Abstract
Background
Sugammadex has been reported to lower the incidence of postoperative residual neuromuscular blockade. Despite the advantages, until recently the effects of sugammadex on postoperative pulmonary complications (PPCs) were controversial. We conducted a systematic review and meta-analysis to determine whether reversal with sugammadex was associated with a lower risk of PPCs compared with neostigmine.
Methods
PubMed, Embase, and Cochrane Central Register of Controlled Trials were searched from inception to May 2022. Randomized controlled trials (RCTs) and observational studies comparing PPCs in patients receiving sugammadex or neostigmine as reversal agent at the end of surgery were included. The primary outcomes focused on PPCs including desaturation, pneumonia, atelectasis, noninvasive ventilation (NIV) and reintubation. Trial sequential analysis was performed on the primary outcomes to confirm whether firm evidence was reached.
Results
Meta-analysis of included studies showed that the rate of desaturation (43.2% vs 45.0%, RR = 0.82; 95% CI 0.63 to 1.05; p = 0.11) were comparable between the two groups. When looking at other primary outcomes, significantly lower risk of pneumonia (1.37% vs 2.45%, RR = 0.65; 95% CI 0.49 to 0.85; p = 0.002), atelectasis (24.6% vs 30.4%, RR = 0.64; 95% CI 0.42 to 0.98; p = 0.04), NIV (1.37% vs 2.33%, RR = 0.65; 95% CI 0.43 to 0.98; p = 0.04) and reintubation (0.99% vs 1.65%, RR = 0.62; 95% CI 0.43 to 0.91; p = 0.01) in the sugammadex group were detected compared with the neostigmine group.
Conclusions
We concluded that sugammadex is more effective at reducing the incidence of PPCs including pneumonia, atelectasis, NIV and reintubation compared with neostigmine. Further evidence, preferably from RCTs, is required to confirm these findings.
Similar content being viewed by others
Background
The use of neuromuscular blocking drugs is considered essential during general anesthesia, but may contribute to residual neuromuscular blockade (NMB) [1]. Numerous studies have shown that residual NMB was associated with impaired upper airway patency, decreased functional residual capacity, and respiratory insufficiency, consequently putting patients at risk of multiple postoperative pulmonary complications (PPCs) [2, 3]. Therefore, full restoration of muscle strength may decrease the risk of PPCs, including hypoxemia, atelectasis, and pneumonia [4, 5].
Acetylcholinesterase (AChE) inhibitors like neostigmine are used to reduce residual NMB through increasing the amount of acetylcholine in the synaptic cleft. However, the application of neostigmine cannot always ensure complete restoration of patients’ muscle strength. In addition, routine reversal with neostigmine at the end of the surgery may not only cause a variety of side effects (e.g. bradycardia, bronchoconstriction, hypersalivation), but also adversely affect neuromuscular functions [6, 7].
Meanwhile, sugammadex, a novel reversal agent, has been reported to lower the incidence of postoperative residual NMB with more rapid reversal [8]. Besides, sugammadex has no endogenous targets, so no major adverse effects will be caused [8]. Despite the advantages, until recently the effects of sugammadex on PPCs were controversial [9,10,11,12,13,14,15]. Therefore, in order to address this question, we performed this systematic review and meta-analysis of current evidence to evaluate whether reversal with sugammadex was associated with a lower risk of postoperative PPCs compared with reversal with neostigmine.
Methods
We followed the Cochrane Handbook for Systematic Reviews of Interventions statement and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) (Supplementary file 1). The protocol for this review was registered on PROSPERO (CRD42021253820) on June 7, 2021. Ethical approval and patient consent are not required in a meta-analysis.
Search strategy
Systematic research was performed on PubMed, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) with retrieval time from inception to May 2022. The search was restricted to articles published in English language and full-text versions. Our search strategy was based on two search themes, using: 1) “Sugammadex” or “selective relaxant binding agent of SRBA” or “org 25,969” or “bridion” and 2) “pulmonary complications” or “pneumonia” or “respiratory complications” (Supplementary file 2). A manual search was also conducted to identify additional relevant studies.
