Abstract
Background
Thoracic epidural analgesia (TEA) has always been the first choice for postoperative pain treatment, but associated complications and contraindications may limit its use. Our study put forward a new analgesic strategy that combines TEA with patient controlled intravenous analgesia (PCIA) to optimize TEA.
Methods
Patients undergoing laparotomy were enrolled in this prospective randomized study. Patients were randomized to one of two groups: TEA/PCIA group and TEA group. Patients in TEA/PCIA group received TEA in the day of surgery and the first postoperative day and PCIA continued to use until the third postoperative day. Patients in TEA group received TEA for three days postoperatively. Visual analogue scale (VSA) pain scores at rest and on movement at 6, 24,48,72 h after surgery were recorded. In addition, the incidence of inadequate analgesia, adverse events, time to first mobilization, time to pass first flatus, time of oral intake recovery, time of urinary catheter removal, postoperative length of hospital stay, cumulative opioid consumption, and the overall cost were compared between the two groups. We examined VAS pain scores using repeated measures analysis of variance; P < 0.05 was considered as statistically significant.
Results
Eighty-six patients were analysed (TEA/PCIA = 44, TEA = 42). The mean VAS pain scores at rest and on movement in TEA/PCIA group were lower than TEA group, with a significant difference on movement and 48 h postoperatively (P < 0.05). The time to first mobilization and pass first flatus were shorter in TEA/PCIA group (P < 0.05). Other measurement showed no statistically significant differences.
Conclusions
The combination of TEA with PCIA for patients undergoing laparotomy, can enhance postoperative pain control and facilitate early recovery without increasing the incidence of adverse effects and overall cost of hospitalization.
Trial registration
Chinese Clinical Trial Registry(www.chictr.org.cn), ChiCTR 1,800,020,308, 13 December 2018.
Similar content being viewed by others
Introduction
Enhanced recovery after surgery (ERAS) is a standardized and evidence-based perioperative care protocol and has been developed to many surgical fields. It largely facilitates postoperative recovery and attenuates peri-operative stress response and thus reduces complications and length of stay [1,2,3]. Adequate postoperative analgesia has always been considered as one of the key components for ERAS programs. Poor pain control would lead to delayed recovery and increased morbidity and bring challenges to subsequent treatment. Several analgesic techniques or drugs have been created and widely used for postoperative acute pain management within the past 20 years, however the outcomes of pain control are not always satisfactory. Correll et al. published a scientometric analysis pointed out that inappropriate use of new technologies and drugs would impede improvement on postoperative acute pain relief [4].
Thoracic epidural analgesia (TEA), as the cornerstone of postoperative pain relief in laparotomy, can provide better effective pain management compared with patient controlled intravenous analgesia (PCIA) [5]. Prior studies have supported that TEA could reduce the incidence of postoperative pulmonary complications and facilitate the recovery of gastrointestinal function [6, 7]. However, some problems still emerge in the application of TEA, such as postoperative hypotension, fluid overload, urinary retention, and motor block. Furthermore, a review summarizes that the failure of epidural anaesthesia and analgesia occurs in up to 30% in clinical practice [8]. Current guidelines for ERAS still emphasize the role of TEA in multimodal analgesia for postoperative pain control. Thus, how to optimize TEA is important to laparotomy.
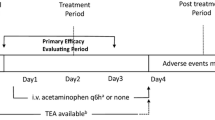
To our knowledge, laparotomy is often characterized by severe trauma, severe pain, and long recovery time. PCIA is not recommended for laparotomy because of its low efficacy and a higher rate of adverse events. However, combination of different classes of analgesics in PCIA, along with the advantage of rapid onset, may improve efficacy or minimize adverse effects. Therefore, under the concept of multimodal analgesia, our study put forward a new analgesic strategy that combines short-term TEA with PCIA on the first two postoperative days and apply PCIA alone afterwards in the subsequent two days (Fig. 1). This strategy could not only maximize the effect of epidural analgesia, but also theoretically reduce the adverse effects [9, 10]. This study attempted to take a multimodal analgesic approach to optimize postoperative analgesia and facilitate enhanced recovery. It is expected that the combination of TEA and PCIA would result in decreased pain scores, but it is uncertain that this approach could reduce pain scores without increasing costs or adverse effects. Therefore, we conducted a prospective non-blinded randomised controlled trial to compare TEA/PCIA with TEA, to explore the feasibility of combination of TEA with PCIA in pain control and early recovery after laparotomy under the goal of ERAS.
