Abstract
Background
Laryngeal masks airway (LMA) has been increasingly used in surgical patients. However, the use of LMA in laparoscopic surgeries remains controversial. The major concerns include the potential risk of esophageal regurgitation, aspiration, and difficulties to achieve effective ventilation. The aim of this study was to evaluate the safety and effectiveness of the LMA® Protector™ in patients undergoing laparoscopic surgery.
Methods
Patients aged 18 to 70 years, scheduled for laparoscopic surgeries were included. The insertion time, successful insertion rate, and oropharyngeal leak pressure were measured. Airway complications and airway manipulations during the procedure were documented. Effective ventilation rate was calculated. Visible bloodstains and reflux content in the drainage channel were documented after the removal of LMA® Protector™.
Results
Three hundred patients were enrolled. The insertion of LMA® Protector™ failed in seven patients resulting with a successful insertion rate of 97.7%. During the maintenance of anesthesia, airway manipulation was required in 19 patients (19/293, 6.48%), in three of whom the LMA was replaced with endotracheal intubation resulting with an effective ventilation rate of 96.7% (290/300). The oropharyngeal leak pressure was 30.18 ± 5.88 cmH2O. Seventy-five patients (25.86%) reported mild sore throat on the first day after surgery. Bloodstains on study devices were noticed in 58 patients (20%). Seventy-five patients (25.86%) reported mild sore throat on the first day after surgery. Gastric reflux was noticed in the drainage tube in 5 patients (1.72%) with no signs of aspiration in any of those patients.
Conclusions
The LMA® Protector™ was shown to be safe and effective in patients undergoing laparoscopic surgeries. Although minor complications that require no further treatment, no clinically diagnosed aspiration was noticed in our study. Gastric reflux was noticed in the drainage tube in five patients undergoing laparoscopic gynecology surgery. Further research is needed to verify whether LMA® Protector™ is suitable for procedures in Trendelenburg position or other situations that a high risk of gastroesophageal reflux exists.
Trial registration
The trial was registered at the Chinese Clinical Trial Registry (ChiCTR1800018300, date of registration: September 2018).
Similar content being viewed by others
Introduction
Laparoscopic surgery is now being used for more varieties of surgery than ever before due to the advantages that it offers over conventional open surgery. General anesthesia with endotracheal intubation is used as a common practice in this kind of surgery. As an alternative to endotracheal intubation, the laryngeal mask airway (LMA) has also been proved to be safe and may have several advantages in these procedures, including improved hemodynamic and respiratory stability, less restricted mucociliary clearance, and a reduced need for anesthetics [1,2,3,4,5,6,7]. However, there remain some concerns with the use of LMA in laparoscopic surgery. The major concerns include the potential risk of gastroesophageal regurgitation, aspiration, and difficulties to achieve effective ventilation due to the influence of artificial pneumoperitoneum and postural changes on airway pressure and pulmonary compliance [1, 2]. Therefore, many anesthesiologists advocate endotracheal intubation and mechanical ventilation for this kind of procedures despite evidences provided by previous studies.
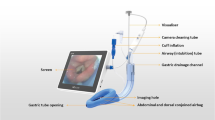
It has been shown that the performance of a laryngeal mask can be affected by different factors, including types of materials, sizes of esophageal drainage tubes, intracuff pressure, and shapes of the cuff [1, 6, 8,9,10]. Numerous attempts have been made to improve the sealing pressure and drainage of reflux, and therefore, to reduce the risk of air leak and aspiration of LMAs [11]. LMA® Protector™ (Teleflex Medical Europe, Westmeath, Ireland) was first used in clinical in 2015. It is made of medical-grade silicone with two large-volume gastric drainage channels and integrated with a cuff pressure indicator, the Cuff Pilot™ [12]. Previous studies have assessed the clinical performance of LMA® Protector™ [12, 13]. This second-generation LMA has enabled the application of higher respiratory pressure, possible drainage of regurgitated material and the introduction of a gastric tube via integrated gastric access [8, 12, 14, 15].
