Abstract
Background
It is uncertain whether the association of the intraoperative driving pressure (ΔP) with postoperative pulmonary complications (PPCs) depends on the surgical approach during abdominal surgery. Our primary objective was to determine and compare the association of time–weighted average ΔP (ΔPTW) with PPCs. We also tested the association of ΔPTW with intraoperative adverse events.
Methods
Posthoc retrospective propensity score–weighted cohort analysis of patients undergoing open or closed abdominal surgery in the ‘Local ASsessment of Ventilatory management during General Anaesthesia for Surgery’ (LAS VEGAS) study, that included patients in 146 hospitals across 29 countries. The primary endpoint was a composite of PPCs. The secondary endpoint was a composite of intraoperative adverse events.
Results
The analysis included 1128 and 906 patients undergoing open or closed abdominal surgery, respectively. The PPC rate was 5%. ΔP was lower in open abdominal surgery patients, but ΔPTW was not different between groups. The association of ΔPTW with PPCs was significant in both groups and had a higher risk ratio in closed compared to open abdominal surgery patients (1.11 [95%CI 1.10 to 1.20], P < 0.001 versus 1.05 [95%CI 1.05 to 1.05], P < 0.001; risk difference 0.05 [95%CI 0.04 to 0.06], P < 0.001). The association of ΔPTW with intraoperative adverse events was also significant in both groups but had higher odds ratio in closed compared to open abdominal surgery patients (1.13 [95%CI 1.12– to 1.14], P < 0.001 versus 1.07 [95%CI 1.05 to 1.10], P < 0.001; risk difference 0.05 [95%CI 0.030.07], P < 0.001).
Conclusions
ΔP is associated with PPC and intraoperative adverse events in abdominal surgery, both in open and closed abdominal surgery.
Trial registration
LAS VEGAS was registered at clinicaltrials.gov (trial identifier NCT01601223).
Similar content being viewed by others
Introduction
The incidence of postoperative pulmonary complications (PPCs) is high and depends on the used definitions and the studied population [1]. Their occurrence is associated with increased morbidity and mortality [2, 3]. PPCs can be prevented by reducing lung strain by using a low tidal volume (VT) [4], ,and by using sufficient positive end–expiratory pressure (PEEP) [5]. Since the driving pressure (ΔP), defined as the difference between plateau pressure and PEEP, is associated with the development of PPCs [5, 6], titrating VT and PEEP to obtain the lowest ΔP could be an effective preventive strategy against PPCs.
The overall behaviour of the respiratory system depends on the properties of its components, i.e., the artificial and native airways, and the lung tissue, but also the chest wall consisting of the rib cage and diaphragm. Most of the force applied during invasive ventilation is needed to expand the chest wall, and only a lesser amount to inflate lung tissue [7]. When the chest wall elastance increases, e.g., during pneumoperitoneum, the ΔP increases, even when VT is left unchanged [8]. This rise in ΔP is often interpreted as ‘innocent’, and therefore accepted during intraoperative pneumoperitoneum. However, the cephalad shift of the diaphragm could induce, or worsen atelectases during intraoperative ventilation, and the resulting increase in ΔP is related with a rise in lung applied force [9]. In other words, it should be questioned if a rise in ΔP during pneumoperitoneum with closed abdominal surgery can be accepted.
To determine and compare the independent associations of ΔP with PPCs in patients undergoing open abdominal surgery versus patients undergoing closed abdominal surgery, we reassessed the database of the ‘Local ASsessment of Ventilatory management during General Anaesthesia for Surgery’ (LAS VEGAS) study [10]. The LAS VEGAS study was a large observational study that included a large proportion of patients at an increased risk for PPCs. The primary hypothesis tested here was that the association of ΔP with PPCs is weaker in closed versus open abdominal surgery patients. The primary objective was to test the association of a time–weighted average driving pressure (ΔPTW) with PPCs. The secondary objective was to test the association of ΔPTW with intraoperative adverse events.
Methods
Study design and setting
This is a posthoc analysis of the LAS VEGAS study [10], carried out following current guidelines and the recommendations of the statement for strengthening the reporting of observational studies in epidemiology (STROBE) (www.strobe-statemenent.org). The statistical analysis plan was predefined, updated, and finalised before data extraction, and is presented as Additional file 1. The LAS VEGAS study is a worldwide international multicentre prospective seven–day observational study describing intraoperative ventilation practice, complications during anaesthesia, PPCs in the first five postoperative days, hospital length of stay, and hospital mortality.
The ethical committee of the Academic Medical Center, Amsterdam, the Netherlands, approved the LAS VEGAS study protocol (W12_190#12.17.0227). Each participating centre obtained approval from their institutional review board if needed, and patients were included after obtaining written informed consent when dictated by national or regional legislation. The LAS VEGAS study was partially funded and endorsed by the European Society of Anaesthesiology and registered at clinicaltrials.gov (study identifier NCT01601223, first posted date: 17/05/2012).
Inclusion and exclusion criteria
The LAS VEGAS study recruited consecutive patients undergoing general anaesthesia with mechanical ventilation during anaesthesia for surgery during a seven–days timeframe between 14 January and 4 March 2013. Exclusion criteria of the LAS VEGAS study were: (1) age < 18 years, (2) having received mechanical ventilation in the preceding month, (3) obstetric or ambulatory surgical interventions, and (4) cardiothoracic surgery cardiopulmonary bypass.
For the current analysis, inclusion was restricted to patients undergoing abdominal surgery. The following additional exclusion criteria were used: (1) insufficient data to calculate ΔP, i.e., on at least two timepoints sufficient data had to be available to calculate the driving pressure for a patient to be included; (2) to increase the homogeneity of the compared patient cohorts and avoid using erroneous data, patients who received intraoperative ventilation through an airway device other than an endotracheal tube as well as patients under an assisted or spontaneous ventilation mode were excluded; (3) patients in whom laparoscopy only assisted the surgery, i.e., surgeries that could not be classified as mere open or mere closed abdominal surgery, were also excluded from the current analysis.
Data recording and calculations
Full details on data collection can be found in the original publication of the LAS VEAGS study [10], and in Additional file 2. In the LAS VEGAS study database, ventilatory parameters at every hour of surgery, from induction up to the last hour of surgery, were recorded. Data in the LAS VEGAS database was validated through two rounds of extensive data cleaning to check for invalid or incomplete data. Local investigators were queried on incorrect or missing data and had to correct those in the cleaning rounds.