Methods
This study was a single-centre prospective non-blinded randomised controlled trial (Chinese Clinical Trial Registry, ChiCTR 1,800,020,308, 13/12/2018). Ethical approval for this study (Ethical Committee No. [2018]265) was provided by the Ethics Committee of the First Affiliated Hospital of Sun Yat-Sen University. The study adhered to the CONSORT guidelines.
Participants
A total of 102 patients undergoing laparotomy in the First Affiliated Hospital of Sun Yat-Sen University were recruited between December 2018 and December 2019. The patients aged 18–75 years, with an ASA I or II, and BMI ranged from 18 to 27 kg m−2, who were undergoing laparotomy (hepatectomy, pancreaticoduodenectomy, gastrointestinal surgery, or colorectal surgery), were eligible for this study. Patients were randomly allocated to group TEA/PCIA or TEA according to a random number table by the Social Sciences software version 20.0 (SPSS Inc, Chicago, IL, USA). All participants must be able to understand the research protocol and signed informed consent. Exclusion criteria included contraindication to epidural analgesia, allergy or sensitivity to local anaesthetics, contraindication to opioid and non-opioid analgesic drugs. The patients with a history of chronic pain or long-time medication with antidepressants, narcotic analgesics or nonsteroidal anti-inflammatory drugs (NSAIDs) were also excluded.
Patients may discontinue participation in the trial at their own request, or be withdrawn if a surgery is not performed, or continuation of the trial may be detrimental to the patient’s health in the investigator’s opinion. Drop-out patients will be included in the final report to ensure complete transparency of the trial.
Preparations in the operation room before surgery
After established intravenous access and continuous monitoring in the operative room, the patients were placed in the lateral position to receive TEA prior to the induction of general anaesthesia. Insertion of an epidural catheter was performed between T8 and T10 in patients undergoing a right sided colon resection or upper abdominal surgery (hepatectomy, pancreaticoduodenectomy, gastrointestinal open surgery), or between T10 and T11 in patients undergoing a left sided colon resection. After the epidural space was identified using the loss of resistance technique with air, standard aseptic insertion procedure was performed. A test dose of 3 mL of 2% lidocaine was injected to ensure the catheter was in the correct space. Sterile device was used to hold the catheter in place after excluding the spinal anaesthesia.
Standard general anaesthesia
All patients in the trial underwent a general anaesthesia. Anaesthesia was induced with sufentanil (0.3–0.5 mcg kg−1), cisatracurium (0.2 mg kg−1) or rocuronium (0.6 mg kg−1), propofol (2–3 mg kg−1). Standard monitoring used in the surgery involved electrocardiogram, blood pressure, respiratory rate, oxygen saturation, end-tidal carbon dioxide, central venous pressure, temperature, and Narcotrend® (MonitorTechnik, Bad Bramstedt, Germany). Anaesthesia was maintained by propofol and sevoflurane, as the depth of anaesthesia showed as Narcotrend® value was kept between 40 and 60.