Recent studies also showed that LMA® Protector™ provides effective and efficient pulmonary ventilation in laparoscopic surgeries, promising as an alternative to endotracheal intubation in such procedures. However, due to the low incidence, adverse events that correlated with the use of LMA® Protector™, such as failed ventilation, esophageal regurgitation, pulmonary aspiration, postoperative sore throat, and other airway complications, were not intensively monitored in previous studies. To evaluate the prevalence of these complications, a large scale research was usually required. Therefore, in this multicenter study, we further investigated the effectiveness and safety of LMA® Protector™ in laparoscopic surgeries in a large sample and intended to provide evidence for LMA to be used in laparoscopic surgery [3, 16].
Materials and methods
Study design
This study was conducted in six hospitals in China (The First Medical Center of Chinese PLA General Hospital, Henan Provincial People’s Hospital, The First Affiliated Hospital of Zhengzhou University, Tianjin Medical University General Hospital, The Second Affiliated Hospital of Air Force Medical University, Daping Hospital) from October 01, 2018, to October 31, 2019. The study was registered at the Chinese Clinical Trial Registry on September 10, 2018 (ChiCTR1800018300, Weidong Mi as the principal investigator). The study adhered to the STROBE Statement and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant before enrollment.
Ethics
This study was approved by the ethics committee of Chinese PLA General Hospital(S2017–034-02), Daping Hospital of Army Medical University (2018–45), the First Affiliated Hospital of Zhengzhou University (SS-2019-038), Tianjin Medical University General Hospital (record), Henan Provincial People’s Hospital (2018–46), the Second Affiliated Hospital of Air Force Medical University (201812–09).
Study population
In this study, we included adult patients who were American Society of Anesthesiology (ASA) class 1 and 2, 18 to 70 years old, and scheduled for laparoscopic surgeries with an expected duration of surgery less than 4 h and expected blood loss less than 300 ml. Exclusion criteria consisted of BMI > 30 kg/m2, patient refusal, a suspected difficult airway, increased risk of aspiration (hiatus hernia, gastroesophageal reflux disease, non-fasting status), recent upper respiratory tract infection, inability to comply with study requirements including follow-up. All the anesthetists who participated in this study were experienced in the use of LMA with more than 200 cases of LMA insertions and were trained in the use of LMA® Protector™ (the study device) and study protocol before the beginning of the study.
Study procedures
All patients received standard monitoring, including electrocardiogram, non-invasive blood pressure, and oxyhemoglobin saturation in the operation room. After pre-oxygenation with a facemask for 3 min, anesthesia was induced with sufentanil 0.2–0.4 μg/kg or fentanyl 1-3 μg/kg, propofol 1-2 mg/kg oretomidate 0.2–0.3 mg/kg, and rocuronium 0.6–0.9 mg/kg or cisatracurium 0.2–0.3 mg/kg. LMA® Protector™ insertion was attempted in all patients. The choice of LMA® Protector™ size was based on the following principles: weight ≤ 50 kg for size 3; weight between 51 and 70 kg for size 4; weight>70 kg for size 5. After deflation and lubrication of the cuff, the LMA® Protector™ was inserted with the distal tip kept in the midline until resistance was felt. Then the cuff was inflated to maintain the intracuff pressure at 40 to 60 cmH2O according to the Cuff Pilot™, the integrated cuff pressure indicator [17]. The anesthesia apparatus was connected to the LMA® Protector™ for mechanical ventilation. Chest wall movement and capnography were used to confirm the successful placement of LMA. Further extension of the head and chin lift performed by the assistant was permitted during the insertion of the study device.