Selection criteria
Inclusion criteria were as follows: 1) design: randomized controlled trials (RCTs) or observational studies; 2) population: adult patients (> 18 years) who received non-depolarizing neuromuscular blocking agents for surgery; 3) intervention: receiving sugammadex reversal of neuromuscular blockade; 4) control: receiving neostigmine reversal of neuromuscular blockade. The dose of sugammadex and neostigmine, and the time-point of administration of the study drug were not limited; 5) outcomes: eligible studies must report at least one type of the pulmonary or respiratory complications. The primary outcomes focused on PPCs including desaturation, pneumonia, atelectasis, noninvasive ventilation (NIV) and reintubation. Secondary outcomes were other PPCs including pleural effusion, aspiration pneumonia, airway obstruction and pneumothorax.
Data extraction
Two investigators independently screened the titles and abstracts of initial search results with compliance to selection criteria and selected studies for the final analysis. Data were extracted with a standard form. The collected information was as follows: first author, year of publication, study design, sample size, surgical procedure, dose of study drugs. Divergences were finally resolved by consensus with the corresponding author.
Validity assessment
The quality of RCTs was assessed by the Cochrane Collaboration risk of bias tool in seven aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other biases. Observational studies were evaluated according to the Newcastle–Ottawa scale (NOS) that contained three parts: patient selections, comparability of the study groups, and assessment of outcomes. The score of each part was 4, 2, and 3, respectively. A high-quality study was defined as an overall quality score ≥ 7 [16].
Quality of evidence
Quality of evidence was evaluated by GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) system using the Guideline Development Tool ( https://www.grade.pro.org).
Statistical analysis
All analyses were performed using computer programs including Review Manager (RevMan) V.5.4. For primary and secondary outcomes, we estimated the risk ratio (RR) with 95% confidence intervals (CIs) using the random effects model. The I2 statistics was used to assess the studies’ heterogeneity. I2 values of 0% to 24.9%, 25% to 49.9%, 50% to 74.9%, and 75% to 100% indicated none, low, moderate, and high thresholds for statistical heterogeneity. Furthermore, we performed subgroup analyses according to study type (RCTs and observational studies) and sensitivity analyses to explore the sources of heterogeneity. A funnel plot was used to estimate potential publication bias for analyses over 10 studies. A pre-specified trial sequential analysis (TSA) was performed on the primary outcomes using TSA software (Copenhagen Trial Unit, Center for Clinical Intervention Research, Copenhagen). Statistical significance was set at a two-sided P value < 0.05.
Results
Literature identification and study characteristics
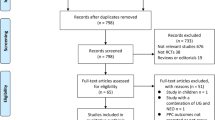
We initially identified 1395 potentially eligible articles in the database searches (343 from Medline/PubMed, 739 from Embase/OVID and 313 from CENTRAL). The results are summarized in the PRISMA diagram (Fig. 1). Eventually, 21 studies were included based on the full text. The characteristics of the eligible studies were described in Table 1.
Ten RCTs [9, 13, 14, 17,18,19,20,21,22,23] (1123 patients) and eleven observational studies [10,11,12, 15, 24,25,26,27,28,29,30] (66,671 patients) were identified for final analyses. The sample size of the included studies ranged from 57 to 45,712 adults. Three studies were multi-centered [10, 22, 23], whereas the rest had a single-center design. The patients in the included studies underwent various surgeries. Seven studies were performed with no surgical procedure restriction [9, 10, 12, 22, 24, 29, 31], four studies on patients undergoing laparoscopic sleeve gastrectomy [11, 15, 17, 25], four on thoracic surgery [18, 20, 21, 30]. Other procedures included laparoscopic cholecystectomy[19], robot-assisted laparoscopic prostatectomy [27], major abdominal surgery[13, 23], da Vinci surgery [28] and treatment of obstructive sleep apnea [14].