Intervention in TEA/PCIA group
Half an hour before the completion of surgery, 0.4 mg of hydromorphone 2 mL and 5 mL of 0.25% ropivacaine were injected into the epidural space as a loading dose. All the patients were then connected with an epidural analgesia pump (Jiangsu REHN Medical Instruments Technology CO., ITD). As for analgesia regimen, 0.125% ropivacaine combined with hydromorphone was used for TEA, with a background infusion rate of 2 mL h−1. TEA was only applied in the day of surgery and the first postoperative day. Hydromorphone combined with flurbiprofen was used for PCIA until third postoperative day. The removal time of an analgesia pump was recorded, and the cumulative opioid consumption was recorded in equivalents of oral morphine equivalents (OMEs) [11]. The types of medications and additional analgesics were documented in detail.
Intervention in TEA group
The patients in TEA group received epidural puncture and catheterization to establish epidural analgesia before anaesthesia induction. TEA was used until third postoperatively day. The analgesia regimen for TEA was the same as that in TEA/PCIA group, with the analgesia pump settings of a background infusion rate of 2 mL h−1. Similarly, detailed recording included removal time of an analgesia pump, cumulative opioid consumption, and additional analgesics.
Date collection
The demographic and operation-related information including age, sex, BMI, ASA grade, comorbidities, surgical type, incision type, and operation time was collected. Postoperative pain at rest and on movement was evaluated with visual analogue scale (VAS) pain score. The primary endpoints were mean VAS pain scores at rest and on movement for three days postoperatively. The secondary endpoints included VAS pain scores at rest and on movement at 6, 24, 48 and 72 h postoperatively, incidence of inadequate analgesia, incidence of opioid-related adverse events, the time to first mobilization, the time to pass first flatus, the time of oral intake recovery, the time of the urinary catheter removal, postoperative length of hospital stay (PLOS), cumulative opioid consumption, and overall cost.
Sample size
The mean VAS pain scores at rest and on movement for three days postoperatively were the primary endpoints in our work. Kelly et al.reported the minimum clinically significant VAS pain score in the management of severe pain was 1 cm [12]. Standard deviations (SD) varying between 1.4 and 1.8 cm have been reported, thus we estimated a SD of 1.5 cm for the study. To achieve 90% power to detect a difference (1 cm) in the primary endpoints with a two-sided 5% level of significance, a sample size of 38 patients in each group of the study is needed. An additional four participants were recruited in each study arm to cover a maximum of 10% losses, thus the sample size required for each group was up to 42 subjects.
Statistical analysis
SPSS software version 20.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. All numerical variables were first examined for normality. Numerical variables were descripted as mean ± SD for data with a normal distribution, otherwise descripted as median and interquartile range (IQR). Independent two-sample t-test was used for the comparison of the normally distributed numerical variables. The non-normally distributed numerical variables were compared by Mann–Whitney U test. Frequency and percentage were used for statistical description of categorical variables, and chi-square test or Fischer’s exact test were used for the comparison of the unordered categorical variables depend on their expected counts. Kruskal Wallis H test was used for ordinal multiple categorical variables. In addition, repeated measured data was analysed using repeated-measures analysis of variance (ANOVA), such as the VAS scores of the two groups at different time points. Bonferroni correction was used to adjust for the increased alpha error in the multiple comparisons. Survival analysis assessed by the Kaplan–Meier method and Breslow test was used to analyse postoperative indicators. P values < 0.05 was considered statistically significant (the level of significance was bilateral).
Results
A total of 102 patients underwent laparotomy at our institution from November 2018 to November 2019 were recruited. Finally, 86 patients (44 patients in the TEA/PCIA group,42 patients in the TEA group) were included in the final statistical analysis, details of dropout reasons are given in Fig. 2. Baseline characteristics of the two groups are presented in Table 1(at the end of the manuscript). There was no significant difference in demographic characteristics, comorbidities, surgical type, and incision type between the two groups (P > 0.05). No significant differences were observed between the two groups in operation time, intraoperative fluid intake, intraoperative blood loss, intraoperative sufentanil consumption, cumulative opioid consumption, as well as length of stay and complications in the post anaesthesia care unit (PACU) (Table 2, at the end of the manuscript).