The insertion time was measured from the moment the anesthetist picked up the LMA® Protector™ until the appearance of the first capnography trace by an assistant. Oropharyngeal leak pressure (OLP) was measured by setting the adjustable pressure limiting valve to 40 cmH2O at a fixed gas flow of 3 L/min and noting the steady-state airway pressure on the monitor [10, 18]. The suprasternal notch test was performed as Eckardt described, [19] and a lubricated 14F gastric tube was then inserted through the female drainage port. Failed insertion of LMA® Protector™ was defined by any of the following criteria: [1] failed passage into the pharynx [2]; malposition (air leaks or end-tidal capnography could not be obtained); and [3] ineffective ventilation (maximum expired tidal volume < 8 ml/kg, end-tidal carbon dioxide > 45 mmHg, or pulse oxygen saturation < 92% if correctly positioned) [13]. We allowed a maximum of three attempts with the allocated device. The easiness of insertion was graded as easy, mild difficulty with resistance, moderate difficulty with resistance, and severe difficulty with resistance.
Anesthesia was maintained with intravenous or intravenous-inhalation combined anesthesia to keep bispectral index (BIS) between 40 and 60. Supplemental doses of neuromuscular blockade (rocuronium 0.1–0.2 mg/kg or cisatracurium 0.02–0.04 mg/kg) was administered intermediately during the operation. The timing of muscle relaxants during the operation was guided by clinical criteria according to the pharmacokinetics of muscle relaxants, the pathophysiological characteristics of patients, and the demand for surgical relaxation. The total dosage of neuromuscular blockade used during the surgery was recorded. Volume control ventilation was set at an inspired tidal volume of 6–8 mL/kg, a respiratory rate of 12/min, and an inspiratory/expiratory ratio of 1:2 to maintain the end-tidal CO2(EtCO2) at around 35–45 mmHg and no positive-end-expiratory pressure was used. Pneumoperitoneum was established by insufflation of carbon dioxide to a pressure of 14 mmHg. During maintenance of anesthesia, airway complications (intermittent obstruction, complete obstruction, airway leak) and airway manipulations during the procedure (position corrections, additional cuff inflation or deflation, device reinsertion) were documented. If effective ventilation could not be achieved after airway manipulations, i.e., maximum expired tidal volume < 8 ml/kg, end-tidal carbon dioxide > 45 mmHg, or pulse oxygen saturation < 92%, the LMA® Protector™ would be replaced with endotracheal intubation. Effective ventilation rate was calculated as the proportion of patients who were effectively ventilated with LMA® Protector™ throughout the procedure in all participants.
On emergence from anesthesia, the LMA® Protector™ was removed when the patient was able to breathe spontaneously and follow verbal instructions. All patients were monitored for at least 30 min in the recovery room before returning to the ward. Visible bloodstains and reflux content in the drainage channel were documented after the removal of LMA® Protector™. The operator’s satisfaction score was rated on a scale of 0 (worst) to 10 (best) by the anesthetists. On the first day after surgery, participants were interviewed to ascertain if they had the following complaints: sore throat, dysphagia, and hoarseness of voice. Vigilance against aspiration was kept especially when the patient presented with symptoms such as coughing, difficulty breathing, choking, or wheezing. Further evaluation would be taken if aspiration was suspected. Adverse events should be reported if the patient was diagnosed as aspiration during hospitalization or re-administered as aspiration pneumonia in 1 month after surgery.
Statistical analysis
The sample size was calculated based on the primary outcome of effective ventilation rate. An estimated minimum sample size of 243 patients would be required to show an effective ventilation rate of 95% with a single-sided type I error of 0.05 and power of 0.8 [20]. As the sample dropout rate was estimated to be 20%, the final determined sample size was 300.
The Kolmogorov-Smirnov test was performed to evaluate the normality of continuous data. Mean and the standard deviation was used to describe symmetrically distributed continuous data. Asymmetrically distributed continuous data were presented as median [interquartile range]. Percentages were used to describe categorical data. Continuous data were analyzed with Student’s t test or the Mann-Whitney U test. The chi-square test or Fisher’s exact test was used for categorical data, as appropriate. All the data were analyzed using SPSS (Version 20, IBM Corp., Chicago, IL, USA).