Additionally, although all studies compared sugammadex and neostigmine, the drugs were administered in different doses. The doses of sugammadex ranged from 1.5 to 4 mg/kg and the doses of neostigmine ranged from 0.02–0.07 mg/kg. All but three studies [11, 23, 26] reported the quantitative neuromuscular monitoring using the train-of-four ratio with a nerve stimulator.
Quality assessment
Quality assessments of included RCTs were shown in Fig. 2 and observational studies were shown in Table 1. Study quality appraisal showed that 7 of 10 RCTs [9, 14, 19,20,21,22,23] described appropriate methods used for randomized sequence generation and allocation concealment and 6 of 10 RCTs [18,19,20,21,22,23] performed the blinding of both participants and personnel and outcome assessment. 5 RCTs [19,20,21,22,23] were of high quality, whereas others lacked important details to appraise the risk of selection, performance, attrition, or detection biases. Quality assessments of observational studies showed all of them were ranked as publications with high quality with a score of 8 or 9.
Primary outcomes
Meta-analysis of 8 RCTs [9, 13, 14, 17, 19,20,21,22] and 5 observational studies [11, 12, 15, 24, 30] showed that the rate of desaturation (43.2% vs 45.0%, RR = 0.82; 95% CI 0.63 to 1.05; p = 0.11; p for heterogeneity = 0.13, I2 = 31%; Fig. 3, publication bias in Supplementary file 3) were comparable between the two groups.
When looking at other primary outcomes, a significantly lower risk of the following PPCs in the sugammadex were detected when compared with neostigmine: pneumonia (1.37% vs 2.45%, RR = 0.65; 95% CI 0.49 to 0.85; p = 0.002; p for heterogeneity = 0.19, I2 = 27%; Fig. 3, publication bias in Supplementary file 3) by pooling results from 5 RCTs [9, 13, 18, 21, 22] and 5 observational studies [10, 11, 26,27,28]; atelectasis (24.6% vs 30.4%, RR = 0.64; 95% CI 0.42 to 0.98; p = 0.04; p for heterogeneity = 0.0002, I2 = 77%; Fig. 3) from 4 RCTs [9, 18, 21, 23] and 3 observational studies [11, 25, 27]; NIV (1.37% vs 2.33%, RR = 0.65; 95% CI 0.43 to 0.98; p = 0.04; p for heterogeneity = 0.003, I2 = 75%; Fig. 3) from 2 RCTs [13, 14] and 3 observational studies [10, 12, 15]; and reintubation (0.99% vs 1.65%, RR = 0.62; 95% CI 0.43 to 0.91; p = 0.01; p for heterogeneity = 0.79, I2 = 0%; Fig. 3) from 5 RCTs [13, 14, 17, 21, 22] and 4 observational studies [15, 25, 26, 29].
Secondary outcomes
Pooled data showed a significant lower rate of pleural effusion (14.6% vs 19.1%, RR = 0.77; 95% CI 0.61 to 0.95; p = 0.02; p for heterogeneity = 0.62, I2 = 0%; Fig. 4) from two observational studies [11, 25] and airway obstruction (4.7% vs 11.4%, RR = 0.44; 95% CI 0.22 to 0.87; p = 0.02; p for heterogeneity = 0.54, I2 = 0%; Fig. 4) from three observational studies [9, 14, 30] in the sugammadex group compared with that in the neostigmine group.
There were no significant reductions with the use of sugammadex compared to neostigmine in aspiration pneumonia (0.14% vs 0.14%, RR = 1.00; 95% CI 0.10 to 9.60; p = 1.00; p for heterogeneity = 0.34, I2 = 0%; Fig. 4) from one RCT [9] and one observational study [11] and pneumothorax (1.40% vs 1.68%, RR = 0.84; 95% CI 0.37 to 1.89; p = 0.67; p for heterogeneity = 0.87, I2 = 0%; Fig. 4) from one RCT [9] and one observational study [11].