The CONSORT Flow Diagram In TEA/PCIA group, three patients failed to receive an epidural puncture, four patients withdraw from the research due to postoperative abdominal infection and haemorrhage. In TEA group, five patients failed to receive an epidural puncture, three were transferred to the ICU due to surgery complications, and one patient withdrew due to the changes in surgical protocols. TEA, thoracic epidural analgesia; PCIA, patient-controlled intravenous analgesia; ICU, intensive care unit
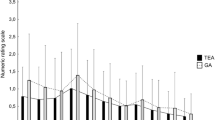
The mean VAS pain scores on movement during postoperative days 0–3 in TEA/PCIA group were significant lower (2.45 ± 0.55 vs 2.68 ± 0.52; P < 0.05) compared with TEA group (Table 3). TEA/PCIA group had lower VAS pain scores at rest and on movement at each time point compared with TEA group, with a significant difference at 48 h postoperatively (P < 0.05). In addition, inadequate analgesia occurred in 9 (20.5%) of the 44 patients in the TEA/PCIA group and in 13 (31%) of 42 patients in TEA group (P = 0.265), but it did not differ significantly between groups (Table 5).
The TEA/PCIA group had earlier time to first mobilization and recovery of gastrointestinal motility (shorter time to first pass flatus) compared with the TEA group (P < 0.05). But no significant differences were observed in the postoperative length of hospital stay, the time to urinary catheter removal, and the time of oral intake recovery between the two groups (Table 4).
There was no significant difference in the incidence of opioid-related adverse events between the two groups (Table 5). The overall cost of hospitalization and the cost of anaesthesia were not different between the groups (Table 6).
Discussion
This study showed there was a significant reduction of mean M-VAS pain scores in TEA/PCIA group. Furthermore, there was significant lower VAS pain scores at 48 h postoperatively both in rest and on movement. In general, TEA/PCIA group provides superior pain control, which is consistent with our initial hypothesis. In past decades, epidural analgesia (EA) was regarded as the gold standard for treating postoperative pain after laparotomy. Previous publications reported that a successful epidural analgesia can provide excellent pain relief. But an epidural catheter was placed before surgery and used for 2–3 days postoperatively, many epidurals are not effective for such long periods [13]. Another literature review showed high analgesia failure rates of EA ranging from 13% to 48.6%, the main reasons reported were catheter dislodgment, malposition, occlusion and unplanned removal [14]. Some of these reasons is hard to detected and prevention. Once epidural failure, the patients need to tolerate the pain for a period, until additional drugs are used for relieving pain. In our study, the combination of short-term TEA with PCIA not only maximized analgesic effect, but also decreases the analgesia failure theoretically. Besides, opioids and NSAID were used for PCIA at the same time. Opioids can treat inadequate analgesia and reduce the regressed risk of sensory level of epidural analgesia, NSAID can effectively make up for the poor effect of TEA on inflammatory pain [15].
In our study, the time to first mobilization in TEA/PCIA group was earlier than that in TEA group, which may be attributed to better pain control. Additionally, early removal of epidural catheter may be another important factor. As we know, TEA has always been recognized for its analgesic effect, but some risks and complications, such as catheter dislodgment, increased risk of infection, adverse to early mobilization, adverse to the prevention of deep vein thrombosis, which could be limiting factors for ERAS programs [13]. Early removal of epidural catheter could decrease the incidence of motor block. At the same time, it also relieved the difficulty of postoperative care and labour-intensive monitoring. The patients will be more comfortable for mobilization without concerning the epidural catheter falling out.
