Abstract
Background
Standardized risk assessment tools can be used to identify patients at higher risk for postoperative complications and death. In this study, we validate the PreOperative Score to predict Post-Operative Mortality (POSPOM) for in-hospital mortality in a large cohort of non-cardiac surgery patients. In addition, the performance of POSPOM to predict postoperative complications was studied.
Methods
Data from the control cohort of the TRACE (routine posTsuRgical Anesthesia visit to improve patient outComE) study was analysed. POSPOM scores for each patient were calculated post-hoc. Observed in-hospital mortality was compared with predicted mortality according to POSPOM. Discrimination was assessed by receiver operating characteristic curves with C-statistics for in-hospital mortality and postoperative complications. To describe the performance of POSPOM sensitivity, specificity, negative predictive values, and positive predictive values were calculated. For in-hospital mortality, calibration was assessed by a calibration plot.
Results
In 2490 patients, the observed in-hospital mortality was 0.5%, compared to 1.3% as predicted by POSPOM. 27.1% of patients had at least one postoperative complication of which 22.4% had a major complication. For in-hospital mortality, POSPOM showed strong discrimination with a C-statistic of 0.86 (95% CI, 0.78–0.93). For the prediction of complications, the discrimination was poor to fair depending on the severity of the complication. The calibration plot showed poor calibration of POSPOM with an overestimation of in-hospital mortality.
Conclusion
Despite the strong discriminatory performance, POSPOM showed poor calibration with an overestimation of in-hospital mortality. Performance of POSPOM for the prediction of any postoperative complication was poor but improved according to severity.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Postoperative mortality has decreased significantly over the last few decades. The European Surgical Outcomes Study (EuSOS) reported an overall in-hospital mortality of 4% in patients undergoing non-cardiac surgery across 28 European nations in 2011 [1]. More specifically for the Dutch surgical population, an all-cause mortality rate of 1.85% was reported for major surgery performed during 1991–2005 [2]. The TRACE investigators published a 30-day mortality of 0.5% in major non-cardiac surgery patients in the period 2016–2018 [3].
Current improvements in perioperative care aim to reduce failure to rescue, thus lowering the proportion of preventable deaths due to unnoticed complications in the postoperative period [4]. Besides death, postoperative complications are associated with adverse functional outcome after surgery. The challenge is to improve postoperative survival and prevent new disability, especially in selected groups of high risk patients. Standardized risk assessment tools can be used to distinguish patients at higher risk for postoperative complications and death, and are recommended by international societies for perioperative risk stratification [5].
Various risk assessment tools for the prediction of adverse postoperative outcome have been published [6]. In 2016, Le Manach and others developed and validated the PreOperative Score to predict Post-Operative Mortality (POSPOM) [7]. A strength of POSPOM is that it is based on a large cohort consisting of more than 5.5 million patients who underwent heterogeneous surgical procedures originating from the French National Hospital Discharge Data Base (NHDBB), which makes it representative for the European surgical population.
The performance of the POSPOM score has been evaluated mostly in specific surgical populations, including patients undergoing emergency abdominal surgery, [8] radical cystectomy, [9] vascular surgery, [10, 11] and elderly patients with hip fractures [12]. Two studies have evaluated POSPOM in large heterogeneous surgical populations, using single center retrospective data [13, 14]. However, both studies did not address the performance of POSPOM in terms of sensitivity, specificity, negative predictive values (NPV) and positive predictive values (PPV), which limits the clinical applicability.
In this study we validated the POSPOM score in a heterogeneous Dutch cohort of nearly 2500 non-cardiac surgery patients by using data from the control cohort of the multicenter stepped-wedge cluster randomized interventional TRACE (routine posTsuRgical Anesthesia visit to improve patient outComE) study [3]. In addition, we studied the potential of the POSPOM score to predict the development of postoperative complications. The aim of the study was to investigate to what extent the POSPOM score is able to predict in-hospital mortality and major postoperative complications in the TRACE cohort.
