Abstract
Background
Video-assisted surgery has become an increasingly used surgical technique in patients undergoing major thoracic and abdominal surgery and is associated with significant perioperative respiratory and cardiovascular changes. The aim of this study was to investigate the effect of intraoperative pneumoperitoneum during video-assisted surgery on respiratory physiology in patients undergoing robotic-assisted surgery compared to patients undergoing classic laparoscopy in Trendelenburg position.
Methods
Twenty-five patients undergoing robotic-assisted surgery (RAS) were compared with twenty patients undergoing classic laparoscopy (LAS). Intraoperative ventilatory parameters (lung compliance and plateau airway pressure) were recorded at five specific timepoints: after induction of anesthesia, after carbon dioxide (CO2) insufflation, one-hour, and two-hours into surgery and at the end of surgery. At the same time, arterial and end-tidal CO2 values were noted and arterial to end-tidal CO2 gradient was calculated.
Results
We observed a statistically significant difference in plateau pressure between RAS and LAS at one-hour (26.2 ± 4.5 cmH2O vs. 20.2 ± 3.5 cmH2O, p = 0.05) and two-hour intervals (25.2 ± 5.7 cmH2O vs. 17.9 ± 3.1 cmH2O, p = 0.01) during surgery and at the end of surgery (19.9 ± 5.0 cmH2O vs. 17.0 ± 2.7 cmH2O, p = 0.02). Significant changes in lung compliance were also observed between groups at one-hour (28.2 ± 8.5 mL/cmH2O vs. 40.5 ± 13.9 mL/cmH2O, p = 0.01) and two-hour intervals (26.2 ± 7.8 mL/cmH2O vs. 54.6 ± 16.9 mL/cmH2O, p = 0.01) and at the end of surgery (36.3 ± 9.9 mL/cmH2O vs. 58.2 ± 21.3 mL/cmH2O, p = 0.01). At the end of surgery, plateau pressures remained higher than preoperative values in both groups, but lung compliance remained significantly lower than preoperative values only in patients undergoing RAS with a mean 24% change compared to 1.7% change in the LAS group (p = 0.01). We also noted a more significant arterial to end-tidal CO2 gradient in the RAS group compared to LAS group at one-hour (12.9 ± 4.5 mmHg vs. 7.4 ± 4.4 mmHg, p = 0.02) and two-hours interval (15.2 ± 4.5 mmHg vs. 7.7 ± 4.9 mmHg, p = 0.02), as well as at the end of surgery (11.0 ± 6.6 mmHg vs. 7.0 ± 4.6 mmHg, p = 0.03).
Conclusion
Video-assisted surgery is associated with significant changes in lung mechanics after induction of pneumoperitoneum. The observed changes are more severe and longer-lasting in patients undergoing robotic-assisted surgery compared to classic laparoscopy.
Similar content being viewed by others
Introduction
Video assisted surgery (VAS) has become extensively used worldwide in cardiothoracic and major abdominal surgery, including gynecological and urological pro-cedures [1, 2]. VAS, in combination with early-recovery after surgery protocols, enhances patient recovery, lowers overall costs, and shortens hospital stay with the same oncologic outcomes as laparotomy [3,4,5]. As patients presenting for VAS are becoming older and with more severe co-morbidities anesthesiologists are presented with new challenges and cases are becoming more difficult to manage. Trends in anesthesia for VAS are changing and anesthesiologists must provide both proper anesthesia to facilitate the surgical technique [6] and to assure patient safety throughout the perioperative period, minimizing perioperative risks [7].
From an anesthesiologists’ point of view the main problems during VAS are related to patient positioning and pneumoperitoneum induced changes in cardiovascular and respiratory physiology. Insufflation of carbon dioxide (CO2) during VAS is associated with an increase in mean arterial pressure and systemic vascular resistance and a decrease cardiac output [8] and renal blood flow [9]. In the pulmonary system, pneumoperitoneum is associated with an increase in plateau pressure and a decrease in lung compliance making mechanical ventilation and effective CO2 removal a potential problem during surgery [10].
