy
References
Rocke DA, Rout CC. Volume preloading, spinal hypotension and caesarean section. Br J Anaesth. 1995;75:257–9.
Butwick AJ, Columb MO, Carvalho B. Preventing spinal hypotension during caesarean delivery: what is the latest? Br J Anaesth. 2015;114:183–6.
Fearon KC, Ljungqvist O, Von Meyenfeldt M, Revhaug A, Dejong CH, Lassen K, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24:466–77.
Chooi C, Cox JJ, Lumb RS, Middleton P, Chemali M, Emmett RS, et al. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst Rev. 2020;7:CD002251.
Fitzgerald JP, Fedoruk KA, Jadin SM, Carvalho B, Halpern SH. Prevention of hypotension after spinal anaesthesia for caesarean section: a systematic review and network meta-analysis of randomised controlled trials. Anaesthesia. 2020;75:109–21.
Biricik E, Ünlügenç H. Vasopressors for the treatment and prophylaxis of spinal induced hypotension during caesarean section. Turk J Anaesthesiol Reanim. 2021;49:3–10.
Itoh S, Arai M, Kuroiwa M, Ando H, Okamoto H. [Effect of preoperative oral rehydration on the hypotension during spinal anesthesia]. Masui. 2016;65:786–9.
Tsutsui M, Ishigaki S, Kanaya A, Kawaguchi S, Ogura T. [The effect of preoperative oral rehydration on hemodynamic changes during induction of anesthesia and intraoperative fluid management]. Masui. 2015;64:362–7.
American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on obstetric anesthesia. Anesthesiology. 2007;106:843–63.
Practice guidelines for. Obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on obstetric anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2016;124:270–300.
Perlas A, Chan VW, Lupu CM, Mitsakakis N, Hanbidge A. Ultrasound assessment of gastric content and volume. Anesthesiology. 2009;111:82–9.
Perlas A, Davis L, Khan M, Mitsakakis N, Chan VW. Gastric sonography in the fasted surgical patient: a prospective descriptive study. Anesth Analg. 2011;113:93–7.
Bouvet L, Mazoit JX, Chassard D, Allaouchiche B, Boselli E, Benhamou D. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology. 2011;114:1086–92.
Perlas A, Mitsakakis N, Liu L, Cino M, Haldipur N, Davis L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116:357–63.
Kruisselbrink R, Arzola C, Endersby R, Tse C, Chan V, Perlas A. Intra- and interrater reliability of ultrasound assessment of gastric volume. Anesthesiology. 2014;121(1):46–51. https://doi.org/10.1097/ALN.0000000000000193.
Bolondi L, Bortolotti M, Santi V, Calletti T, Gaiani S, Labò G. Measurement of gastric emptying time by real-time ultrasonography. Gastroenterology. 1985;89:752–9.
WHO. World Health Organization Programme for control of diarrheal diseases; a manual for the treatment of diarrhea. Geneva: WHO; 1990.
World Health Organization. Oral rehydration salts (ORS): a new reduced osmolarity formulation. Geneva, Switzerland: World Health Organization; 2002.
Mauer AM, Dweck HS, Finberg L, Holmes F, Reynolds JW, Suskind RM, et al. American Academy of Pediatrics Committee on Nutrition: use of oral fluid therapy and posttreatment feeding following enteritis in children in a developed country. Pediatrics. 1985;75:358–61.
Itou K, Fukuyama T, Sasabuchi Y, Yasuda H, Suzuki N, Hinenoya H, et al. Safety and efficacy of oral rehydration therapy until 2 h before surgery: a multicenter randomized controlled trial. J Anesth. 2012;26:20–7.
Taniguchi H, Sasaki T, Fujita H. Oral rehydration therapy for preoperative fluid and electrolyte management. Int J Med Sci. 2011;8:501–9.
Sladen GE, Dawson AM. Interrelationships between the absorptions of glucose, sodium and water by the normal human jejunum. Clin Sci. 1969;36:119–32.
Nalin DR, Levine MM, Mata L, de Cespedes C, Vargas W, Lizano C, et al. Comparison of sucrose with glucose in oral therapy of infant diarrhoea. Lancet. 1978;2:277–9.
Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931–43.
Buccigrossi V, Lo Vecchio A, Bruzzese E, Russo C, Marano A, Terranova S, et al. Potency of oral rehydration solution in inducing fluid absorption is related to glucose concentration. Sci Rep. 2020;10:7803.