Methods
Study design and participants
This study was based on the TRACE (routine posTsuRgical Anesthesia visit to improve patient outComE) study. Details on design and analysis of the TRACE study have been previously reported [15]. TRACE was a prospective, multicenter, stepped-wedge, cluster-randomized interventional study performed between October 2016 and August 2019 in nine academic and non-academic hospitals in The Netherlands. The effects of a routine postoperative visit by an anesthesiologist on the incidence of postoperative complications and mortality was assessed. For the current study, participants that originated from the control cohort (non-intervention) of the TRACE study were included. The study was ethically approved by the Human Subjects Committee of Amsterdam UMC, location VUmc Amsterdam (number NL56004.029.16, 29-06-2016) and registered with the Netherlands Trial Register (NTR5506). The Clinical Research Unit of the Amsterdam UMC took responsibility for the monitoring of patient inclusion and data registration. All study participants signed informed consent.
Study data and variables
The POSPOM score has been originally developed by Le Manach and others and is composed of a set of preoperative variables including age, medical history and type of surgery [7]. For each variable a certain number of points is assigned to the final score, which varies between 0 and 70 points and equates to a probability of in-hospital mortality. To calculate a POSPOM score for each patient, relevant preoperative variables were extracted from the TRACE database.
Type of surgery was defined in the original TRACE database, however the list of procedures was less detailed compared to POSPOM. Therefore appropriate modifications were made in the surgical classification of POSPOM to calculate the POSPOM score for all study patients. First, because of the in- and exclusion criteria of the TRACE study, day case procedures and cardiac and orthopedic trauma surgery were not included. Due to the absence of day case procedures, the POSPOM surgery groups ‘other orthopedic’ and ‘minor gastrointestinal’ were not represented in the study cohort. Second, within TRACE no subdivision was made between minor and major surgery. Hence, for urologic, vascular and hepatic procedures this subdivision was based on duration of surgery; > 120 min was considered as major surgery for urologic and vascular procedures and > 180 min for hepatic procedures.
Study endpoints
The primary study endpoint was in-hospital mortality. The secondary study endpoints were the occurrence of any complication, severity of a complication graded according to the Clavien-Dindo classification, [16] and type of complication (i.e. infectious, cardiac/transfusion, pulmonary, venous thromboembolic, renal, neurological, surgical, ileus, delirium and other).
Statistical analysis
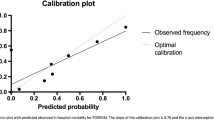
The size of the cohort was determined within the sample size calculation of the TRACE study of which detailed descriptions have been published previously [15]. Continuous variables were expressed as mean ± standard deviation (SD) and in case of a non-symmetric distribution as median with interquartile range (IQR). Categorical variables were described with frequencies and percentages. For the calculation of the discriminating power of POSPOM, receiver operating characteristic (ROC) curves with corresponding C-statistics (i.e. area under the curve (AUC) values) were calculated for primary and secondary study endpoints [17]. For the description of the performance of the POSPOM score sensitivity, specificity, negative predictive values (NPV), and positive predictive values (PPV) were calculated from true-positive, false-positive, true-negative, and false-negative values for the outcome variables in-hospital mortality and major complication (Clavien-Dindo grade III-V). To assess the mean calibration (or calibration-in-the-large) the average predicted risk of in-hospital mortality was compared with the overall observed rate of in-hospital mortality [18]. In addition, the difference between predicted and observed in-hospital mortality rates per POSPOM score group were calculated. A calibration plot for in-hospital mortality was constructed with corresponding calibration curve intercept and slope; the predicted probability was based on the average POSPOM score per tentile. All analyses were performed using SPSS Statistics version 26.0 (IBM, Armonk, NY, USA) and GraphPad Prism version 8.2.1 (GraphPad Software, San Diego, California, USA).
Results
Study population
In total, 5473 patients were included in the TRACE study cohort. After removal of 283 drop-outs (i.e. withdrawal of informed consent before operation, operation cancelled and/or not meeting inclusion criteria) and 2700 patients originating from the intervention cohort, 2490 patients were included in the analysis. Median age was 65 (IQR 12) years, 46.9% of patients were female, and a majority (61.7%) was classified as ASA class 2. Active cancer or diabetes mellitus were the most common comorbidities. Major gastrointestinal surgery was the most frequently performed procedure, followed by arthroplasty and spine surgery, and minor urologic surgery (Table 1).