Results
Figure 1 shows patient enrollment and flow chart. Three hundred patients were enrolled. The characteristics of these patients are presented in Table 1. The insertion of LMA® Protector™ failed after three attempts in seven patients (7/300, 2.41%). During the maintenance of anesthesia, airway manipulation was required in 19 patients (19/293, 6.48%), in three of whom the LMA was replaced with endotracheal intubation. The effective ventilation rate of LMA® Protector™ was 96.7% (290/300). Comparison of demographic parameters between successful and failed cases showed no difference (Table 1).
The performance of the LMA® Protector™ is shown in Table 2. The recommended size of the LMA® Protector™ was suitable in 285 patients (285/300, 95%). The median insertion time was 29.5 s. The oropharyngeal leak pressure was ≥25 cmH2O in 85% of patients. The anesthetists’ satisfaction score of LMA® Protector™ was 8.59 ± 1.02.
Table 3 shows the most frequent complications of LMA® Protector™ observed in this study. Bloodstains on study devices were noticed in 58 patients (20%). Seventy-five patients (25.86%) reported mild sore throat on the first day after surgery. All of them recovered and no further treatment was required. Gastric reflux was noticed in the drainage tube in 5 patients (1.72%) with no signs of aspiration in any of those patients.
Discussion
In the present study, we found that LMA® Protector™ was an effective supraglottic airway device (SAD) in laparoscopic surgeries. The initial successful insertion rate was 97.7%, which is relatively high in comparison to other SADs from the literature review. During the maintenance of anesthesia, the airway manipulation frequency was as low as 6.48%. The oropharyngeal leak pressure was 30.18 ± 5.88 cmH2O and could achieve effective ventilation in 96.7% of patients throughout the procedure.
The mean oropharyngeal leak pressure of LMA® Protector™ in our study was 30 cmH2O, which was in keeping with the previous findings on LMA® Protector™ by Zaballos, M., [21] and van Zundert [18]. The oropharyngeal leak pressure of 30 cmH2O was better than most of the other LMAs that were commonly used in clinical practice and meet the needs of laparoscopic surgeries [22, 23]. In our study, once the LMA® Protector™ was placed properly, the frequency of airway manipulation was very low during the maintenance of anesthesia. Adequate ventilation could be achieved with LMA® Protector™ in patients in the Trendelenburg position as well. The advantage of the study device in high oropharyngeal leak pressure and low frequency of airway manipulation may partially be attributed to the silicone material that is used in LMA® Protector™, which makes the device soft and flexible [11]. The integrated Cuff Pilot™ with a cuff pressure indicator that provides easier adjustment of the intracuff pressure may also be beneficial in reducing LMA displacement and air leak [12, 17].
A major concern of using LMAs in laparoscopic surgery is the risk of regurgitation and aspiration [2]. The incidence of regurgitation varies from 0.2 to 16.7% in previous reports with different SADs [9, 24, 25]. In this study, gastric reflux was noticed in five patients (1.72%) in the drainage tube of the device at the end of surgery. All of the five patients were undergoing laparoscopic gynecology surgery in the Trendelenburg position. We believe that the patient’s postural status and pneumoperitoneum during surgery were major causes of regurgitation, although previous studies have proved that LMA could be used safely in laparoscopic gynecology surgery in a head-down position [1, 10]. None of these patients experienced any signs of aspiration or pulmonary complications. It is believed that the large volume drainage channels built in LMA® Protector™ had possibly made the reflux easier to drain out and therefore reduced the risk of aspiration [26]. Further research is needed to verify this point of view. However, our findings suggested that attention should be given to gastroesophageal regurgitation especially under head-down positions and adequate suction should be conducted before removal of SADs. The incidence of sore throat (25.86%) observed in our study on the first day after surgery was relatively higher in comparison with 6% in Zaballos, M.’s study [21] and 23.1% in Sng’s study [13]. However, all 75 patients experienced mild symptoms and recovered without any further intervention.