Sensitivity analyses
Sensitivity analyses were performed according to study design (RCTs and observational studies) (Fig. 3 and Fig. 4). The pooled data of the desaturation, pneumonia, NIV and reintubation from observational studies had no change. However, the pooled data of all primary outcomes from RCTs showed no significance between the two groups.
Quality of evidence
GRADE system grades of evidence are low certainty for desaturation, pneumonia, and very low certainty for atelectasis, NIV and reintubation (Supplementary file 4).
Trial sequential analysis
The cumulative Z-score curve (blue line) of desaturation neither crossed the traditional significance boundary nor reached the required information size, which indicated that more trials were needed to detect the effect of sugammadex on the incidence of desaturation compared to neostigmine. The Z-score curves (blue line) of pneumonia and NIV crossed both the required information size (RIS) (vertical red line) and the conventional statistical significance boundary (dotted red lines), which indicated that the observed reduction in rate of pneumonia and NIV in patients using sugammadex could be considered conclusive with the existing evidence. The Z-score curve (blue line) of atelectasis and reintubation crossed the conventional statistical significance boundary (dotted red lines), but did not reach the RIS, which indicated that more trials were needed to reliably detect a plausible effect of sugammadex on the incidence of atelectasis and reintubation (Supplementary file 5). TSA for secondary outcomes were ignored due to too little information used to calculate RIS.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to collect all available data from clinical trials to find out whether reversal with sugammadex was associated with a lower risk of PPCs compared with reversal with neostigmine. In the current study, we performed a comprehensive search with broad search terms, without limiting the search to RCTs or by surgical type.
The major findings of our study indicated that the use of sugammadex as a reversal agent compared to neostigmine decreased the incidence of pneumonia, atelectasis, NIV, reintubation, pleural effusion and airway obstruction. However, the incidence of desaturation, aspiration pneumonia and pneumothorax did not differ significantly between the two groups.
The use of intermediate acting NMB agents during anesthesia was associated with an increased risk of clinically meaningful respiratory complications [7]. However, neostigmine, a commonly used AChE inhibitors, administered after full recovery of neuromuscular function may result in paradoxical muscle weakness [32]. However, sugammadex, a novel reversal agent, acts 10 times more rapidly than neostigmine, which indicates that it can reverse neuromuscular blockade more rapidly and completely than neostigmine and lower the incidence of residual paralysis [8, 33]. Therefore, sugammadex was considered to have the potential to reduce PPCs compared to neostigmine.
Previous meta-analysis [34] comparing sugammadex with neostigmine in older patients found that the application of sugammadex was associated with lower incidence of pneumonia, which was consistent with our study. However, there existed differences between that study and our meta-analysis. First, that study included only three RCTs (n = 510), introducing risk of bias. Second, previous studies showed that sugammadex was related to rapid and complete restoration of muscle strength [8, 33, 35], improving muscle tone of upper airway and chest wall, thus enabling patients to cough, decreased alveolar collapse and prevented microaspiration [36]. Therefore, older patients with worse metabolic function may benefit more from sugammadex [9, 37], which suggested that the conclusion of Carron’s study [34] was less generalizable than our meta-analysis.
In the current meta-analysis, the pooled data of pneumonia, atelectasis, NIV and reintubation all favored sugammadex. Application of TSA indicated that we can draw a confirm conclusion that sugammadex appears superior to neostigmine in decreasing the risk of pneumonia and NIV. These studied outcomes, pneumonia, NIV and reintubation, could represent reliable and impactful pulmonary complications, unlike less severe but more frequent events such as desaturation, and atelectasis [38]. What is noteworthy of this finding is that the superiority of sugammadex might have been largely influenced by the results from Kheterpal et al. (a multicenter matched cohort study including 45,712 participants) as they found sugammadex was associated with a 47% reduced risk of pneumonia, and a 55% reduced risk of respiratory failure compared to neostigmine [10].