Incidence of mortality and postoperative complications
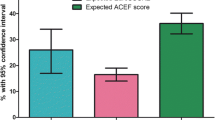
The distribution of in-hospital mortality according to POSPOM score is presented in Fig. 1. Based on the median POSPOM score of 24 (IQR 8) points, the predicted risk of in-hospital mortality was 1.3% in our study population. The observed in-hospital mortality rate was 0.5% (14 patients). Of these patients POSPOM scores varied between 24 and 36 points, which equates a probability of in-hospital mortality of 1.3 and 32.9% respectively. POSPOM values less than 24 points were associated with 0% observed mortality (Fig. 1).
Six hundred and seventy-six patients (27.1%) had at least one postoperative complication. A majority (39.3%) of complications was scored Clavien-Dindo (CD) grade II at highest (requiring pharmacological treatment), followed by 33.4% CD grade I (any deviation from the normal postoperative course, without the need for pharmacological treatment or surgical, endoscopic and radiological interventions) and 15.7% CD grade III (requiring surgical, endoscopic or radiological intervention). Forty-two patients (6.2%) had a life-threating complication requiring ICU-management. The distribution of the observed in-hospital complication rates among POSPOM scores is presented in Fig. 2. Of all postoperative complications, the incidence of other complications (not further defined) was highest (45.1%), followed by infectious (36.5%) and cardiac/transfusion (27.5%).
Validation of POSPOM for mortality and postoperative complications
Discrimination
The concordance between POSPOM calculated mortality and observed mortality was strong (C-statistic 0.86 (95% CI, 0.78–0.93). The corresponding Receiver Operating Characteristics (ROC) curve is shown in Fig. 3. A cutoff value of a POSPOM score of 24 points equated to a sensitivity of 100%, a specificity of 48%, a PPV of 1.1% and an NPV of 100%. Despite a strong C-statistic for postoperative mortality, the low incidence of in-hospital mortality resulted in low PPV and high NPV values. Other cutoff values of the POSPOM score did not result in improvement of the PPV (Table 2).
ROC curve: predicted versus observed in-hospital mortality for POSPOM. ROC curve with a corresponding C-statistic of 0.86 (95% CI, 0.78–0.93), which implies strong discriminating power. Abbreviations: ROC Receiver Operating Characteristics, POSPOM PreOperative Score to predict PostOperative Mortality
The overall discriminatory ability of POSPOM for any postoperative complication was poor (C-statistic 0.63 (95% CI, 0.60–0.65)) but improved according to severity (C-statistic 0.65 (95% CI, 0.61–0.70) for CD grade III-V and C-statistic 0.73 (95% CI, 0.67–0.80) for CD grade IV-V; Fig. 4). For different types of complications, the discrimination was lowest for neurological complications (C-statistic 0.54 (95% CI, 0.40–0.68)) and highest for pulmonary complications (C-statistic 0.73 (95% CI, 0.69–0.78)). For the prediction of major complications, the cutoff value of 38 points showed the highest PPV of 20.0%, nevertheless with a corresponding high NPV of 93.5% (Table 3).
Calibration
The average predicted risk of in-hospital mortality was higher than the overall observed mortality rate, which indicated that POSPOM overestimated risk in general (‘calibration-in-the-large’). In addition, for each particular POSPOM score group the predicted risk of in-hospital mortality exceeded the observed percentage of in-hospital mortality (Table 4 in Appendix), especially in the groups with higher POSPOM score. The calibration plot for in-hospital mortality (Fig. 5) showed poor calibration of POSPOM with a fitting curve slope of 0.17 and a negative y-axis interception, which suggests too extreme estimated risks and overestimation respectively.
Discussion
In this large cohort of Dutch non-cardiac surgery patients, we found a strong discrimination for the prediction of in-hospital mortality with POSPOM. This suggests that POSPOM scores are capable to rank patients from low to high risk for in-hospital mortality during their perioperative course [17]. On the other hand, the poor agreement between predicted and observed in-hospital mortality rates indicated poor calibration; i.e. POSPOM systematically overestimated the risk of in-hospital mortality in general [18]. For the prediction of complications, the discrimination of POSPOM was poor to fair according to the severity of the complication.