Two VAS techniques are generally used during major abdominal surgery: classic laparoscopic surgery (LAS) or robotic assisted surgery (RAS). Although most studies demonstrate similar surgical outcomes between the two techniques [11], no study to date has focused on the comparative effects of LAS and RAS on pulmonary mechanics. The primary outcome was to assess the effects of pneumoperitoneum on lung compliance and airway pressure during video-assisted surgery (VAS) in patients undergoing RAS compared to patients undergoing LAS in Trendelenburg position. The secondary objective was to assess the effect of this changes on arterial to end-tidal CO2 gradient and the return of both lung compliance and airway pressure to pre-pneumoperitoneum values at the end of surgery.
Methods
The ethical approval for the present study was provided by the Ethical Committee of Fundeni Clinical Institute, Bucharest, Romania, and all patients signed the informed consent.
Twenty-five consecutive patients who underwent RAS were matched based on age, body mass index, American Society of Anesthesiologists (ASA) score, duration of surgery and of pneumoperitoneum and baseline lung mechanics parameters (Table 1) with a second group of twenty patients who underwent LAS in the Department of General Surgery and Liver Transplantation at Fundeni Clinical Institute. The decision to perform either RAS or LAS was made by the attending surgeon prior to patient inclusion. All patients underwent VAS in Trendelenburg position for endometrial cancer or rectal carcinoma. Exclusion criteria consisted of age under 18 years, a body mass index > 35 kg/m2, conversion of VAS to laparotomy and severe preoperative pulmonary or cardiovascular co-morbidities.
All surgeries were performed under general anesthesia by four surgeons experienced in VAS. Induction of anesthesia was performed using propofol, fentanyl and atracurium and maintained of anesthesia was achieved with Sevoflurane and fentanyl. Subsequent doses of atracurium were administered during surgery guided by train of four monitoring. The lungs were ventilated using a Perseus A500 Anesthesia Machine (Dräger Medical®, Lübeck, Germany) in a volume-controlled mode with an oxygen/air mixture of 0.5. Ventilator settings were tidal volume of 7 mL/kg, inspiratory/expiratory ratio 1:2, an inspiratory fresh gas flow of 2.0 L/min and an end-expiratory positive pressure of 5 mmHg. Respiratory rate was adjusted to maintain an EtCO2 pressure of 36 ± 4 mmHg. An arterial catheter was inserted on the radial artery before induction of anesthesia for blood sample collection and hemodynamic monitoring. Pneumoperitoneum was obtained by CO2 insufflation after induction of anesthesia and was automatically maintained at 12–14 mmHg in both groups.
Patient age, sex, height, and weight were collected by the attending anesthesiologist before surgery. Arterial blood samples and ventilatory parameters were obtained at five specific time points: after induction of anesthesia (T0), after induction of pneumoperitoneum (T1), one-hour into surgery (T2), two-hours into surgery (T3) and at the end of surgery (T4). Arterial blood gases analysis was performed on ABL 800 Radiometer (Medical APS®, Brǿnshǿj, Denmark). Lung compliance (Lc) was defined as pulmonary compliance during periods without gas flow, such as during an inspiratory pause. The following ventilatory parameters were recorded at the five timepoints: plateau airway pressure (Pplat), Lc after performing an inspiratory hold maneuver and end-tidal CO2 (EtCO2). The arterial to EtCO2 gradient (ΔCO2) was calculated as the arithmetic difference between the measured arterial oxygen pressure (PaO2) and the mean EtCO2 during the minute before obtaining the arterial blood sample. Percentual change in either Lc or Pplat was calculated by the following formula: [(parameter at a time “x” – parameter at time “x + 1”)/parameter at time “x”] *100 and results were recorded as absolute values. Haemodynamic variables, mean arterial blood pressure measured invasively – MAP and heart rate – HR, were recorded at the same time points.