Our results showed that the time to pass first flatus in TEA/PCIA patients was earlier than that in TEA patients. Previous studies have demonstrated that better pain control brings earlier mobilization, and thus contributes to recovery of gastrointestinal function and reduction of the risks of pulmonary and cardiovascular events [7, 16]. However, the result seems to be contradictory to the beneficial effect of TEA on the recovery of gastrointestinal function [6]. EA may promote a faster return in gastrointestinal motility via various mechanisms including decrease in opioid administration, blockade of the relevant sympathetic nerve and reduction of inflammatory reactions, but this advantage being increasingly doubted in recent reviews. The benefits of TEA in the recovery of gastrointestinal motility after colorectal surgery have been confirmed in several clinical trials, but for other types of laparotomies, its role on recovery of bowel function remains questionable. In a retrospective analysis of open surgery for gynecological tumors, EA was correlated with a higher incidence of ileus risk (odds ratio: 2.6; P = 0.03) [17]. In another prospective observational study on gastrointestinal function recovery after upper abdominal surgery, the first gas-out time was not different between the TEA group and PCIA group [18]. The study explained that the failure of TEA to promote gastrointestinal function recovery may attribute to the combination administration of local anesthetics and opioids in the epidural analgesia regimen. Moreover, the study also pointed out that the implementation of ERAS programs may also make TEA become less important on gastrointestinal function. Postoperative ileus is multifactorial and represents a limiting factor for the implementation of ERAS. The correlation between EA and postoperative ileus may require further research.
In the present study, the combination of TEA/PCIA required higher dosage of opioids compared with TEA alone. Therefore, whether the risk of opioid-related adverse events would increase was also the focus of this study. Our results showed that no significant difference was observed in the incidences of opioid-related adverse events between the two groups. The incidence of postoperative nausea and vomiting was less in both TEA/PCIA group (20.5%) and TEA group (19.0%), which is consistent with that incidence in our institution and lower than the generally reported incidence of 30–50% [19, 20]. Hypotension is an unwanted side effect of epidural analgesia. TEA/PCIA group did not show a lower incidence. Further analysis found that postoperative hypotension mainly occurred in the night of the first postoperative day. Postoperative hypotension of a major laparotomy is common and multifactorial. Due to the lack of a control group receiving PCIA alone, it is difficult to determine whether postoperative hypotension is attributable to epidural analgesia.
Study limitations
There are several limitations in this study. The first is that this study was not blinded, as it was difficult to blind the observers and patients to the intervention they belonged. Secondly, given the lack of a PCIA control group, we cannot be sure if the adverse effects were attributed to the TEA itself. In addition, we utilized the scapula as a landmark rather than ultrasound guidance for the localization of an epidural catheter, and postoperative imagological examination was not routinely given to determine the location of the catheter. Therefore, we could not guarantee whether the catheter was located at the target place. Finally, the current study focused on patients undergoing major open surgery. Although the number of subjects included in the final analysis reached the requirement of sample size, the number of some subgroups was too small for further subgroup analysis. More attention will be paid to hepatobiliary surgery in our future clinical research.
Conclusion
In summary, the combination of TEA and PCIA for patients underwent major open abdominal surgery, can provide superior postoperative analgesia and facilitate early rehabilitation without increasing the incidence rate of adverse effects and the overall cost of hospitalization.
Availability of data and materials
The full study protocol and raw data set can be obtained from the corresponding author (anke@mail.sysu.edu.cn).
Abbreviations
- TEA:
-
Thoracic epidural analgesia
- PCIA:
-
Patient controlled intravenous analgesia
- VAS:
-
Visual analogue scale
- R-VAS:
-
VAS score at rest
- M-VAS:
-
VAS score on movement
- ERAS:
-
Enhanced recovery after surgery
- ASA:
-
American Society of Anesthesiology
- BMI:
-
Body mass index
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- OMEs:
-
Oral morphine equivalents
- PLOS:
-
Postoperative length of hospital stay
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- PACU:
-
Post anaesthesia care unit
- ICU:
-
Intensive care unit
- EA:
-
Epidural analgesia
References
Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606–17.
Greco M, Capretti G, Beretta L, et al. Enhanced recovery program in colorectal surgery: a meta-analysis of randomised controlled trials. World J Surg. 2014;38(6):1531–41.