The strong C-statistic (> 0.8) of POSPOM for the prediction of in-hospital mortality found in this surgical cohort is in line with two previously published validation studies [8, 9]. Two studies reported a mean observed mortality rate (‘calibration-in-the-large’) lower than predicted by POSPOM which is in accordance with our results [9, 12]. One study found an observed mortality similar to predicted [11]. Of all preceding validation studies, two studies examined calibration more closely via a calibration plot [8, 14]. Both studies found underestimation of in-hospital mortality in the POSPOM risk groups with a low risk of in-hospital mortality, whereas in the higher risk groups POSPOM overestimated mortality. In our patients, an overestimation of in-hospital mortality for all POSPOM groups was observed. The subgroup of patients with high POSPOM scores were overrepresented compared to the derivation cohort of Le Manach and others, nevertheless the observed mortality rate of 0.5% in the TRACE cohort was comparable with the mortality rate of 0.47% in the derivation cohort. An explanation for this finding may be the decrease in surgery-related risks over the years; patients in the original POSPOM cohort underwent surgery in 2010 compared to 2016 until 2018 in the TRACE cohort.
The low incidence of postoperative in-hospital death resulted in a clinically irrelevant low PPV for the prediction of mortality. For the prediction of a major complication, the PPV values were homogenous for the majority of the POSPOM cutoff value groups, which resulted in poor differentiation for the prediction of a major complication. The highest PPV of 20% was found for the POSPOM cutoff value of 38 points. Nevertheless, because of the low number of patients in this subgroup, the use of this cutoff value to distinguish patients at higher risk will not be of added value in clinical practice.
Our study was limited by the low absolute number of deaths which negatively influences the quality of the validation of the POSPOM score in this cohort. Validation of the POSPOM score in a population with a higher a priori chance of a complicated postoperative course may have led to different results. As described in the method section, unavoidable modifications of the surgical classification were made which may have influenced the calculation of the final POSPOM score. In addition, cardiac and orthopedic trauma patients were absent in our cohort. Strengths of the TRACE control cohort are its prospective and multicenter design, large size and heterogeneous surgery patient group.
In our study cohort the performance of the POSPOM score to predict in-hospital mortality and complications was poor. In a population with a higher a priori chance of a complicated postoperative hospital stay, using POSPOM as a standardized risk assessment tool may be of added value. However, despite all the risk models that have been developed in the last couple of decades, there is a lack of evidence with regard to the clinical consequences of a higher predicted risk of in-hospital death or complications. The role of these risk models in clinical practice has still to be determined. Besides, because of the low mortality rates in current European healthcare, it may be more interesting to focus on the prediction of complications and relevant outcomes which lead to disability instead of mortality.
Conclusion
In this study POSPOM showed strong discrimination for the prediction of in-hospital mortality, however the calibration of POSPOM was poor. In addition, for the prediction of complications the performance of POSPOM was poor. This limits the use of the risk score in clinical practice.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- CD:
-
Clavien-Dindo
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- EuSOS:
-
The European Surgical Outcomes Study
- IQR:
-
Interquartile range
- NHDBB:
-
The French National Hospital Discharge Data Base
- NPV:
-
Negative predictive value
- POSPOM:
-
PreOperative Score to predict Post-Operative Mortality
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation
- TRACE:
-
Routine posTsuRgical Anesthesia visit to improve patient outComE
References
Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380(9847):1059–65.
Noordzij PG, Poldermans D, Schouten O, Bax JJ, Schreiner FA, Boersma E. Postoperative mortality in The Netherlands: a population-based analysis of surgery-specific risk in adults. Anesthesiology. 2010;112(5):1105–15.
Buhre WFFA, Boer C, Boer DK, Stolze A, Posthuma LM, Smit-Fun VM, et al. Routine Postsurgical Anesthesia Visit to Improve 30-Day Morbidity and Mortality: A Multicenter, Stepped-Wedge Cluster Randomized Interventional Study (the TRACE Study). Ann Surg. 2021. Epub ahead of print.
Lafonte M, Cai J, Lissauer ME. Failure to rescue in the surgical patient: a review. Curr Opin Crit Care. 2019;25(6):706–11.
Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–431.
Mureddu GF. Current multivariate risk scores in patients undergoing non-cardiac surgery. Monaldi Arch Chest Dis. 2017;87(2):848.
Le Manach Y, Collins G, Rodseth R, Le Bihan-Benjamin C, Biccard B, Riou B, et al. Preoperative Score to Predict Postoperative Mortality (POSPOM): Derivation and Validation. Anesthesiology. 2016;124(3):570–9.