In order to detect clinically significant 15% change in lung compliance and airway pressure, based on mean variables cited in the literature, 24 patients were included in the RAS group in order to obtain a 75% statical power. The 15% change was based on previously published data from our study group, as well as that demonstrated by other studies [10, 12] These patients were matched on a 0.8 ratio to 20 patients undergoing LAS. Statistical analyses were performed using SPSS 19.0 (SPSS Inc®, Chicago, IL, USA). Data are presented as mean ± standard deviation of the mean. Data distribution was examined for normality using Kolmogorov Smirnov test to insure the proper statistical examination. Categorical variables were analyzed by utilizing the Chi-square test and quantitative data were analyzed with independent samples t-test. Mann–Whitney test was used when the analyzed data did not follow a normal distribution. All P values are two-tailed and a P value ≤ 0.05 was considered statistically signdicant.
Results
Twenty-five patients were included in the RAS group and twenty patients in the LAS group. No statistically significant differences regarding preoperative variables were identified between the two groups (Table 1). Pplat increased and Lc decreased after the induction of pneumoperitoneum, but no significant difference was observed between the two groups. We observed a statistically significant difference in Pplat and Lc between RAS and LAS at one-hour and two-hour intervals during surgery and at the end of surgery. Data are presented in Table 2.
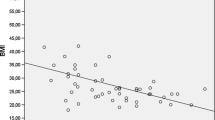
Pplat significantly increased to 25.0 ± 5.3 cmH2O in the RAS group after induction of pneumoperitoneum compared to 15.1 ± 3.4 cmH2O postinduction of anesthesia (p = 0.05) but did not change compared to this level at both one-hour (26.2 ± 4.5 cmH2O, p = 0.48) and two-hours into surgery (25.2 ± 5.7 cmH2O, p = 0.11) or at the end of surgery (19.9 ± 5.0 cmH2O, p = 0.36). Pplat remained significantly higher at the end of surgery compared to postinduction of anesthesia (p = 0.05). In the LAS group, Pplat increased to 22.2 ± 4.6 cmH2O after induction of pneumoperitoneum compared to 14.9 ± 2.7 cmH2O postinduction of anesthesia (p = 0.01) but did not change intraoperatively at one-hour (20.2 ± 3.5 cmH2O, p = 0.28) and two-hours into surgery (17.9 ± 3.1 cmH2O, p = 0.59) or at the end of surgery (17.0 ± 2.7 cmH2O, p = 0.69). Pplat remained significantly higher at the end of surgery compared to postinduction of anesthesia (p = 0.02)—Fig. 1A.
In the RAS group, Lc decreased significantly to 26.4 ± 6.4 mL/cmH2O after insufflation of pneumoperitoneum compared to 48.1 ± 8.8 mL/cmH2O postinduction of anesthesia (p = 0.03) but did not significantly change com-pared to this level at one-hour (28.2 ± 8.5 mL/cmH2O, p = 0.104) or two-hours into surgery (26.2 ± 7.8 mL/cmH2O, p = 0.71) or between the second hour and the end of surgery (36.3 ± 9.9 mL/cmH2O, p = 0.43). Lc remained statistically significantly lower at end of anesthesia compared postinduction of anesthesia (p = 0.04). In the LAS group, Lc decreased significantly to 33.0 ± 7.2 mL/cmH2O after induction of pneumoperitoneum compared to 58.0 ± 9.8 mL/cmH2O postinduction of anesthesia (p = 0.02), then significantly increased at one-hour into surgery compared to postinduction of pneumoperitoneum (40.5 ± 13.9 mL/cmH2O, p = 0.02) and remained constant at two-hours into surgery (54.6 ± 16.9 cL/cmH2O, p = 0.90) and at the end of surgery (58.2 ± 21.3 mL/cmH2O,,p = 0.91). There was no statistical difference in Lc at the end of surgery compared to postinduction of anesthesia (p = 0.23) – Fig. 1B. The difference in percentual change in Lc and Pplat between the two groups at the five specific timepoints are presented in Table 3.