Muller S, Zalunardo MP, Hubner M, et al. Zurich Fast Track Study Group. A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology. 2009 Mar;136(3):842–7.
Correll DJ, Vlassakov KV, Kissin I. No evidence of real progress in treatment of acute pain, 1993–2012: scientometric analysis. J Pain Res. 2014;11(7):199–210.
Salicath JH, Yeoh EC, Bennett MH. Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults. ochrane Database Syst Rev. 2018;8(8):CD010434.
Guay J, Nishimori M, Kopp S. Epidural local anaesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting and pain after abdominal surgery. Cochrane Database Syst Rev. 2016;7(7):CD001893.
Pöpping DM, Elia N, Marret E, et al. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: a meta-analysis. Arch Surg. 2008 Oct;143(10):990–9; discussion 1000.
Hermanides J, Hollmann MW, Stevens MF, Lirk P. Failed epidural: causes and management. Br J Anaesth. 2012;109(2):144–54.
Baldini G, Bagry H, Aprikian A, et al. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology. 2009;110(5):1139–57.
Wheatley RG, Schug SA, Watson D. Safety and efficacy of postoperative epidural analgesia. Br J Anaesth. 2001;87(1):47–61.
Nielsen S, Degenhardt L, Hoban B, et al. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733–7.
Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18(3):205–7.
Rawal N. Epidural analgesia for postoperative pain: Improving outcomes or adding risks? Best Pract Res Clin Anaesthesiol. 2021;35(1):53–65.
Suksompong S, von Bormann S, von Bormann B. Regional Catheters for Postoperative Pain Control: Review and Observational Data. Anesth Pain Med. 2020;10(1):e99745.
Woolf CJ. American College of Physicians; American Physiological Society. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441–51.
Holte K, Kehlet H. Effect of postoperative epidural analgesia on surgical outcome. Minerva Anestesiol. 2002;68(4):157–61.
Boitano TKL, Smith HJ, Rushton T, Johnston MC, et al. Impact of enhanced recovery after surgery (ERAS) protocol on gastrointestinal function in gynecologic oncology patients undergoing laparotomy. Gynecol Oncol. 2018;151(2):282–6.
Ahn JH, Ahn HJ. Effect of thoracic epidural analgesia on recovery of bowel function after major upper abdominal surgery. J Clin Anesth. 2016;34:247–52.
American Society of PeriAnesthesia Nurses PONV/PDNV Strategic Work Team. ASPAN'S evidence-based clinical practice guideline for the prevention and/or management of PONV/PDNV. J Perianesth Nurs. 2006 Aug;21(4):230–50.
Gan TJ, Diemunsch P, Habib AS, et al. Society for Ambulatory Anesthesia. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113.
Acknowledgements
Assistance with the study: We would like to acknowledge and express our deepest gratitude to the participants of this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
#Wenwen Xu: designed the study and collected data, wrote the part of introduction, method, and discussion, and revised the paper. #Youpei Li: collected data, wrote the part of results, and made tables and figures. #: The two authors contribute to this work equally as co-first authors. Nanqi Li: data collection. Yu Sun: data collection. Wang Chao: data collection. Ke An: designed the study together with Wenwen Xu and revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study (Ethical Committee No. [2018]265) was provided by the Ethics Committee of the First Affiliated Hospital of Sun Yat-Sen University (Chairperson Prof Churong Yan) on 24 October 2018. All methods were carried out in accordance with relevant guidelines and regulations (Declaration of Helsinki). Written informed consent was obtained from all participating subjects prior to enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, W., Li, Y., Li, N. et al. Combination of thoracic epidural analgesia with patient-controlled intravenous analgesia versus traditional thoracic epidural analgesia for postoperative analgesia and early recovery of laparotomy: a prospective single-centre, randomized controlled trial. BMC Anesthesiol 22, 341 (2022). https://doi.org/10.1186/s12871-022-01891-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-022-01891-3