This study showed that the median insertion time was 29.5 s, which seems to be longer than what was reported in previous studies (4.8 s to 19 s) [12, 13]. The reasons for this discrepancy may include the following: First, a different definition of insertion time was used. In our study, the insertion time was defined as from picking up the LMA® Protector™ until the first capnography trace appeared, which included insertion, inflation, and connection of the ventilation machine, other than inserting alone [12]. Second, LMA® Protector™ was designed according to the anatomical characteristics of Caucasians. At the same time, the silicone material and double drainage channel integrated in LMA® Protector™ had made it softer and wider. In our survey of the operators, the difficulty of insertion was more than moderate in 34.81% of cases. A small amount of blood was noticed on the device in 58 (20%) patients after the removal of LMA® Protector™. These indicated that the difference in airway anatomy between Asians and Caucasians might have affected the difficulty of insertion. Third, although all the operators were experienced with LMA and were trained before the beginning of the study, there was still a learning curve in the use of LMA® Protector™. As the number of cases increased, the insertion time began to shorten gradually.
There were several limitations in our study. First, the position of LMA® Protector™ was not determined by fiberoptic bronchoscopy, which may provide a more objective diagnosis of malposition than the clinical signs that were used in our study. Second, we used clinical criteria to guide the use of neuromuscular blockade rather than the neuromuscular block monitoring, which may lead to different degrees of muscle relaxation between patients. Third, we did not compare the LMA® Protector™ with other SADs or endotracheal intubation in this study. However, for our intention to evaluate the efficacy and safety of LMA protector in laparoscopic surgeries, we have well controlled the quality of our study and have make sure that all investigators were trained prior to the study. The oropharyngeal leak pressure in our study was shown to be comparable to other LMAs reported in previously published articles [22, 23]. We believe that our results, including the prevalence of failed ventilation, airway manipulation, and postoperative complications, are of great help to clinicians in choosing airway management tools for patients undergoing laparoscopic surgeries.
In conclusion, LMA® Protector™ was shown to be safe and effective in patients undergoing laparoscopic surgeries. The high sealing pressure provided by the study device guarantees effective mechanical ventilation during pneumoperitoneum. No clinically diagnosed aspiration was noticed in our study except for minor complications, such as sore throat and bloodstaining on devices. Gastric reflux was noticed in the drainage tube in five patients undergoing laparoscopic gynecology surgery. Further research is needed to verify whether LMA® Protector™ is suitable for procedures in the Trendelenburg position or other situations that a high risk of gastroesophageal reflux exists.
Conclusions
The LMA® Protector™ was shown to be safe and effective in patients undergoing laparoscopic surgeries. Although minor complications that require no further treatment, such as sore throat were reported, no clinically diagnosed aspiration was noticed in our study.
Availability of data and materials
The datasets and materials related to the current study are available from the corresponding author upon reasonable request.
Abbreviations
- LMA:
-
Laryngeal masks airway
- SAD:
-
Supraglottic airway device
- OLP:
-
Oropharyngeal leak pressure
- ASA:
-
American Society of Anesthesiologists
References
Mishra SK, Sivaraman B, Balachander H, Naggappa M, Parida S, Bhat RR, et al. Effect of pneumoperitoneum and Trendelenberg position on oropharyngeal sealing pressure of I-gel and ProSeal LMA in laparoscopic gynecological surgery: a randomized controlled trial. Anesth Essays Res. 2015;9(3):353–8. https://doi.org/10.4103/0259-1162.159771.
Lemos J, De Oliveira GS, Jr., de Pereira Cardoso HE, Lemos LD, de Carvalho LR, Modolo NS. Gastric regurgitation in patients undergoing gynecological laparoscopy with a laryngeal mask airway: a prospective observational study. J Clin Anesth. 2017;36:32–5. https://doi.org/10.1016/j.jclinane.2016.07.038 Epub Nov 11.
Kang SH, Park M. Comparison of early postoperative recovery between laryngeal mask airway and endotracheal tube in laparoscopic cholecystectomy: a randomized trial. Medicine. 2019;98(25):e16022.