We observed a non-significant difference between the RAS and LAS groups in ΔCO2 after induction of anesthesia (4.6 ± 2.1 mmHg vs. 5.0 ± 1.8 mmHg, p = 0.41) and after induction of pneumoperitoneum (7.9 ± 3.9 mmHg vs. 6.1 ± 4.5 mmHg, p = 0.36) and a statistically significant difference one-hour (12.9 ± 4.5 mmHg vs. 7.4 ± 4.4 mmHg, p = 0.02) and two-hours into the surgery (15.2 ± 4.5 mmHg vs. 7.7 ± 4.9 mmHg, p = 0.02) and at the end of surgery (11.0 ± 6.6 mmHg vs. 7.0 ± 4.6 mmHg, p = 0.03). Data are presented in Table 2. No significant differences were observed between the two groups in terms of measured hemodynamic parameters (MAP and HR) and PaO2 during the same time points. Data are presented in supplementary table 1.
Discussion
Our results show that induction of pneumoperitoneum is associated with an increase in plateau pressure and decrease in lung compliance in patients undergoing VAS in Trendelenburg position independent of surgical technique. Our results are in accordance with previously published data in patients undergoing pelviscopic surgery [13].
The increase in Pplat during surgery observed in both groups may be explained by the increase in intra-abdominal pressure due to induction of pneumoperitoneum and Trendelenburg position that cause an upward shift of the diaphragm. This increases intrathoracic pressure and is responsible for observed changes in the distribution of ventilation and subsequent increase in ventilation-perfusion mismatch [14, 15]. However, in our study group, the Pplat failed to return to preoperative levels at the end of surgery after pneumoperitoneum was released. This is in accordance with the study published by Lian et al. [16] who showed that peak pressures remain higher than perioperative values in patients undergoing laparoscopic hysterectomy regardless of the ventilation mode applied and this may be attributed to retention of secretions and basal atelectasis that persist after pneumoperitoneum is released.
In a study by Choi et al. [17], patients who had a peak airway pressure ≥ 30 cm H2O had a fivefold greater incidence of postoperative respiratory complications, longer postanesthesia care unit stays, greater alveolar dead space-to-tidal volume ratios and a lower arterial partial pressure of oxygen. We consider that anesthetic strategies aimed at lowering airway pressure below this threshold are important to improve both intraoperative respiratory function and to decrease the incidence of postoperative complications. In another study, Sroussi et al. [18] showed that the use of a new insufflation system that uses a lower intra-abdominal pressure is associated with improved hemodynamics and lower peak lower pressures. The development of such surgical techniques may be useful in lowering the effects of pneumoperitoneum on lung mechanics while maintaining adequate surgical access.
The changes observed in lung compliance were more long-lasting during RAS. In this group we observed that Lc decreased after induction of pneumoperitoneum, remained low throughout surgery, and did not return to preoperative values at the end of surgery. By comparison, in the LAS group Lc decreased after induction of pneumoperitoneum, gradually increased during surgery and there was no statistically significant difference between preoperative and end-of surgery values. The decrease in Lc is mostly due to basal atelectasis, decrease in functional residual capacity and a decrease in diaphragmatic excursion during pneumoperitoneum [19]. Application of positive end-expiratory pressure may slightly recover the ventilation-perfusion mismatch in the Trendelenburg position and improve both oxygenation and lung mechanics [20]. However, studies did not find an appropriate level of positive end-expiratory pressure to improve intraoperative ventilation and lower the incidence of postoperative hypoxia [21] and further research is still needed.
Although in our study there was no significant difference in terms of length of surgery, this may represent a reason for the persistence of decreased Lc and increased Pplat at the end of surgery.