Juul S, Kokotovic D, Degett TH, Oreskov JO, Ekeloef S, Gogenur I, et al. Validation of the preoperative score to predict postoperative mortality (POSPOM) in patients undergoing major emergency abdominal surgery. Eur J Trauma Emerg Surg. 2021;47(6):1721-7.
Froehner M, Koch R, Hubler M, Heberling U, Novotny V, Zastrow S, et al. Validation of the Preoperative Score to Predict Postoperative Mortality in Patients Undergoing Radical Cystectomy. Eur Urol Focus. 2019;5(2):197–200.
Reis P, Lopes AI, Leite D, Moreira J, Mendes L, Ferraz S, et al. Predicting mortality in patients admitted to the intensive care unit after open vascular surgery. Surg Today. 2019;49(10):836–42.
Reis P, Lopes AI, Leite D, Moreira J, Mendes L, Ferraz S, et al. Incidence, predictors and validation of risk scores to predict postoperative mortality after noncardiac vascular surgery, a prospective cohort study. Int J Surg. 2020;73:89–93.
Niessen R, Bihin B, Gourdin M, Yombi JC, Cornu O, Forget P. Prediction of postoperative mortality in elderly patient with hip fractures: a single-centre, retrospective cohort study. BMC Anesthesiol. 2018;18(1):183.
Hill BL, Brown R, Gabel E, Rakocz N, Lee C, Cannesson M, et al. An automated machine learning-based model predicts postoperative mortality using readily-extractable preoperative electronic health record data. Br J Anaesth. 2019;123(6):877–86.
Layer YC, Menzenbach J, Layer YL, Mayr A, Hilbert T, Velten M, et al. Validation of the Preoperative Score to Predict Postoperative Mortality (POSPOM) in Germany. PLoS One. 2021;16(1):e0245841.
Smit-Fun VM, de Korte-de BD, Posthuma LM, Stolze A, Dirksen CD, Hollmann MW, et al. TRACE (Routine posTsuRgical Anesthesia visit to improve patient outComE): a prospective, multicenter, stepped-wedge, cluster-randomized interventional study. Trials. 2018;19(1):586.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Pencina MJ, D'Agostino RB Sr. Evaluating Discrimination of Risk Prediction Models: The C Statistic. Jama. 2015;314(10):1063–4.
Van Calster B, McLernon DJ, van Smeden M, Wynants L, Steyerberg EW, Topic Group 'Evaluating diagnostic t, et al. Calibration: the Achilles heel of predictive analytics. BMC Med. 2019;17(1):230.
Acknowledgements
The TRACE Study investigators
Wolfgang F. F. A. Buhre4, wolfgang.buhre@mumc.nl
Christa Boer1, c.boer@amsterdamumc.nl
Dianne de Korte-de Boer4, dianne.de.korte@mumc.nl
Annick Stolze1, a.stolze@amsterdamumc.nl
Linda M. Posthuma3, l.m.posthuma@amsterdamumc.nl
Valérie M. Smit-Fun4, v.smit.fun@mumc.nl
Sander van Kuijk13,14, sander.van.kuijk@mumc.nl
Peter G. Noordzij5, p.noordzij@antoniusziekenhuis.nl
Myra Rinia6, mrinia@rijnstate.nl
Jens-Peter Hering7, j.p.hering@westfriesgasthuis.nl
Bas in ‘t Veld8, b.in.t.veld@haaglandenmc.nl
Gert-Jan Scheffer9, gertjan.scheffer@radboudumc.nl
Carmen Dirksen13,14, c.dirksen@mumc.nl
Marja Boermeester10, m.a.boermeester@amsterdamumc.nl
Jaap Bonjer11, j.bonjer@amsterdamumc.nl
Cees Dejong12, chc.dejong@mumc.nl
Markus W. Hollmann3, m.w.hollmann@amsterdamumc.nl
Collaborators:
J. S. Breel3, T. Bouw10, I. van den Brink9, F. van Dijk6, J. Geurts6, W. Glas7, R. van Gorp4, A. Jwair1, F. Koca3, I. Lange5, B. Preckel3, J. P. van Roy7, M. Theunissen4, A. G. C. L. Wensing3 and A. Werger8.