Our secondary outcomes, which included pleural effusion, aspiration pneumonia, airway obstruction and pneumothorax, were also likely to be related to neuromuscular blockade. However, these pulmonary outcomes were poorly described in our included studies and a firm conclusion could not be drawn.
There are several potential limitations as our findings are limited by the quality and quantity of available evidence in the included trials. First, according to the GRADE system, the certainty of our findings ranked very low to low across different outcomes. The main limiting factors that contribute to the low overall quality included the high risk of bias and observational study design of included studies. Second, some of the included studies poorly described the outcomes of interest (such as PPCs defined as secondary outcomes). Third, the sample size of included RCTs was limited. Therefore, the findings might be largely influenced by the observational studies. Fourth, the study design of included studies varied in surgical type, time and dosage of drug administration and definition of PPCs, but we pooled data of individual pulmonary complications. Also, the calculated heterogeneity related to the clinical outcomes was very low and sensitivity analyses were also performed. Further evidence, preferably from RCTs, is required to confirm our findings. Fifth, neuromuscular monitoring is strongly recommended whenever NMB agents are administered to decrease the risk of PPCs [39]. However, among 21 studies, 17 studies applied neuromuscular monitoring. And the population who was not monitored may have higher incidence of PPCs, which would obfuscate the results. Furthermore, we did not test for publication bias with datasets of less than 10 data, so publication bias could not be excluded for some outcomes.
Conclusions
The results from this systematic review and meta-analysis suggested that reversal of neuromuscular block with sugammadex decreased the incidence of PPCs including pneumonia, atelectasis, NIV, reintubation, pleural effusion and airway obstruction. The evidence was not strong enough to draw firm conclusions about other PPCs including desaturation, aspiration pneumonia and pneumothorax. More sufficiently powered, prospective randomized studies are warranted to confirm this effect size.
Availability of data and material
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AChE:
-
Acetylcholinesterase
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- CIs:
-
Confidence intervals
- GRADE:
-
Grades of Recommendation, Assessment, Development, and Evaluation
- NIV:
-
Noninvasive ventilation
- NMB:
-
Neuromuscular blockade
- NOS:
-
Newcastle–Ottawa scale
- PPCs:
-
Postoperative pulmonary complications
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCTs:
-
Randomized controlled trials
- RIS:
-
Required information size
- RR:
-
Risk ratio
- TSA:
-
Trial sequential analysis
References
Brueckmann B, Sasaki N, Grobara P, Li MK, Woo T, de Bie J, et al. Effects of sugammadex on incidence of postoperative residual neuromuscular blockade: a randomized, controlled study. Br J Anaesth. 2015;115(5):743–51. https://doi.org/10.1093/bja/aev104.
Sundman E, Witt H, Olsson R, Ekberg O, Kuylenstierna R, Eriksson LI. The incidence and mechanisms of pharyngeal and upper esophageal dysfunction in partially paralyzed humans: pharyngeal videoradiography and simultaneous manometry after atracurium. Anesthesiol. 2000;92(4):977–84. https://doi.org/10.1097/00000542-200004000-00014.
Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg. 2010;111(1):120–8. https://doi.org/10.1213/ANE.0b013e3181da832d
Mirzakhani H, Williams JN, Mello J, Joseph S, Meyer MJ, Waak K, et al. Muscle weakness predicts pharyngeal dysfunction and symptomatic aspiration in long-term ventilated patients. Anesthesiol. 2013;119(2):389–97. https://doi.org/10.1097/ALN.0b013e31829373fe.
Martinez-Ubieto J, Ortega-Lucea S, Pascual-Bellosta A, Arazo-Iglesias I, Gil-Bona J, Jimenez-Bernardó T, et al. Prospective study of residual neuromuscular block and postoperative respiratory complications in patients reversed with neostigmine versus sugammadex. Minerva Anestesiol. 2016;82(7):735–42.