The following calculations were performed. ΔP was calculated by subtracting PEEP from plateau pressure or inspiratory pressure at every hour in volume–controlled and pressure–controlled ventilated patients, respectively. ΔPTW, i.e., the pressure that is proportional to the amount of time spent at each driving pressure in relation to the total time, was calculated by summing the mean values between consecutive time points multiplied by the time between those points and then dividing by the entire time [11]. Similarly, time–weighted average peak pressure and PEEP were determined. Details on calculations are provided in the Additional file 2 Figure S1.
Definitions
PPCs were defined as a collapsed composite of the following events: unexpected postoperative invasive or non–invasive ventilation, acute respiratory failure, acute respiratory distress syndrome, pneumonia, and pneumothorax. The occurrence of each type of complication was monitored until hospital discharge but restricted to the first five postoperative days.
Intraoperative adverse events were defined as an ordinal composite of the following events: any oxygen desaturation or lung recruitment manoeuvres performed to rescue from hypoxemia, any need for adjusting ventilator settings for reducing airway pressures or correction of expiratory flow limitation, any hypotension or need for vasoactive drugs, and any new cardiac arrhythmia.
A detailed list of definitions of the composites of PPCs and intraoperative adverse events is provided in Additional file 2 Table S1 and Table S2.
Endpoints
The primary endpoint was the composite of PPCs. The secondary endpoint was the composite of intraoperative adverse events.
Analysis plan
The analysis plan was prespecified before data access, and we used data of all available patients in the LAS VEGAS database without formal sample size calculation. Also, as the purpose of the analysis was exploring a physiological hypothesis, we did not specify any a priori effect size.
Continuous variables were reported as median and interquartile ranges; categorical variables expressed as n (%). Normality of distributions was assessed by inspecting quantile–quantile plots. If variables were normally distributed, the two–sample t–test was used; if not, the Wilcoxon rank sum test was used. We used the Chi–square test or Fisher’s exact test for categorical variables, or when appropriate, as relative risks. Statistical uncertainty was expressed by showing the 95%–confidence intervals (CI). Since the simultaneous occurrence of various intraoperative adverse events is frequent, we analysed them as an ordinal variable with a range spanning from zero to seven adverse events.
To control for confounding effects, we estimated the association of ΔPTW with PPC with a weighted mixed–effect logistic regression, and the association of ΔPTW with intraoperative adverse events with a weighted mixed ordinal regression. To fit the models, we introduced centres as a random intercept, and an inverse probability weighting factor computed from the covariate–balancing propensity score (CBPS) method to simultaneously optimise treatment assignment prediction, i.e., ΔPTW as a continuous variable, and confounders influence [12]. The CBPS procedure sets mean independence between treatment, i.e., ΔPTW, and covariates to ensure covariate balancing and estimates the propensity score with the generalised method of moments method. For both outcomes, we fitted the model for each of the compared patient cohorts respectively, i.e., patients who underwent open surgery intervention and those who underwent closed surgical intervention. We used a Wald z-test to test the difference between odds ratios from models fitted on closed and open surgery cohort. Models’ goodness of fit was assessed by residual diagnosis based on scaled quantile residuals (R DHARMa package v. 0.2.4) and simulated residuals (R sure package v 0.2.0) for logistic and ordinal models, respectively.
To build the CBPS to relate exposure variable, i.e., ΔPTW, with potential confounders, we included by clinical judgment the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) risk class [13, 14], and the average intraoperative VT. Then we performed feature selection with an augmented backward elimination selection method introducing 37 preoperative and intraoperative variables (Additional file 2:Statistics for a detailed list). The selection was based on a sequential process where initially all variables entered the model and finally those preoperative and intraoperative factors that yielded a change in the effect estimate > 0.1 and a significance criterion (alpha) < 0.1 were included. The algorithm stopped when all variables left in the model complied with both criteria [15]. We carried out a selection process of potential variables to avoid bias in the effect estimates using a comprehensive strategy to prevent the drawbacks of simple stepwise methods [16]. The model’s internal validation was assessed by bootstrap using 5 hundred generated samples and estimating the Area Under Curve (AUC) of the full and stepwise–selected variables models.
To further unravel the effect of the surgical approach on PPCs, we performed a sensitivity analysis fitting a mixed logistic regression with a random intercept for centre on a propensity score matched cohort. The propensity score was used to match patients with a similar covariable structure using the R matchit package carrying out the matching with the nearest neighbour method with a caliper of 0.1 with a ratio of patients in the open surgery arm of 2 to 1. Full details on the covariables introduced in the propensity score matching procedure can be found in the Additional file 2: Statistics. To assess the type of surgery as an effect modifier, we carried out another sensibility analysis fitting a weighted mixed logistic regression model on all data, i.e., both surgery cohorts, introducing the type of surgery as an independent variable and an interaction term between ΔPTW and type of surgery.
Statistical significance was considered for two–tailed P < 0.05. No imputation routine of missing values and no correction for multiple comparisons was prespecified; thus, all the findings should be viewed as exploratory. All analyses were performed with R 3.5.2 (The R Foundation for Statistical Computing, www.r-project.org). Additional explanation on the used methods can be found in the Additional file 2: Statistics.
Results
Patients
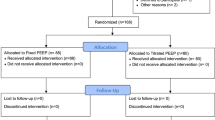
Of a total of 3265 patients undergoing abdominal surgery in the LAS VEGAS study, 1231 had insufficient data for calculating the ΔP (37.7%).
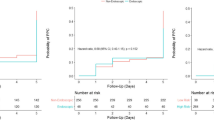
Out of the remaining 2034 patients, 1218 (60%) patients underwent an open abdominal intervention, and 906 (40%) patients, a closed abdominal surgical procedure (Fig. 1). ΔP could be calculated on two different timepoints in 34.4 and 53.7% of patients in the open and closed surgery group, respectively (Fig. 2 and Table S3). In 87% of patients, ΔP could be calculated on up to four timepoints.
Baseline demographic data, surgery–related and intraoperative ventilation characteristics are presented in Tables 1 and 2, and Fig. 2. Open abdominal surgery patients had higher ASA class and ARISCAT risk score, lower functional status, and fewer elective procedures, longer surgery times, less neuromuscular reversals, and received more intraoperative transfusions and fluids. Lower abdomen surgeries were the most frequently performed in the open abdominal surgery patients, while upper abdomen interventions were performed more often in closed abdominal surgery patients. ΔPTW was not different between the open and closed surgery groups (Table 2).