1Department of Anesthesiology, Amsterdam University Medical Centre, VU University, Amsterdam, The Netherlands.
3Department of Anesthesiology, Amsterdam University Medical Centre, University of Amsterdam, Amsterdam, The Netherlands.
4Department of Anesthesiology and Pain Medicine, Maastricht University Medical Centre+, Maastricht, The Netherlands.
5Department of Anesthesiology, Intensive Care and Pain management, St. Antonius Nieuwegein, Nieuwegein, The Netherlands.
6Department of Anesthesiology, Rijnstate Hospital, Arnhem, The Netherlands.
7Department of Anesthesiology and Operations, Dijklander Hospital, Hoorn, The Netherlands.
8Department of Anesthesiology and Pain Management, Haaglanden Medical Centre, The Hague, The Netherlands.
9Department of Anesthesiology, Pain and Palliative Medicine, Radboud University Medical Centre, Nijmegen, The Netherlands.
10Department of Surgery, Amsterdam University Medical Centre, University of Amsterdam, Amsterdam, The Netherlands.
11Department of Surgery, Amsterdam University Medical Centre, VU University, Amsterdam, The Netherlands.
12Department of Surgery, Maastricht University Medical Centre+, Maastricht, The Netherlands.
13Department of Clinical Epidemiology and Medical Technology Assessment, Maastricht University Medical Centre+, Maastricht, The Netherlands.
14Care and Public Health Research Institute CAPHRI, Maastricht University, Maastricht, The Netherlands.
Funding
The TRACE study was supported by The Netherlands Organization for Health Research and Development (ZonMw), the Dutch Society for Anesthesia (NVA), and the individual participating medical centers. The TRACE study was also supported by a grant from the European Society of Anesthesiology and Intensive Care (ESAIC). It is furthermore supported by the Dutch Society for Surgery (NVvH) and the umbrella organization of nine health insurers in The Netherlands (Zorgverzekeraars Nederland). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Consortia
Contributions
AS: acquisition of data, conception and design of study, analysis and interpretation of data, drafting the article, final approval of the version to be submitted. EMWG: interpretation of data, critically revising the article, final approval of the version to be submitted. LMP: critically revising the article, interpretation of data, final approval of the version to be submitted. MWH: critically revising the article, interpretation of data, final approval of the version to be submitted. DKB: critically revising the article, interpretation of data, final approval of the version to be submitted. VMSF: critically revising the article, interpretation of data, final approval of the version to be submitted. WFFAB: critically revising the article, interpretation of data, final approval of the version to be submitted. CB: conception and design of study, interpretation of data, critically revising the article, final approval of the version to be submitted. PGN: conception and design of study, interpretation of the data, drafting the article, final approval of the version to be submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was ethically approved by the Human Subjects Committee of Amsterdam UMC, location VUmc Amsterdam (number NL56004.029.16, 29-06-2016) and registered with the Netherlands Trial Register (NTR5506). The Clinical Research Unit of the Amsterdam UMC took responsibility for the monitoring of patient inclusion and data registration. All study participants signed informed consent. The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
MWH reports grants from ZonMW & Zorgverzekeraars Nederland, grants from Dutch Society of Anesthesiology (NvA), grants from European Society of Anesthesiology and Intensive Care (ESAIC), during the conduct of the study. He is executive section editor for Pharmacology for Anesthesia & Analgesia, section editor for Anesthesiology of the Journal of Clinical Medicine, received grants, honoraria for lectures and is advisory board member for Eurocept BV, received grants and honoraria for lectures from BBraun, received grants and honoraria for lectures from Edwards and received grants and honoraria for lectures from Behring, outside the submitted work. WFFAB reports grants from ZonMW & Zorgverzekeraars Nederland and grants from ESAIC during the conduct of the study. He has received funding from the European Commission (EU-Horizon 2020) and InterReg EU outside the presented study. His department has received funding from Medtronic and WFFAB is member of an advice committee of Medtronic. All honorarium is directed to the department and not to him as a person. All other authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Stolze, A., van de Garde, E.M.W., Posthuma, L.M. et al. Validation of the PreOperative Score to predict Post-Operative Mortality (POSPOM) in Dutch non-cardiac surgery patients. BMC Anesthesiol 22, 58 (2022). https://doi.org/10.1186/s12871-022-01564-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-022-01564-1