Hayes AH, Mirakhur RK, Breslin DS, Reid JE, McCourt KC. Postoperative residual block after intermediate-acting neuromuscular blocking drugs. Anesth. 2001;56(4):312–8. https://doi.org/10.1046/j.1365-2044.2001.01921.x.
Grosse-Sundrup M, Henneman JP, Sandberg WS, Bateman BT, Uribe JV, Nguyen NT, et al. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. Bmj. 2012;345:e6329. https://doi.org/10.1136/bmj.e6329
Hristovska AM, Duch P, Allingstrup M, Afshari A. The comparative efficacy and safety of sugammadex and neostigmine in reversing neuromuscular blockade in adults. A Cochrane systematic review with meta-analysis and trial sequential analysis. Anaesthesia. 2018;73(5):631–41.doi: https://doi.org/10.1111/anae.14160
Togioka BM, Yanez D, Aziz MF, Higgins JR, Tekkali P, Treggiari MM. Randomised controlled trial of sugammadex or neostigmine for reversal of neuromuscular block on the incidence of pulmonary complications in older adults undergoing prolonged surgery. Br J Anaesth. 2020;124(5):553–61. https://doi.org/10.1016/j.bja.2020.01.016.
Kheterpal S, Vaughn MT, Dubovoy TZ, Shah NJ, Bash LD, Colquhoun DA, et al. Sugammadex versus Neostigmine for Reversal of Neuromuscular Blockade and Postoperative Pulmonary Complications (STRONGER): A Multicenter Matched Cohort Analysis. Anesthesiol. 2020;132(6):1371–81. https://doi.org/10.1097/ALN.0000000000003256.
Han J, Ryu JH, Koo BW, Nam SW, Cho SI, Oh AY. Effects of Sugammadex on Post-Operative Pulmonary Complications in Laparoscopic Gastrectomy: A Retrospective Cohort Study. J Clin Med. 2020;9(4). https://doi.org/10.3390/jcm9041232
Krause M, McWilliams SK, Bullard KJ, Mayes LM, Jameson LC, Mikulich-Gilbertson SK, et al. Neostigmine Versus Sugammadex for Reversal of Neuromuscular Blockade and Effects on Reintubation for Respiratory Failure or Newly Initiated Noninvasive Ventilation: An Interrupted Time Series Design. Anesth Analg. 2020;131(1):141–51. https://doi.org/10.1213/ANE.0000000000004505.
Alday E, Munoz M, Planas A, Mata E, Alvarez C. Effects of neuromuscular block reversal with sugammadex versus neostigmine on postoperative respiratory outcomes after major abdominal surgery: a randomized-controlled trial. Can J Anaesth. 2019;66(11):1328–37. https://doi.org/10.1007/s12630-019-01419-3.
Ünal DY, Baran İ, Mutlu M, Ural G, Akkaya T, Özlü O. Comparison of Sugammadex versus Neostigmine Costs and Respiratory Complications in Patients with Obstructive Sleep Apnoea. Turk J Anaesthesiol Reanim. 2015;43(6):387–95. https://doi.org/10.5152/tjar.2015.35682.
Ezri T, Evron S, Petrov I, Schachter P, Berlovitz Y, Shimonov M. Residual Curarization and Postoperative Respiratory Complications Following Laparoscopic Sleeve Gastrectomy. The Effect of Reversal Agents: Sugammadex vs. Neostigmine. J Crit Care Med (Targu Mures). 2015;1(2):61–7.doi: https://doi.org/10.1515/jccm-2015-0009
Wells G SB, O’Connell B, et al. The New- castle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http:// www.ohri.ca/programs/clinical_epidemiology/ oxford.asp. Accessed 10 Dec 2020.