Primary and secondary outcome rates
In 102 (5%) patients, one or more PPC occurred, with a higher prevalence in open surgery patients than in patients who underwent a closed surgical procedure (7 versus 3%; P < 0.001). Hypotension, or need for vasopressors was more frequently observed during open surgery, while the need for airway pressure reduction was more often needed during closed surgery (Table 3).
Propensity score estimation variables
The variables that finally entered the propensity score and covariate balance assessment are detailed in the Additional file 2: Statistics and Fig. S2 and S3.
Association of ΔPTW with PPCs
ΔPTW was significantly associated with PPCs in both surgical groups. The association was stronger in closed abdominal surgery patients (odds ratio (OR), 1.17 [95%CI 1.16 to 1.19]; P < 0.001; risk ratio (RR), 1.11 [95%CI 1.10 to 1.20], P < 0.001) than in patients who underwent an open abdominal surgical intervention (OR, 1.07 [95%CI 1.06 to 1.08]; P < 0.001; RR 1.05 [95% CI 1.05 to 1.05]), with a significant difference (difference between ORs: 0.09 [95%CI 0.07 to 0.10]; P < 0.001; risk difference 0.05: [95%CI 0.04 to 0,06]), P < 0.001. Residuals plots are reported in Additional file 2: Figure S4.
Association of ΔPTW with the occurrence of adverse events
ΔPTW was significantly associated with intraoperative adverse events in both open and closed surgery patients. Also, here the association was stronger in closed surgery patients (1.13 [95%CI 1.12 to 1.14]) than in patients who underwent an open abdominal intervention (1.07 [95%CI 1.05 to 1.10]), difference between ORs 0.05 [95%CI 0.03 to 0.07]; P < 0.001.
Sensitivity analyses
ΔPTW was significantly associated with PPCs (OR, 1.08 [95%CI 1.06 to 1.09], P < 0.001) with closed surgery patients having a lower probability of occurrence (OR, 0.14 [95%CI 0.12 to 0.16, P < 0.001) with a significant interaction between ΔPTW and closed surgery (OR, 1.09 [95%CI 1.08 to 1.11], P < 0.001). The marginal effect of ΔPTW by type of surgery on PPCs probability is showed in Fig. 3. A rise in ΔPTW was associated with an increased probability of PPCs in both surgery types, with a steeper increase in closed surgery patients for ΔPTW above 20 cmH2O ∙ hour− 1.
After matching, the resulting cohort consisted of 344 open surgery patients and 254 closed surgery patients. Baseline characteristics between groups were well balanced (Additional file 2: Table S4 and S5). Type of surgery at matched levels of driving pressure was not associated with either outcome. (Additional file 2: Table S5 and S6).
Discussion
The main findings of this posthoc analysis of the LAS VEGAS study can be summarised as follows: (i.) the intraoperative ΔPTW was not different between open and closed surgery groups; (ii.) ΔPTW was associated with PPCs in both closed and open surgery patients; (iii.) ΔPTW was associated with intraoperative adverse events in both closed and open surgery patients; and (iv.) the type of surgery had a modifying effect on the association between ΔPTW and PPCs, with an increasing probability of PPCs at high ΔPTW in closed surgery. The last finding, though, was not confirmed in the matched cohort analysis.
This analysis uses the database of a worldwide international multicentre prospective observational study as a convenience sample [10], strictly followed a plan, and was characterised by a robust method accounting for the multilevel data structure and allowing precise estimation and confounder control, even with seven or fewer events per confounder [17, 18]. Also, the outcome of interest, i.e., PPCs, was predefined, well–described, and largely followed the European Perioperative Clinical Outcome (EPCO) group definitions [19]. Furthermore, the study population was defined to minimise information and selection bias and to have a sufficient number of patients while keeping an acceptable number of timepoints at which ΔPTW could be calculated per patient.
A recent metanalysis of individual trials on protective ventilation during general anaesthesia for cardiac or thoracic surgery found a significant association between ΔPTW and PPCs (OR 1.16, 95% CI 1.13 to 1.19; p < 0·0001) [5]. We found an almost identical association in patients undergoing closed abdominal surgery. Thus, our results confirm that ΔPTW is a promising target for interventions to prevent PPCs after closed abdominal surgery. The sensitivity analysis showed that the association between ΔPTW and PPCs was lower in patients who underwent a closed surgical procedure. However, this was not confirmed in the propensity score matched analysis, probably because of smaller sample size due to the matching procedure.
ΔP is an indicator of the amount of strain delivered to the respiratory system during mechanical ventilation [7]. Several studies investigated the effect of pneumoperitoneum on respiratory mechanics. Pneumoperitoneum was consistently found to decrease chest wall compliance, whereas lung compliance seems mostly spared by it [20,21,22,23,24,25,26,27]. Thus, inferring the amount of lung strain from plateau pressure and PEEP during pneumoperitoneum is challenging, since the part of the rise in plateau pressure caused by chest wall stiffening should not be regarded as a rise in lung strain [28]. Consequently, a higher ΔP during closed abdominal surgery is often seen as innocent. The current analysis results reject this assumption, as the association of ΔP with PPCs was stronger in patients undergoing closed abdominal surgery than in patients undergoing open abdominal surgery.
Pneumoperitoneum can affect lung mechanics in several ways [20,21,22,23,24,25,26,27]. A cranial shift of the diaphragm during laparoscopic surgery increases alveolar collapse, especially in lung parts close to the diaphragm. This is particularly true in upper abdominal surgery, which was the most common surgical procedure in patients undergoing closed surgery in the here studied cohort [29, 30]. PEEP may partially prevent this, and usually only when using high PEEP [31]. In the patients studied here, mostly low PEEP was used, regardless of the group. Additional studies are needed to test how high PEEP affects the association between ΔP with PPCs during pneumoperitoneum. Also, we found that ΔP was higher in patients undergoing closed surgery than in patients undergoing open abdominal surgery. However, open abdominal surgery lasted longer, resulting in a comparable ΔPTW in the two groups. The higher absolute ΔP was compensated for by a shorter duration of intraoperative ventilation, and vice versa. Using the ΔPTW allowed us to estimate an exposure limit threshold to an injurious factor as in occupational health. The steeper increase in probability of PPCs above a 20 cm H2O∙hour− 1 found in the sensitivity analysis can be related to an increase in collapsed lung tissue.
As expected, PPCs occurred more frequently in open abdominal surgery patients. An increased baseline risk could explain this due to typical differences in patient characteristics and the duration and the type of surgery. However, this finding strengthens the current analysis since we observed the association even in a cohort of patients, i.e., closed abdominal surgery, at low risk for PPCs and even after controlling for confounding effects with propensity score analysis.