The observed differences in both Lc and Pplat between RAS and LAS were statistically significant with better lung mechanics in patients undergoing classic laparoscopy. Two main reasons can be responsible for the observed changes. The first would be a much higher insufflation pressure to maintain pneumoperitoneum during surgery. However, no difference between intraabdominal pressure was observed between the two groups. (12–14 mmHg). The second reason would be a steeper Trendelenburg position applied during RAS to improve surgical access [22]. In a study published by Mitsuhashi et al. [23] even a slightly higher increase of 5°, from 20° to 25°, in head-down position was associated with a significant increase in airway pressures in obese patients undergoing robotic-assisted hysterectomy. Interesting, in a recent survey on randomly selected active members of the American Society of Anesthesiology, more than two-thirds did not limit the duration or inclination angle during VAS [24]. This crucial of patient positioning is mostly decided by surgeons in order to improve surgical access, especially in RAS where the robustness of the device may impose a steeper position up to 45° in order to facilitate arm movement [25, 26]. From an anesthesiologic point of view, pulmonary changes associated with induction of pneumoperitoneum may be different between laparoscopic and robotic-assisted surgery and a more personalized, patient-based approach should be applied to improve lung mechanics.
One of the most important aspects of any observed physiological changes during anesthesia is the impact on patient outcome. In a recently published systematic review, Katayama et al. [27] found no correlation between steep Trendelenburg position and incidence of cardiac, cerebrovascular complications, as well as an increased risk of venous thromboembolism. However, a third of patients may experience postoperative pulmonary complications that require admission to an intensive care unit [28] and, hence, appropriate intraoperative management and correction of ventilatory alterations becomes a crucial issue. Intraoperative recruitment maneuvers and the addition of positive end-expiratory pressure (PEEP) are well documented in the literature. Kudoh et al. [29], demonstrated a significant increase in lung compliance after performing a 30 s recruitment maneuver of sustained inflation to 30 cmH2O alongside applying a PEEP of 5 cmH2O. However, due to the low number of patients we cannot assess if this is sufficient to decrease the incidence of postoperative pulmonary complications. The use of recruitment maneuvers has also been investigated in a meta-analysis published by Pei et al. [30]. Their results show a significant impact in improving intraoperative lung mechanics and reducing postoperative pulmonary complications but the exact effect of such a technique on cardiocirculatory physiology, and especially venous return and cardiac output needs further research. Using higher PEEP values was assessed by Shono et al. [31] in a randomized control trial and have demonstrated that the application of 15 cm H2O of PEEP resulted in a better ventilation profile and favorable physiologic effects during RAS prostatectomy, however this did not improve postoperative lung function.
The mode of mechanical ventilation may also represent a key factor in lung mechanics during VAS. When comparing pressure-controlled ventilation to volume controlled-ventilation, pressure-control was associated with higher Lc and lower peak airway pressure but did not have any overall advance in terms of respiratory mechanics and hemodynamics [32]. Dual-controlled ventilation may offer the combined benefits of both volume- and pressure-controlled ventilation. In their study, Park et al. [33], demonstrated that using Autoflow they were able to also decrease the peak inspiratory airway pressure. However, due to the low number of patients no estimate on the potential impact on postoperative pulmonary could be made. Based on these studies, it seems that the best strategy should combine recruitment maneuvers, optimal PEEP, and pressure-controlled ventilation. However, to date, there are no conclusive evidence to support the best anesthetic management during VAS to improve ventilatory parameters and future research is urgently needed in order to decrease the high incidence of postoperative pulmonary strategy.
The induction of pneumoperitoneum was associated with an increase in arterial to end-tidal CO2 difference. Absorption of CO2 during surgery and increased ventilation-perfusion mismatch is responsible for the higher CO2 gradient [34]. Kamine et al. [35] showed that higher abdominal pressure values were associated with decreased end-tidal CO2 values. Although abdominal pressure was identical between the two groups, we observed that patients in the RAS group had both a higher CO2 gradient and a decreased lung compliance. This may be related with increased atelectasis and a higher shunt fraction that is responsible for the difference in arterial to end-tidal CO2, and thus making ΔCO2 a useful marker in the assessment of ventilation-perfusion mismatch.