Evron S, Abelansky Y, Ezri T, Izakson A. Respiratory events with sugammadex vs. neostigmine following laparoscopic sleeve gastrectomy: a prospective pilot study assessing neuromuscular reversal strategies. Rom J Anaesth Intensive Care. 2017;24(2):111–4. https://doi.org/10.21454/rjaic.7518.242.evr
Baysal Çitil A, Alıcıkuş Tuncel Z, Yapıcı N, Kudsioğlu T, Aykaç Z, Kavaklı AS. Reversal of rocuronium induced neuromuscular blockade in lung resection surgery: a comparison of sugammadex and neostigmine. J Cardio-Vascular-Thoracic Anesth Intensive Care Soc. 2019. https://doi.org/10.5222/gkdad.2019.49369.
Lee YJ, Oh AY, Koo BW, Han JW, Park JH, Hong JP, et al. Postoperative residual neuromuscular blockade after reversal based on a qualitative peripheral nerve stimulator response: A randomised controlled trial. Eur J Anaesthesiol. 2020;37(3):196–202. https://doi.org/10.1097/EJA.0000000000001157.
Moon TS, Reznik S, Pak T, Jan K, Pruszynski J, Kim A, et al. Sugammadex versus neostigmine for reversal of rocuronium-induced neuromuscular blockade: A randomized, double-blinded study of thoracic surgical patients evaluating hypoxic episodes in the early postoperative period. J Clin Anesth. 2020;64:109804. https://doi.org/10.1016/j.jclinane.2020.109804
Lee TY, Jeong SY, Jeong JH, Kim JH, Choi SR. Comparison of postoperative pulmonary complications between sugammadex and neostigmine in lung cancer patients undergoing video-assisted thoracoscopic lobectomy: a prospective double-blinded randomized trial. Anesth Pain Med. 2021;16(1):60–7. https://doi.org/10.17085/apm.20056.
Ledowski T, Szabó-Maák Z, Loh PS, Turlach BA, Yang HS, de Boer HD, et al. Reversal of residual neuromuscular block with neostigmine or sugammadex and postoperative pulmonary complications: a prospective, randomised, double-blind trial in high-risk older patients. Br J Anaesth. 2021;127(2):316–23. https://doi.org/10.1016/j.bja.2021.04.026.
Leslie K, Chan MTV, Darvall JN, De Silva AP, Braat S, Devlin NJ, et al. Sugammadex, neostigmine and postoperative pulmonary complications: an international randomised feasibility and pilot trial. Pilot and feasibility studies. 2021;7(1). https://doi.org/10.1186/s40814-021-00942-9
Ledowski T, Hillyard S, O’Dea B, Archer R, Vilas-Boas F, Kyle B. Introduction of sugammadex as standard reversal agent: Impact on the incidence of residual neuromuscular blockade and postoperative patient outcome. Indian J Anaesth. 2013;57(1):46–51. https://doi.org/10.4103/0019-5049.108562.
Llaurado S, Sabate A, Ferreres E, Camprubi I, Cabrera A. Postoperative respiratory outcomes in laparoscopic bariatric surgery: comparison of a prospective group of patients whose neuromuscular blockade was reverted with sugammadex and a historical one reverted with neostigmine. Rev Esp Anestesiol Reanim. 2014;61(10):565–70. https://doi.org/10.1016/j.redar.2013.11.009.
Li G, Freundlich RE, Gupta RK, Hayhurst CJ, Le CH, Martin BJ, et al. Postoperative Pulmonary Complications’ Association with Sugammadex versus Neostigmine. Anesthesiol. 2021. https://doi.org/10.1097/aln.0000000000003735.
Yu J, Park JY, Lee Y, Hwang JH, Kim YK. Sugammadex versus neostigmine on postoperative pulmonary complications after robot-assisted laparoscopic prostatectomy: a propensity score-matched analysis. J Anesth. 2021. https://doi.org/10.1007/s00540-021-02910-2.