Several intraoperative ventilation approaches, like the use of recruitment manoeuvres and higher PEEP, may result in a lower ΔP [32, 33]. Findings of a metanalysis including clinical trials on intraoperative ventilation suggest that PEEP titrations that resulted in a ΔP rise increased the risk of PPCs [5]. One randomised clinical trial showed an intraoperative PEEP strategy targeting the best compliance to reduce PPCs, though this was only a secondary endpoint in that study [34]. Thus, the best approach to minimise PPCs remains a matter of debate.
ΔPTW was associated with intraoperative adverse events in both closed and open surgery patients. Among all adverse events, airway pressure reduction was more frequently needed in closed surgery group underlining the need for ventilation strategies to lower peak and plateau pressures in this group of patients reflecting unacceptable high airway pressure during surgery.
Several limitations must be acknowledged. We used the parent LAS VEGAS definition of PPCs. This definition differs from what was somewhat recently proposed [1], but they remain reasonably comparable. The protocol of the LAS VEGAS study did not include the collection of oesophageal pressure recordings. Information regarding surgical positioning was not collected, and intra–abdominal pressure levels were also not recorded in the database of the LAS VEGAS study. Both could influence ΔPTW, though [35,36,37]. Due to the additional strict exclusion criteria, we excluded a considerable number of patients. Thus, the findings of this analysis need confirmation in other studies. Also, some patients had only a few timepoints at which ΔP could be calculated. Furthermore, we only included patients with an endotracheal tube and patients who received controlled ventilation, limiting our focus on a specific type of intraoperative airway device and ventilation mode. Of note, 25% of patients had a Body Mass Index (BMI) > 30 kg∙m− 2. Extrapolating this analysis’s findings to obese or morbidly obese patients should be done with some caution. Also, the original LAS VEGAS study was performed 7 years ago. Since then, there could have been changes in clinical practice, e.g., in the use of ‘Enhanced Recovery After Surgery’ (ERAS) pathways and muscle relaxant monitoring during and reversal at the end of surgery. Although the time gap between research findings and practice changes usually lasts longer than a decade [38,39,40], still could be that more immediate changes may affect the associations. Finally, we did not set any a priori effect threshold nor multiple comparisons correction; hence the results’ statistical significance and the exploratory nature of secondary outcome analysis must be confirmed in future trials.
Conclusions
ΔPTW is associated with the occurrence of PPCs and intraoperative adverse events in abdominal surgery. These associations are present regardless of the type of surgical approach and depend on the duration and actual ΔP. Both in patients undergoing open or closed abdominal surgery, the ΔP is a promising target for future strategies to reduce PPCs.
Availability of data and materials
The data as well as the code used for analysis are available from the corresponding author upon reasonable request.
Abbreviations
- ΔP:
-
Driving pressure
- ΔPTW :
-
Time–weighted average ΔP
- VT :
-
Tidal volume
- PEEP:
-
Positive end–expiratory pressure
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
- ARISCAT:
-
Assess Respiratory Risk in Surgical Patients in Catalonia
- AUC:
-
Area Under Curve
- RR:
-
Risk ratio
- OR:
-
Odds ratio
- BMI:
-
Body Mass Index
- EPCO:
-
European Perioperative Clinical Outcome
- ERAS:
-
Enhanced Recovery After Surgery’
References
Abbott TEF, Fowler AJ, Pelosi P, et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth. 2018;120:1066–79.
Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118:317–34.
Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–43.
Serpa Neto A, Hemmes SNT, Barbas CSV, et al. Protective versus conventional ventilation for surgery. Anesthesiology. 2015;123:66–78.
Neto AS, Hemmes SNT, Barbas CSV, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med. 2016;4:272–80.
Ladha K, Melo MFV, McLean DJ, Wanderer JP, Grabitz SD, Kurth T, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. Br Med J. 2015;351:h3646.
Williams EC, Motta-Ribeiro GC, Vidal Melo MF. Driving pressure and Transpulmonary pressure: how do we guide safe mechanical ventilation? Anesthesiology. 2019;131:155–63.
De Jong MAC, Ladha KS, Melo MFV, Staehr-Rye AK, Bittner EA, Kurth T, et al. Differential effects of intraoperative positive end-expiratory pressure (PEEP) on respiratory outcome in major abdominal surgery versus craniotomy. Ann Surg. 2016;264:362–9.
Wirth S, Biesemann A, Spaeth J, Schumann S. Pneumoperitoneum deteriorates intratidal respiratory system mechanics: an observational study in lung-healthy patients. Surg Endosc. 2017;31:753–60.
LAS VEGAS investigators. Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS - an observational study in 29 countries. Eur J Anaesthesiol. 2017;34:492–507.
Nichol A, Bailey M, Egi M, et al. Dynamic lactate indices as predictors of outcome in critically ill patients. Crit Care. 2011;15:R242.
Imai L, Ratkovic M. CBPS: covariate balancing propensity score. J R I State Dent Soc. 2014;76:243–63.
Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesilogy. 2010;113:1338–50.
Mazo V, Sabaté S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219–31.
Dunkler D, Plischke M, Leffondré K, Heinze G. Augmented backward elimination: a pragmatic and purposeful way to develop statistical models. PLoS One. 2014;9:1–19.
Myers J, Rassen J, Gagne J, et al. Effects of adjusting for instrumental variables on bias and precision of effect estimates. Am J Epidemiol. 2011;174:1213–22.
Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. 2003;158:280–7.
Austin PC. Assessing the performance of the generalised propensity score for estimating the effect of quantitative or continuous exposures on binary outcomes. Stat Med. 2018;37:1874–94.
Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European perioperative clinical outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measur. Eur J Anaesthesiol. 2015;32:88–105.
Cinnella G, Grasso S, Spadaro S, et al. Effects of recruitment maneuver and positive end-expiratory pressure on respiratory mechanics and transpulmonary pressure during laparoscopic surgery. Anesthesiology. 2013;118:114–22.
Fahy BG, Nagle SE, Njoku MJ, Flowers JL, Barnas GM. The effects of increased abdominal pressure on Lung and Chest Wall mechanics during laparoscopic surgery. Anesth Analg. 1995;81:744–50.
Maracajá-Neto LF, Verçosa N, Roncally AC, Giannella A, Bozza FA, Lessa MA. Beneficial effects of high positive end-expiratory pressure in lung respiratory mechanics during laparoscopic surgery. Acta Anaesthesiol Scand. 2009;53:210–7.