The present study has some limitations. First, this was an observational, retrospective, single-center study and all patients received the same ventilatory strategy independent of the video-assisted technique use and so, the observed difference in ventilatory mechanics, may be minimized by a more personalized approach on ventilation and positive end-expiratory pressure titration. The authors are aware of the fact that these strategies can very between centers and our remarks may apply only in patients who undergo VAS under similar conditions of mechanical ventilation. Secondary, some parameters, like the steepness of Trendelenburg position and shunt fraction, that may have an important effect on lung physiology, could not be assessed. Thirdly, the low number of patients was insufficient to assess the effects of intraoperative lung mechanics on postoperative outcome. Future studies are needed to investigate the composite effect of surgical position, type of VAS used and intraoperative recruitment maneuvers on perioperative lung mechanics.
Conclusion
In conclusion VAS, regardless of whether RAS or LAS was used, or is associated with increased airway pressure and decreased lung compliance. The effects of pneumoperitoneum on lung mechanics are more pronounced in patients undergoing robotic-assisted surgery compared to classic laparoscopy. Although airway pressures failed to return to preoperative values in both groups at the end of surgery, changes in lung compliance were minimal compared to preoperative values in patients undergoing classic laparoscopy compared to RAS after the pneumoperitoneum was released. The decrease in lung compliance and increase in plateau pressure was associated with a greater arterial to end-tidal CO2 gradient.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- VAS:
-
Video-assisted surgery
- RAS:
-
Robotic-assisted surgery
- LAS:
-
Classic laparoscopic surgery
- ASA score:
-
American Society of Anesthesiologists score
- CO2 :
-
Carbon dioxide
- EtCO2 :
-
End-tidal carbon dioxide
- PaO2 :
-
Arterial partial pressure of oxygen
- Lc:
-
Lung compliance
- Pplat:
-
Plateau pressure
References
Roh HF, Nam SH, Kim JM. Robot-assisted laparoscopic surgery versus conventional laparoscopic surgery in randomized controlled trials: A systematic review and meta-analysis. PLoS ONE. 2018;13(1):e0191628.
O’Sullivan KE, Kreaden US, Hebert AE, Eaton D, Redmond KC. A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact Cardiovasc Thorac Surg. 2019;28(4):526–34.
Song XJ, Liu ZL, Zeng R, Ye W, Liu CW. A meta-analysis of laparoscopic surgery versus conventional open surgery in the treatment of colorectal cancer. Medicine (Baltimore). 2019;98(17):e15347.
Pisarska M, Torbicz G, Gajewska N, Rubinkiewicz M, Wierdak M, Major P, et al. Compliance with the ERAS Protocol and 3-Year Survival After Laparoscopic Surgery for Non-metastatic Colorectal Cancer. World J Surg. 2019;43(10):2552–60.
Pędziwiatr M, Wierdak M, Nowakowski M, Pisarska M, Stanek M, Kisielewski M, et al. Cost minimization analysis of laparoscopic surgery for colorectal cancer within the enhanced recovery after surgery (ERAS) protocol: a single-centre, case-matched study. Wideochir Inne Tech Maloinwazyjne. 2016;11(1):14–21.
Bruintjes MH, van Helden EV, Braat AE, Dahan A, Scheffer GJ, van Laarhoven CJ, et al. Deep neuromuscular block to optimize surgical space conditions during laparoscopic surgery: a systematic review and meta-analysis. BJA. 2017;118(6):834–42.
Bajwa SJ, Kulshrestha A. Anaesthesia for laparoscopic surgery: General vs regional anaesthesia. J Minim Access Surg. 2016;12(1):4–9.
Atkinson TM, Giraud GD, Togioka BM, Jones DB, Cigarroa JE. Cardiovascular and Ventilatory Consequences of Laparoscopic Surgery. Circulation. 2017;135:700–10.
O’Leary E, Hubbard K, Tormey W, Cunningham AJ. Laparoscopic cholecystectomy: haemodynamic and neuroendocrine responses after pneumoperitoneum and changes in position. Br J Anaesth. 1996;76:640–4.
Tomescu DR, Popescu M, Dima SO, Bacalbașa N, Bubenek-Turconi Ș. Obesity is associated with decreased lung compliance and hypercapnia during robotic assisted surgery. J Clin Monit Comput. 2017;31(1):85–92.