Arzola C, Cubillos J, Perlas A, Downey K, Carvalho JC. Interrater reliability of qualitative ultrasound assessment of gastric content in the third trimester of pregnancy. Br J Anaesth. 2014;113:1018–23.
Roukhomovsky M, Zieleskiewicz L, Diaz A, Guibaud L, Chaumoitre K, Desgranges FP, et al. Ultrasound examination of the antrum to predict gastric content volume in the third trimester of pregnancy as assessed by MRI: a prospective cohort study. Eur J Anaesthesiol. 2018;35:379–89.
Desgranges FP, Chassard D, Zieleskiewicz L, Bouvet L. Ultrasound assessment of gastric contents at the end of pregnancy. Int J Obstet Anesth. 2018;35:116–7.
Shi Y, Dong B, Dong Q, Zhao Z, Yu Y. Effect of preoperative oral carbohydrate administration on patients undergoing cesarean section with epidural anesthesia: a pilot study. J Perianesth Nurs. 2021;36:30–5.
Sarhan K, Hasanin A, Melad R, Fouad R, Elhadi H, Elsherbeeny M, et al. Correction to: evaluation of gastric contents using ultrasound in full-term pregnant women fasted for 8 h: a prospective observational study. J Anesth. 2022;36:143.
Taniguchi H, Sasaki T, Fujita H, Takamori M, Kawasaki R, Momiyama Y, et al. Preoperative fluid and electrolyte management with oral rehydration therapy. J Anesth. 2009;23:222–9.
Sanders G, Mercer SJ, Saeb-Parsey K, Akhavani MA, Hosie KB, Lambert AW. Randomized clinical trial of intravenous fluid replacement during bowel preparation for surgery. Br J Surg. 2001;88:1363–5.
Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–8.
Madi-Jebara S, Ghosn A, Sleilaty G, Richa F, Cherfane A, Haddad F, et al. Prevention of hypotension after spinal anesthesia for cesarean section: 6% hydroxyethyl starch 130/0.4 (Voluven) versus lactated Ringer’s solution. J Med Liban. 2008;56:203–7.
Mercier FJ, Diemunsch P, Ducloy-Bouthors AS, Mignon A, Fischler M, Malinovsky JM, et al. 6% hydroxyethyl starch (130/0.4) vs Ringer’s lactate preloading before spinal anaesthesia for caesarean delivery: the randomized, double-blind, multicentre CAESAR trial. Br J Anaesth. 2014;113:459–67.
Khosravi F, Alishahi M, Khanchemehr Y, Jarineshin H. A comparison between the effects of preloading with Ringer’s solution and voluven on hemodynamic changes in patients undergoing elective cesarean section under spinal anesthesia. Med Arch. 2019;73:44–8.
Ljungqvist O, Thorell A, Gutniak M, Häggmark T, Efendic S. Glucose infusion instead of preoperative fasting reduces postoperative insulin resistance. J Am Coll Surg. 1994;178:329–36.
Mimura F, Sakurai Y, Uchida M, Aiba J, Yamaguchi M. [Safety of preoperative oral rehydration therapy]. Masui. 2011;60:615–20.
Acknowledgements
We would like to express our sincere gratitude to all the participants for their support and contribution to this research. We greatly appreciate the assistance from Obstetrics and Gynecology physicians, operating room medical staff, and 4 A ward medical staff of Kushiro Red Cross Hospital. Part of the data in this study was presented at the American Society of Anesthesiologists Annual meeting (Chicago, 2016).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
E.I. and T.S. substantially contributed to the study conceptualization. E.I. and T.S. significantly contributed to data analysis and interpretation. C.M. substantially contributed to the manuscript drafting. All authors critically reviewed and revised the manuscript draft and approved the final version for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Kushiro Red Cross Hospital (Hokkaido, Japan) and is registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000019825). All the methods in our study were conducted in accordance with the Declaration of Helsinki. The trial was conducted from February 2014 to July 2017 at Kushiro Red Cross Hospital, Japan, which is affiliated with the Department of Anesthesiology and Critical Care Medicine of Asahikawa Medical University. Written informed consent was obtained from all participants at least 24 h before the operation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ijiri, E., Mori, C. & Sasakawa, T. Effect of preoperative oral rehydration before cesarean section on ultrasound assessment of gastric volume and intraoperative hemodynamic changes: a randomized controlled trial. BMC Anesthesiol 23, 293 (2023). https://doi.org/10.1186/s12871-023-02250-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-023-02250-6