Mutoh T, Lamm WJE, Embree LJ, Hildebrandt J, Albert RK. Abdominal distension alters regional pleural pressures and chest wall mechanics in pigs in vivo. J Appl Physiol. 1991;70:2611–8.
Brandão JC, Lessa MA, Motta-Ribeiro G, et al. Global and regional respiratory mechanics during robotic-assisted laparoscopic surgery. Anesth Analg. 2019;129:1564–73.
Pelosi P, Foti G, Cereda M, Vicardi P, Gattinoni L. Effects of carbon dioxide insufflation for laparoscopic cholecystectomy on the respiratory system. Anaesthesia. 1996;51:744–9.
Kubiak BD, Gatto LA, Jimenez EJ, et al. Plateau and Transpulmonary pressure with elevated intra-abdominal pressure or atelectasis. J Surg Res. 2010;159:e17–24.
Cortes-Puentes GA, Gard KE, Adams AB, Faltesek KA, Anderson CP, Dries DJ, et al. Value and limitations of transpulmonary pressure calculations during intra-abdominal hypertension. Crit Care Med. 2013;41:1870–7.
Loring SH, Topulos GP, Hubmayr RD. Transpulmonary pressure: the importance of precise definitions and limiting assumptions. Am J Respir Crit Care Med. 2016;194:1452–7.
Andersson LE, Bååth M, Thörne A, Aspelin P, Odeberg-Wernerman S. Effect of carbon dioxide pneumoperitoneum on development of atelectasis during anesthesia, examined by spiral computed tomography. Anesthesiology. 2005;102:293–9.
Pereira SM, Tucci MR, Morais CCA, et al. Individual positive end-expiratory pressure settings optimise intraoperative mechanical ventilation and reduce postoperative atelectasis. Anesthesiology. 2018;129:1070–81.
Mazzinari G, Diaz-Cambronero O, Alonso-Iñigo JM, et al. Intraabdominal pressure targeted positive end-expiratory pressure during laparoscopic surgery: an open-label, nonrandomized, crossover, clinical trial. Anesthesiology. 2020;132:667–77.
D’Antini D, Rauseo M, Grasso S, et al. Physiological effects of the open lung approach during laparoscopic cholecystectomy: focus on driving pressure. Minerva Anestesiol. 2018;84:159–67.
Eichler L, Truskowska K, Dupree A, Busch P, Goetz AE, Zöllner C. Intraoperative ventilation of morbidly obese patients guided by Transpulmonary pressure. Obes Surg. 2018;28:122–9.
Ferrando C, Soro M, Unzueta C, et al. Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): a randomised controlled trial. Lancet Respir Med. 2018;6:193–203.
Gainsburg DM. Anesthetic concerns for robotic-assisted laparoscopic radical prostatectomy. Minerva Anestesiol. 2012;78:596–604.
Sharma KC, Brandstetter RD, Brensilver JM, Lung LD. Cardiopulmonary physiology and pathophysiology as a consequence of laparoscopic surgery. Chest. 1996;51:810–5.
Fahy BG. Cardiopulmonary effects of laparoscopic surgery, revisited. Chest. 1997;111:1787–8.
Lane-Fall MB, Cobb BT, Cené CW, Beidas RS. Implementation science in perioperative care. Anesthesiol Clin. 2018;36:1–15.
Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510–20.
Wong A. Closing the gap: applying the theoretical domains framework to improve knowledge translation. Can J Anaesth. 2017;64:569–73.
Acknowledgments
The LAS VEGAS–investigators
AUSTRIA
LKH Graz, Graz: Wolfgang Kroell, Helfried Metzler, Gerd Struber, Thomas Wegscheider
AKH Linz, Linz: Hans Gombotz
Medical University Vienna: Michael Hiesmayr, Werner Schmid, Bernhard Urbanek
BELGIUM
UCL-Cliniques Universitaires Saint Luc Brussels: David Kahn, Mona Momeni, Audrey Pospiech, Fernande Lois, Patrice Forget, Irina Grosu
Universitary Hospital Brussels (UZ Brussel): Jan Poelaert, Veerle van Mossevelde, Marie-Claire van Malderen
Het Ziekenhuis Oost Limburg (ZOL), Genk: Dimitri Dylst, Jeroen van Melkebeek, Maud Beran
Ghent University Hospital, Gent: Stefan de Hert, Luc De Baerdemaeker, Bjorn Heyse, Jurgen Van Limmen, Piet Wyffels, Tom Jacobs, Nathalie Roels, Ann De Bruyne
Maria Middelares, Gent: Stijn van de Velde
European Society of Anaesthesiology, Brussels : Brigitte Leva, Sandrine Damster, Benoit Plichon
BOSNIA HERZEGOVINA
General Hospital ‘prim Dr Abdulah Nakas’ Sarajevo: Marina Juros-Zovko, Dejana Djonoviċ- Omanoviċ
CROATIA
General Hospital Cakovec, Cakovec: Selma Pernar
General Hospital Karlovac, Karlovac: Josip Zunic, Petar Miskovic, Antonio Zilic
University Clinical Hospital Osijek, Osijek: Slavica Kvolik, Dubravka Ivic, Darija Azenic-Venzera, Sonja Skiljic, Hrvoje Vinkovic, Ivana Oputric
University Hospital Rijeka, Rijeka: Kazimir Juricic, Vedran Frkovic
General Hospital Dr J Bencevic, Slavonski Brod: Jasminka Kopic, Ivan Mirkovic
University Hospital Center Split, Split: Nenad Karanovic, Mladen Carev, Natasa Dropulic
University Hospital Merkur, Zagreb: Jadranka Pavicic Saric, Gorjana Erceg, Matea Bogdanovic Dvorscak
University Hospital Sveti Duh, Zagreb: Branka Mazul-Sunko, Anna Marija Pavicic, Tanja Goranovic
University Hospital, Medical school, “Sestre milosrdnice” (Sister of Charity), Zagreb: Branka Maldini, Tomislav Radocaj, Zeljka Gavranovic, Inga Mladic-Batinica, Mirna Sehovic
CZECH REPUBLIC
University Hospital Brno, Brno: Petr Stourac, Hana Harazim, Olga Smekalova, Martina Kosinova, Tomas Kolacek, Kamil Hudacek, Michal Drab
University Hospital Hradec Kralove, Hradec Kralove: Jan Brujevic, Katerina Vitkova, Katerina Jirmanova