Griffiths JD, Nguyen M, Lau H, Grant S, Williams DI. A prospective randomised comparison of the LMA ProSeal versus endotracheal tube on the severity of postoperative pain following gynaecological laparoscopy. Anaesth Intensive Care. 2013;41(1):46–50. https://doi.org/10.1177/0310057X1304100109.
Ye Q, Wu D, Fang W, Wong GTC, Lu Y. Comparison of gastric insufflation using LMA-supreme and I-gel versus tracheal intubation in laparoscopic gynecological surgery by ultrasound: a randomized observational trial. BMC Anesthesiol. 2020;20(1):136.
Park SY, Rim JC, Kim H, Lee JH, Chung CJ. Comparison of i-gel(R) and LMA supreme(R) during laparoscopic cholecystectomy. Korean J Anesthesiol. 2015;68(5):455–61. https://doi.org/10.4097/kjae.2015.68.5.455 Epub Sep 30.
Maltby JR, Beriault MT, Watson NC, Liepert D, Fick GH. The LMA-ProSeal is an effective alternative to tracheal intubation for laparoscopic cholecystectomy. Can J Anaesth. 2002;49(8):857–62.
Rustagi P, Patkar GA, Ourasang AK, Tendolkar BA. Effect of Pneumoperitoneum and lateral position on Oropharyngeal seal pressures of Proseal LMA in laparoscopic urological procedures. J Clin Diag Res. 2017;11(2):Uc05–uc9.
Belena JM, Ochoa EJ, Nunez M, Gilsanz C, Vidal A. Role of laryngeal mask airway in laparoscopic cholecystectomy. World J Gastrointest Surg. 2015;7(11):319–25. https://doi.org/10.4240/wjgs.v7.i11.319.
Lopez AM, Agusti M, Gambus P, Pons M, Anglada T, Valero R. A randomized comparison of the Ambu AuraGain versus the LMA supreme in patients undergoing gynaecologic laparoscopic surgery. J Clin Monit Comput. 2017;31(6):1255–62. https://doi.org/10.1007/s10877-016-9963-0 Epub 2016 Nov 26.
Moser B, Keller C, Audigé L, Bruppacher HR. Oropharyngeal leak pressure of the LMA protector™ vs the LMA supreme™; a prospective, randomized, controlled clinical trial. Acta Anaesthesiol Scand. 2019;63(3):322–8.
Moser B, Audige L, Keller C, Brimacombe J, Gasteiger L, Bruppacher HR. A prospective, randomized trial of the Ambu AuraGain laryngeal mask versus the LMA(R) protector airway in paralyzed, anesthetized adult men. Minerva Anestesiol. 2018;84(6):684–92. https://doi.org/10.23736/S0375-9393.17.12254-6 Epub 2017 Nov 17.
Sng BL, Ithnin FB, Mathur D, Lew E, Han NR, Sia AT. A preliminary assessment of the LMA protector in non-paralysed patients. BMC Anesthesiol. 2017;17(1):26. https://doi.org/10.1186/s12871-017-0323-5.
Qamarul Hoda M, Samad K, Ullah H. ProSeal versus classic laryngeal mask airway (LMA) for positive pressure ventilation in adults undergoing elective surgery. Cochrane Database Syst Rev. 2017;7(7):Cd009026.
Belena JM, Nunez M, Anta D, Carnero M, Gracia JL, Ayala JL, et al. Comparison of laryngeal mask airway supreme and laryngeal mask airway Proseal with respect to oropharyngeal leak pressure during laparoscopic cholecystectomy: a randomised controlled trial. Eur J Anaesthesiol. 2013;30(3):119–23. https://doi.org/10.1097/EJA.0b013e32835aba6a.
Kim D, Park S, Kim JM, Choi GS, Kim GS. Second generation laryngeal mask airway during laparoscopic living liver donor hepatectomy: a randomized controlled trial. Sci Rep. 2021;11(1):3532.