Mäenpää MM, Nieminen K, Tomás EI, Laurila M, Luukkaala TH, Mäenpää JU. Robotic-assisted vs traditional laparoscopic surgery for endometrial cancer: a randomized controlled trial. Am J Obstet Gynecol. 2016;215(588):e1-7.
Tanskanen P, Kyttä J, Randell T. The effect of patient positioning on dynamic lung compliance. Acta Anaesthesiol Scand. 1997;41(5):602–6.
Suh MK, Seong KW, Jung SH, Kim SS. The effect of pneumoperitoneum and Trendelenburg position on respiratory mechanics during pelviscopic surgery. Korean J Anesthesiol. 2010;59(5):329–34.
Rashwan DA, Mahmoud HE, Nofal WH, Sabek EA. Ultrasonographic evaluation of the effect of positive end-expiratory pressure on diaphragmatic functions in patients undergoing laparoscopic colorectal surgery: a prospective randomized comparative study. J Anesth Clin Res. 2018;9(7):843–51.
He X, Jiang J, Liu Y, Xu H, Zhou S, Yang S, et al. Electrical Impedance Tomography-guided PEEP Titration in Patients Undergoing Laparoscopic Abdominal Surgery. Medicine (Baltimore). 2016;95(14):e3306.
Lian M, Zhao X, Wang H, Chen L, Li S. Respiratory dynamics and dead space to tidal volume ratio of volume-controlled versus pressure-controlled ventilation during prolonged gynecological laparoscopic surgery. Surg Endosc. 2017;31:3605–13.
Choi SB, Park HK, Hong JH, Kim BG, Kang H. Postoperative respiratory complications and peak airway pressure during laparoscopic colectomy in patients with colorectal cancer. Surg Laparosc Endosc Percutan Tech. 2015;25:83–8.
Sroussi J, Elies A, Rigouzzo A, Louvet N, Mezzadri M, Fazel A, et al. Low pressure gynecological laparoscopy (7 mmHg) with AirSeal® System versus a standard insufflation (15 mmHg): a pilot study in 60 patients. J Gynecol Obstet Hum Reprod. 2017;46(2):155–8.
Kim K, Jang DM, Park JY, Yoo H, Kim HS, Choi WJ. Changes of diaphragmatic excursion and lung compliance during major laparoscopic pelvic surgery: A prospective observational study. PLoS ONE. 2018;13(11):e0207841.
Kudoh O, Satoh D, Hori N, Kawagoe I, Inada E. The effects of a recruitment manoeuvre with positive end-expiratory pres-sure on lung compliance in patients undergoing robot-assisted laparoscopic radical prostatectomy. J Clin Monit Comput. 2020;34(2):303–10.
Van Hecke D, Bidgoli JS, Van der Linden P. Does Lung Compliance Optimization Through PEEP Manipulations Reduce the Incidence of Postoperative Hypoxemia in Laparoscopic Bariatric Surgery? A Randomized Trial Obes surg. 2019;29(4):1268–75.
Tharp WG, Murphy S, Breidenstein MW, Love C, Booms A, Rafferty MN, et al. Body Habitus and Dynamic Surgical Conditions Independently Impair Pulmonary Mechanics during Robotic-assisted Laparoscopic Surgery A Cross-Sectional Study. Anesthesiology. 2020;133(4):750–63.
Mitsuhashi A, Ishikawa H, Habu Y, Usui H. The effect of steep head-down tilt on respiratory status in endometrial cancer patients with obesity during robot-assisted hysterectomy. Gynecol Oncol Rep. 2022;41:101014. https://doi.org/10.1016/j.gore.2022.101014.
Souki FG, Rodriguez-Blanco YF, Polu SR, Eber S, Candiotti KA. Survey of anesthesiologists’ practices related to steep Trendelenburg positioning in the USA. BMC Anesthesiol. 2018;18(1):1–6.