University Hospital Ostrava, Ostrava: Ivana Volfova, Paula Dzurnakova, Katarina Liskova
Nemocnice Znojmo, Znojmo: Radovan Dudas, Radek Filipsky
EGYPT
El Sahel Teaching hospital, Cairo: Samir el Kafrawy
Kasr Al-Ainy Medical School, Cairo University: Hisham Hosny Abdelwahab, Tarek Metwally, Ahmed Abdel-Razek
Beni Sueif University Hospital, Giza: Ahmed Mostafa El-Shaarawy, Wael Fathy Hasan, Ahmed Gouda Ahmed
Fayoum University Hospital, Giza: Hany Yassin, Mohamed Magdy, Mahdy Abdelhady
Suis medical Insurance Hospital, Suis: Mohamed Mahran
ESTONIA
North Estonia Medical Center, Tallinn: Eiko Herodes, Peeter Kivik, Juri Oganjan, Annika Aun
Tartu University Hospital, Tartu: Alar Sormus, Kaili Sarapuu, Merilin Mall, Juri Karjagin
FRANCE
University Hospital of Clermont-Ferrand, Clermont-Ferrand: Emmanuel Futier, Antoine Petit, Adeline Gerard
Institut Hospitalier Franco-Britannique, Levallois-Perret: Emmanuel Marret, Marc Solier
Saint Eloi University Hospital, Montpellier: Samir Jaber, Albert Prades
GERMANY
Fachkrankenhaus Coswig, Coswig: Jens Krassler, Simone Merzky
University Hospital Carl Gustav Carus, Dresden: Marcel Gama de Abreu, Christopher Uhlig, Thomas Kiss, Anette Bundy, Thomas Bluth, Andreas Gueldner, Peter Spieth, Martin Scharffenberg, Denny Tran Thiem, Thea Koch
Duesseldorf University Hospital, Heinrich-Heine University: Tanja Treschan, Maximilian Schaefer, Bea Bastin, Johann Geib, Martin Weiss, Peter Kienbaum, Benedikt Pannen
Diakoniekrankenhaus Friederikenstift, Hannover: Andre Gottschalk, Mirja Konrad, Diana Westerheide, Ben Schwerdtfeger
University of Leipzig, Leipzig: Hermann Wrigge, Philipp Simon, Andreas Reske, Christian Nestler
GREECE
‘Alexandra’ general hospital of Athens, Athens: Dimitrios Valsamidis, Konstantinos Stroumpoulis
General air force hospital, Athens: Georgios Antholopoulos, Antonis Andreou, Dimitris Karapanos
Aretaieion University Hospital, Athens: Kassiani Theodoraki, Georgios Gkiokas, Marios-Konstantinos Tasoulis
Attikon University Hospital, Athens: Tatiana Sidiropoulou, Foteini Zafeiropoulou, Panagiota Florou, Aggeliki Pandazi
Ahepa University Hospital Thessaloniki, Thessaloniki: Georgia Tsaousi, Christos Nouris, Chryssa Pourzitaki
ISRAEL
The Lady Davis Carmel Medical Center, Haifa: Dmitri Bystritski, Reuven Pizov, Arieh Eden
ITALY
Ospedale San Paolo Bari, Bari: Caterina Valeria Pesce, Annamaria Campanile, Antonella Marrella
University of Bari ‘Aldo Moro’, Bari: Salvatore Grasso, Michele De Michele
Institute for Cancer Research and treatment, Candiolo, Turin: Francesco Bona, Gianmarco Giacoletto, Elena Sardo
Azienda Ospedaliera per l’emergenza Cannizzaro, Catania: Luigi Giancarlo, Vicari Sottosanti
Ospedale Melegnano, Cernuso, Milano: Maurizio Solca
Azienda Ospedaliera – Universitaria Sant’Anna, Ferrara: Carlo Alberto Volta, Savino Spadaro, Marco Verri, Riccardo Ragazzi, Roberto Zoppellari
Ospedali Riuniti Di Foggia - University of Foggia, Foggia: Gilda Cinnella, Pasquale Raimondo, Daniela La Bella, Lucia Mirabella, Davide D’antini
IRCCS AOU San Martino IST Hospital, University of Genoa, Genoa: Paolo Pelosi, Alexandre Molin, Iole Brunetti, Angelo Gratarola, Giulia Pellerano, Rosanna Sileo, Stefano Pezzatto, Luca Montagnani
IRCCS San Raffaele Scientific Institute, Milano: Laura Pasin, Giovanni Landoni, Alberto Zangrillo, Luigi Beretta, Ambra Licia Di Parma, Valentina Tarzia, Roberto Dossi, Marta Eugenia Sassone
Istituto europeo di oncologia – ieo, Milano: Daniele Sances, Stefano Tredici, Gianluca Spano, Gianluca Castellani, Luigi Delunas, Sopio Peradze, Marco Venturino
Ospedale Niguarda Ca'Granda Milano, Milano: Ines Arpino, Sara Sher
Ospedale San Paolo - University of Milano, Milano: Concezione Tommasino, Francesca Rapido, Paola Morelli
University of Naples “Federico II” Naples: Maria Vargas, Giuseppe Servillo
Policlinico ‘P. Giaccone’, Palermo: Andrea Cortegiani, Santi Maurizio Raineri, Francesca Montalto, Vincenzo Russotto, Antonino Giarratano
Azienda Ospedaliero-Universitaria, Parma: Marco Baciarello, Michela Generali, Giorgia Cerati
Santa Maria degli Angeli, Pordenone: Yigal Leykin
Ospedale Misericordia e Dolce - Usl4 Prato, Prato: Filippo Bressan, Vittoria Bartolini, Lucia Zamidei
University hospital of Sassari, Sassari: Luca Brazzi, Corrado Liperi, Gabriele Sales, Laura Pistidda
Insubria University, Varese: Paolo Severgnini, Elisa Brugnoni, Giuseppe Musella, Alessandro Bacuzzi
REPUBLIC OF KOSOVO
Distric hospital Gjakova, Gjakove: Dalip Muhardri
University Clinical Center of Kosova, Prishtina: Agreta Gecaj-Gashi, Fatos Sada
Regional Hospital ‘Prim.Dr. Daut Mustafa’, Prizren: Adem Bytyqi
LITHUANIA
Medical University Hospital, Hospital of Lithuanian University of Health Sciences, Kaunas: Aurika Karbonskiene, Ruta Aukstakalniene, Zivile Teberaite, Erika Salciute
Vilnius University Hospital - Institute of Oncology, Vilnius: Renatas Tikuisis, Povilas Miliauskas
Vilnius University Hospital - Santariskiu Clinics, Vilnius: Sipylaite Jurate, Egle Kontrimaviciute, Gabija Tomkute
MALTA