Cheng K, Tse J, Li TY. The Strategy to Use Sugammadex to Reduce Postoperative Pulmonary Complications after da Vinci Surgery: A Retrospective Study. Journal of Personalized Medicine. 2022;12(1). https://doi.org/10.3390/jpm12010052
Goodner JA, Likar EJ, Hoff AL, Quedado JM, Kohli A, Ellison P. Clinical Impact of Sugammadex in the Reversal of Neuromuscular Blockade. Cureus. 2021;13(6):e15413. https://doi.org/10.7759/cureus.15413
Murphy GS, Avram MJ, Greenberg SB, Bilimoria S, Benson J, Maher CE, et al. Neuromuscular and Clinical Recovery in Thoracic Surgical Patients Reversed With Neostigmine or Sugammadex. Anesth Analg. 2021;133(2):435–44. https://doi.org/10.1213/ANE.0000000000005294.
Li G, Freundlich RE, Gupta RK, Hayhurst CJ, Le CH, Martin BJ, et al. Postoperative Pulmonary Complications’ Association with Sugammadex versus Neostigmine: A Retrospective Registry Analysis. Anesthesiol. 2021;134(6):862–73. https://doi.org/10.1097/ALN.0000000000003735.
Phillips S, Stewart PA. Catching a Unicorn: Neostigmine and Muscle Weakness-Not Neostigmine for All, but Quantitative Monitoring for Everyone! Anesthesiol. 2018;129(2):381–2. https://doi.org/10.1097/aln.0000000000002295.
Hristovska AM, Duch P, Allingstrup M, Afshari A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database Syst Rev. 2017;8(8):Cd012763. https://doi.org/10.1002/14651858.Cd012763
Carron M, Tessari I, Linassi F. Sugammadex compared with neostigmine in reducing postoperative pulmonary complications in older patients: a meta-analysis. Br J Anaesth. 2022;128(4):e259–62. https://doi.org/10.1016/j.bja.2021.12.038.
Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiol. 2008;109(5):816–24. https://doi.org/10.1097/ALN.0b013e31818a3fee.
Bulka CM, Terekhov MA, Martin BJ, Dmochowski RR, Hayes RM, Ehrenfeld JM. Nondepolarizing Neuromuscular Blocking Agents, Reversal, and Risk of Postoperative Pneumonia. Anesthesiol. 2016;125(4):647–55. https://doi.org/10.1097/aln.0000000000001279.
Ledowski T, Szabó-Maák Z, Loh PS, Turlach BA, Yang HS, de Boer HD, et al. Reversal of residual neuromuscular block with neostigmine or sugammadex and postoperative pulmonary complications: a prospective, randomised, double-blind trial in high-risk older patients. Br J Anaesth. 2021;127(2):316–23. https://doi.org/10.1016/j.bja.2021.04.026.
Abbott TEF, Fowler AJ, Pelosi P, Gama de Abreu M, Møller AM, Canet J, et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth. 2018;120(5):1066–79. https://doi.org/10.1016/j.bja.2018.02.007
Carvalho H, Verdonck M, Cools W, Geerts L, Forget P, Poelaert J. Forty years of neuromuscular monitoring and postoperative residual curarisation: a meta-analysis and evaluation of confidence in network meta-analysis. Br J Anaesth. 2020;125(4):466–82. https://doi.org/10.1016/j.bja.2020.05.063.
Acknowledgements
None.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HY and PL planned the study. HML and YDZ performed the literature search, data extraction. HY and HML performed the statistical analysis and drafted the manuscript. PL critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary file 1.
PRISMA Checklist.
Additional file 2: Supplementary file
2. Electronic search strategies.
Additional file 3: Supplementary file 3.
Funnel plot.
Additional file 4: Supplementary file 4.
Quality of evidence by GRADE.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, HM., Yu, H., Zuo, YD. et al. Postoperative pulmonary complications after sugammadex reversal of neuromuscular blockade: a systematic review and meta-analysis with trial sequential analysis. BMC Anesthesiol 23, 130 (2023). https://doi.org/10.1186/s12871-023-02094-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-023-02094-0