Takechi K, Kitamura S, Shimizu I, Yorozuya T. Lower limb perfusion during robotic-assisted laparoscopic radical prostatectomy evaluated by near-infrared spectroscopy: an observational prospective study. BMC Anesthesiol. 2018;18(1):1–5.
Lestar M, Gunnarsson L, Lagerstrand L, Wiklund P, Odeberg- WS. Hemodynamic perturbations during robot assisted lapa- roscopic radical prostatectomy in 45-degree Trendelenburg position. Anesth Analg. 2011;113:1069–75.
Katayama S, Mori K, Pradere B, Yanagisawa T, Mostafaei H, Quhal F, et al. Influence of steep Trendelenburg position on postoperative complications: A systematic review and meta-analysis. J Robot Surg 2021; doi: https://doi.org/10.1007/s11701-021-01361-x.
Yu J, Park JY, Kim DH, Kim S, Hwang JH, Seo H, Kim YK. Incidence and risk factors of pulmonary complications after robot-assisted laparoscopic prostatectomy: a retrospective observational analysis of 2208 patients at a large single center. J Clin Med. 2019;8(10):1509.
Kudoh O, Satoh D, Hori N, Kawagoe I, Inada E. The effects of a recruitment manoeuvre with positive end-expiratory pressure on lung compliance in patients undergoing robot-assisted laparoscopic radical prostatectomy. J Clin Monit Comput. 2020;34(2):303–10.
Pei S, Wei W, Yang K, Yang Y, Pan Y, Wei J, et al. Recruitment Maneuver to Reduce Postoperative Pulmonary Complications after Laparoscopic Abdominal Surgery: A Systematic Review and Meta-Analysis. J Clin Med. 2022;11(19):5841.
Shono A, Katayama N, Fujihara T, Böhm SH, Waldmann AD, Ugata K, et al. Positive end-expiratory pressure and distribution of ventilation in pneumoperitoneum combined with steep Trendelenburg position. Anesthesiology. 2020;132(3):476–90.
Choi EM, Na S, Choi SH, An J, Rha KH, Oh YJ. Comparison of volume-controlled and pressure-controlled ventilation in steep Trendelenburg position for robot-assisted laparoscopic radical prostatectomy. J Clin Anesth. 2011;23(3):183–8.
Park JH, Park IK, Choi SH, Eum D, Kim MS. Volume-controlled versus dual-controlled ventilation during robot-assisted laparoscopic prostatectomy with steep trendelenburg position: a randomized-controlled trial. J Clin Med. 2019;8(12):2032.
Strang CM, Ebmeyer U, Maripuu E, Hachenberg T, Hedenstierna G. Improved ventilation-perfusion matching by abdominal insufflation (pneumoperitoneum) with CO2 but not with air. Minerva Anestesiol. 2013;79(6):617–25.
Kamine TH, Elmadhun NY, Kasper EM, Papavassiliou E, Schneider BE. Abdominal insufflation for laparoscopy increases intracranial and intrathoracic pressure in human subjects. Surg Endosc. 2016;30(9):4029–32.
Acknowledgements
Not applicable
Funding
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.P. and D.T.; methodology, D.T..; validation, M.M., and D.T.; formal analysis, M.R.O. and R.M.S.; investigation, M.O.S. and M.R.O.; data curation, M.P.; writing—original draft preparation, M.M.; writing—review and editing, D.T., M.O.S., M.P.; supervision, D.T.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethical approval for the present study was provided by the Ethical Committee of Fundeni Clinical Institute, Bucharest, Romania (NCT04513262). The study was performed on human patients in accordance with the Declaration of Helsinki. All patients signed an informed consent.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
Supplementary Table 1. Comparison of hemodynamics parameters and PaO2 between the two groups.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Popescu, M., Olita, M.R., Stefan, M.O. et al. Lung mechanics during video-assisted abdominal surgery in Trendelenburg position: a cross-sectional propensity-matched comparison between classic laparoscopy and robotic-assisted surgery. BMC Anesthesiol 22, 356 (2022). https://doi.org/10.1186/s12871-022-01900-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-022-01900-5