Mater Dei Hospital, Msida: John Xuereb, Maureen Bezzina, Francis Joseph Borg
THE NETHERLANDS
Academic Medical Centre, University of Amsterdam: Sabrine Hemmes, Marcus Schultz, Markus Hollmann, Irene Wiersma, Jan Binnekade, Lieuwe Bos
VU University Medical Center, Amsterdam: Christa Boer, Anne Duvekot
MC Haaglanden, Den Haag: Bas in ‘t Veld, Alice Werger, Paul Dennesen, Charlotte Severijns
Westfriesgasthuis, Hoorn: Jasper De Jong, Jens Hering, Rienk van Beek
NORWAY
Haukeland University Hospital, Bergen: Stefan Ivars, Ib Jammer
Førde Central Hospital /Førde Sentral Sykehus, Førde: Alena Breidablik
Martina Hansens Hospital, Gjettum: Katharina Skirstad Hodt, Frode Fjellanger, Manuel Vico Avalos
Bærum Hospital, Vestre Viken, Rud: Jannicke Mellin-Olsen, Elisabeth Andersson
Stavanger University Hospital, Stavanger: Amir Shafi-Kabiri
PANAMA
Hospital Santo Tomás, Panama: Ruby Molina, Stanley Wutai, Erick Morais
PORTUGAL
Hospital do Espírito Santo - Évora, E.P.E, Évora: Glória Tareco, Daniel Ferreira, Joana Amaral
Centro Hospitalar de Lisboa Central, E.P.E, Lisboa: Maria de Lurdes Goncalves Castro, Susana Cadilha, Sofia Appleton
Centro Hospitalar de Lisboa Ocidental, E.P.E. Hospital de S. Francisco Xavier, Lisboa: Suzana Parente, Mariana Correia, Diogo Martins
Santarem Hospital, Santarem: Angela Monteirosa, Ana Ricardo, Sara Rodrigues
ROMANIA
Spital Orasenesc, Bolintin Vale: Lucian Horhota
Clinical Emergency Hospital of Bucharest, Bucharest: Ioana Marina Grintescu, Liliana Mirea, Ioana Cristina Grintescu
Elias University Emergency Hospital, Bucharest: Dan Corneci, Silvius Negoita, Madalina Dutu, Ioana Popescu Garotescu
Emergency Institute of Cardiovascular Diseases Inst. ''Prof. C. C. Iliescu'', Bucharest: Daniela Filipescu, Alexandru Bogdan Prodan
Fundeni Clinical institute - Anaesthesia and Intensive Care, Bucharest: Gabriela Droc, Ruxandra Fota, Mihai Popescu
Fundeni Clinical institute - Intensive Care Unit, Bucharest: Dana Tomescu, Ana Maria Petcu, Marian Irinel Tudoroiu
Hospital Profesor D Gerota, Bucharest: Alida Moise, Catalin-Traian Guran
Constanta County Emergency Hospital, Constanta: Iorel Gherghina, Dan Costea, Iulia Cindea
University Emergency County Hospital Targu Mures, Targu Mures: Sanda-Maria Copotoiu, Ruxandra Copotoiu, Victoria Barsan, Zsolt Tolcser, Magda Riciu, Septimiu Gheorghe Moldovan, Mihaly Veres
RUSSIA
Krasnoyarsk State Medical University, Krasnoyarsk: Alexey Gritsan, Tatyana Kapkan, Galina Gritsan, Oleg Korolkov
Burdenko Neurosurgery Institute, Moscow: Alexander Kulikov, Andrey Lubnin
Moscow Regional Research Clinical Institute, Moscow: Alexey Ovezov, Pavel Prokoshev, Alexander Lugovoy, Natalia Anipchenko
Municipal Clinical Hospital 7, Moscow: Andrey Babayants, Irina Komissarova, Karginova Zalina
Reanimatology Research Institute n.a. Negovskij RAMS, Moscow: Valery Likhvantsev, Sergei Fedorov
SERBIA
Clinical Center of Vojvodina, Emergency Center, Novisad: Aleksandra Lazukic, Jasmina Pejakovic, Dunja Mihajlovic
SLOVAKIA
National Cancer Institute, Bratislava: Zuzana Kusnierikova, Maria Zelinkova
F.D. Roosevelt teaching Hospital, Banská Bystrica: Katarina Bruncakova, Lenka Polakovicova
Faculty Hospital Nové Zámky, Nové Zámky: Villiam Sobona
SLOVENIA
Institute of Oncology Ljubljana, Ljubljana: Barbka Novak-Supe, Ana Pekle-Golez, Miroljub Jovanov, Branka Strazisar
University Medical Centre Ljubljana, Ljubljana: Jasmina Markovic-Bozic, Vesna Novak-Jankovic, Minca Voje, Andriy Grynyuk, Ivan Kostadinov, Alenka Spindler-Vesel
SPAIN
Hospital Sant Pau, Barcelona: Victoria Moral, Mari Carmen Unzueta, Carlos Puigbo, Josep Fava
Hospital Universitari Germans Trias I Pujol, Barcelona: Jaume Canet, Enrique Moret, Mónica Rodriguez Nunez, Mar Sendra, Andrea Brunelli, Frederic Rodenas
University of Navarra, Pamplona: Pablo Monedero, Francisco Hidalgo Martinez, Maria Jose Yepes Temino, Antonio Martínez Simon, Ana de Abajo Larriba
Corporacion Sanitaria Parc Tauli, Sabadell: Alberto Lisi, Gisela Perez, Raquel Martinez
Consorcio Hospital General Universitario de Valencia, Valencia: Manuel Granell, Jose Tatay Vivo, Cristina Saiz Ruiz, Jose Antonio de Andrés Ibañez
Hospital Clinico Valencia, Valencia: Ernesto Pastor, Marina Soro, Carlos Ferrando, Mario Defez
Hospital Universitario Rio Hortega, Valladolid: Cesar Aldecoa Alvares-Santullano, Rocio Perez, Jesus Rico
SWEDEN
Central Hospital in Kristianstad: Monir Jawad, Yousif Saeed, Lars Gillberg
TURKEY
Ufuk University Hospital Ankara, Ankara: Zuleyha Kazak Bengisun, Baturay Kansu Kazbek
Akdeniz University Hospital, Antalya: Nesil Coskunfirat, Neval Boztug, Suat Sanli, Murat Yilmaz, Necmiye Hadimioglu
Istanbul University, Istanbul medical faculty, Istanbul: Nuzhet Mert Senturk, Emre Camci, Semra Kucukgoncu, Zerrin Sungur, Nukhet Sivrikoz
Acibadem University, Istanbul: Serpil Ustalar Ozgen, Fevzi Toraman
Maltepe University, Istanbul: Onur Selvi, Ozgur Senturk, Mine Yildiz
Dokuz Eylül Universitesi Tip Fakültesi, Izmir: Bahar Kuvaki, Ferim Gunenc, Semih Kucukguclu, Şule Ozbilgin
Şifa University Hospital, İzmir: Jale Maral, Seyda Canli