Yonehara S, Komasawa N, Watanabe N, Minami T. Application of laryngeal mask protector™ cuff pilot™ for safe recovery from general anesthesia in a patient with difficult mask ventilation. J Clin Anesth. 2018;45:2–3.
van Zundert AAJ, Wyssusek KH, Pelecanos A, Roets M, Kumar CM. A prospective randomized comparison of airway seal using the novel vision-guided insertion of LMA-supreme(R) and LMA-protector(R). J Clin Monit Comput. 2019;5(10):019–00301.
Eckardt F, Engel J, Tw Mann S, Muller M, Zajonz T, Koerner CM, et al. LMA(R) ProtectorTM airway. First experience with a new second generation laryngeal mask. Minerva Anestesiol. 2018;10(18):12421–7.
Pandit JJ. If it hasn't failed, does it work? On 'the worst we can expect' from observational trial results, with reference to airway management devices. Anaesthesia. 2012;67(6):578–83.
Zaballos M, Zaballos J, López S, Fernández-Dïez AI, Lluch-Oltra A, Mexedo C, et al. The LMA® ProtectorTM in anaesthetised, non-paralysed patients: a multicentre prospective observational study. Anaesthesia. 2019;74(3):333–9.
Belena JM, Nunez M, Vidal A, Gasco C, Gilsanz C, Alcojor A, et al. Use of second generation supra-glottic airway devices during laparoscopic cholecystectomy: a prospective, randomized comparison of LMA Proseal, LMA SupremeTM and igel. Acta Anaesthesiol Belg. 2016;67(3):121–8.
Anand LK, Goel N, Singh M, Kapoor D. Comparison of the supreme and the ProSeal laryngeal mask airway in patients undergoing laparoscopic cholecystectomy: a randomized controlled trial. Acta Anaesthesiol Taiwanica. 2016;54(2):44–50. https://doi.org/10.1016/j.aat.2016.03.001 Epub Apr 19.
Verghese C, Brimacombe JR. Survey of laryngeal mask airway usage in 11,910 patients: safety and efficacy for conventional and nonconventional usage. Anesth Analg. 1996;82(1):129–33.
Khazin V, Ezri T, Yishai R, Sessler DI, Serour F, Szmuk P, et al. Gastroesophageal regurgitation during anesthesia and controlled ventilation with six airway devices. J Clin Anesth. 2008;20(7):508–13.
Van Zundert AA, Skinner MW, Van Zundert TC, Luney SR, Pandit JJ. Value of knowing physical characteristics of the airway device before using it. Br J Anaesth. 2016;117(1):12–6.
Acknowledgments
We would like to thank Yandong Jiang, M.D., Ph.D., Professor and Vice-Chair for Clinical Research, Department of Anesthesiology, McGovern Medical School, University of Texas, Houston Health Science Center, Houston, Texas, for his suggestions in manuscript preparation.
Funding
This work was supported by a grant from the National Key Research and Development Program of China lead by Dr. Weidong Mi (Project No.2018YFC2001900), which provided financial support for the study design, training of investigators, and statistical analysis.
Author information
Authors and Affiliations
Contributions
YL and YS contributed equally to this work. YL and YS have participated to design the study, collect the data, analysing data, write, edit the final version of the manuscript. MW, MY, and HS, ZW, LC, JY, SG, YY, ZS, WZ, XZ, XS, YW, QF, JC have participated to collect data, edit the final version of the manuscript. WM has participated to design the study, visualize and administer the project, acquire funding, write, edit the final version of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Chinese PLA General Hospital(S2017–034-02), Daping Hospital of Army Medical University (2018–45), the First Affiliated Hospital of Zhengzhou University (SS-2019-038), Tianjin Medical University General Hospital (Recorded), Henan Provincial People’s Hospital (2018–46), the Second Affiliated Hospital of Air Force Medical University (201812–09) and written informed consents have been obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The study devices were provided free of charge by the manufacture, Teleflex Medical Europe, Westmeath, Ireland.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Song, Y., Wang, M. et al. LMA® protector™ in patients undergoing laparoscopic surgeries: a multicenter prospective observational study. BMC Anesthesiol 21, 318 (2021). https://doi.org/10.1186/s12871-021-01535-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-021-01535-y