Selcuk University faculty of medicine, Konya: Oguzhan Arun, Ali Saltali, Eyup Aydogan
Fatih Sultan Mehmet Eğitim Ve Araştirma Hastanesi, Istanbul: Fatma Nur Akgun, Ceren Sanlikarip, Fatma Mine Karaman
UKRAINE
Institute Of Surgery And Transplantology, Kiev: Andriy Mazur
Zaporizhzhia State Medical University, Zaporizhzhia: Sergiy Vorotyntsev
UNITED KINGDOM
SWARM Research Collaborative: for full list of SWARM contributors please see www.ukswarm.com
Northern Devon Healthcare NHS Trust, Barnstaple: Guy Rousseau, Colin Barrett, Lucia Stancombe
Golden Jubilee National Hospital, Clydebank, Scotland: Ben Shelley, Helen Scholes
Darlington Memorial Hospital, County Durham and Darlington Foundation NHS Trust, Darlington: James Limb, Amir Rafi, Lisa Wayman, Jill Deane
Royal Derby Hospital, Derby: David Rogerson, John Williams, Susan Yates, Elaine Rogers
Dorset County Hospital, Dorchester: Mark Pulletz, Sarah Moreton, Stephanie Jones
The Princess Alexandra NHS Hospital Trust, Essex: Suresh Venkatesh, Maudrian Burton, Lucy Brown, Cait Goodall
Royal Devon and Exeter NHS Foundation Trust, Exeter: Matthew Rucklidge, Debbie Fuller, Maria Nadolski, Sandeep Kusre
Hospital James Paget University Hospital NHS Foundation Trust, Great Yarmouth: Michael Lundberg, Lynn Everett, Helen Nutt
Royal Surrey County Hospital NHS Foundation Trust, Guildford: Maka Zuleika, Peter Carvalho, Deborah Clements, Ben Creagh-Brown
Kettering General Hospital NHS Foundation Trust, Kettering: Philip Watt, Parizade Raymode
Barts Health NHS Trust, Royal London Hospital, London: Rupert Pearse, Otto Mohr, Ashok Raj, Thais Creary
Newcastle Upon Tyne Hospitals NHS Trust The Freeman Hospital High Heaton, Newcastle upon Tyne: Ahmed Chishti, Andrea Bell, Charley Higham, Alistair Cain, Sarah Gibb, Stephen Mowat
Derriford Hospital Plymouth Hospitals NHS Trust, Plymouth: Danielle Franklin, Claire West, Gary Minto, Nicholas Boyd
Royal Hallamshire Hospital, Sheffield: Gary Mills, Emily Calton, Rachel Walker, Felicity Mackenzie, Branwen Ellison, Helen Roberts
Mid Staffordshire NHS, Stafford: Moses Chikungwa, Clare Jackson
Musgrove Park Hospital, Taunton: Andrew Donovan, Jayne Foot, Elizabeth Homan
South Devon Healthcare NHS Foundation Trust /Torbay Hospital, Torquay, Torbay: Jane Montgomery, David Portch, Pauline Mercer, Janet Palmer
Royal Cornwall Hospital, Truro: Jonathan Paddle, Anna Fouracres, Amanda Datson, Alyson Andrew, Leanne Welch
Mid Yorkshire Hospitals NHS Trust; Pinderfields Hospital, Wakefield: Alastair Rose, Sandeep Varma, Karen Simeson
Sandwell and West Birmingham NHS Trust, West Bromich: Mrutyunjaya Rambhatla, Jaysimha Susarla, Sudhakar Marri, Krishnan Kodaganallur, Ashok Das, Shivarajan Algarsamy, Julie Colley
York Teaching Hospitals NHS Foundation Trust, York: Simon Davies, Margaret Szewczyk, Thomas Smith
UNITED STATES
University of Colorado School of Medicine/University of Colorado Hospital, Aurora: Ana Fernandez- Bustamante, Elizabeth Luzier, Angela Almagro
Massachusetts General Hospital, Boston: Marcos Vidal Melo, Luiz Fernando, Demet Sulemanji
Mayo Clinic, Rochester: Juraj Sprung, Toby Weingarten, Daryl Kor, Federica Scavonetto, Yeo Tze
Funding
The LAS VEGAS study was endorsed and partly funded by a restricted research grant from the European Society of Anesthesiology through their Clinical Trial Network.
Author information
Authors and Affiliations
Consortia
Contributions
GM, ASN and MJS: Designed the study; GM, ASN, SNTH: Wrote the protocol; GM, ASN, LB, MJS: Collected the data from the original database; GM, ASN, ODC: Analyzed the data; GH, SJ, MH, GHM, MFVM, RMP, CP, WS, PS, HW, MWH, PP, MGdA, MJS: made substantial contribution to data interpretation; GM, wrote the manuscript under the supervision of PP and MJS. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The original study protocol was approved by the ethical committee of the Academic Medical Center, Amsterdam, the Netherlands (W12_190#12.17.0227). Each participating centre obtained approval from their institutional review board if needed, and patients were included after obtaining written informed consent when dictated by national or regional legislation.
Consent for publication
Not applicable.
Competing interests
G. Mazzinari: No interest declared; A. Serpa Neto: No interest declared; S.N.T. Hemmes: No interest declared; G. Hedenstierna: No interest declared; S. Jaber: No interest declared; M. Hiesmayr: No interest declared; M.W. Hollmann: Executive Section Editor Pharmacology with Anesthesia & Analgesia, Section Editor Anesthesiology with Journal of Clinical Medicine, and CSL Behring, no conflict of interest with the current work; G.H. Mills: No interest declared; M.F. Vidal Melo: is funded by NIH/NHLBI grant UH3-HL140177; R.M. Pearse: No interest declared; C. Putensen: No interest declared; W. Schmid: No interest declared; P. Severgnini: No interest declared; H.Wrigge: No interest declared; O. Diaz–Cambronero: had received a Merck Sharp & Dohme investigator–initiated grant (protocol code #53607). Sponsors and funders have no roles in study design, analysis of data or reporting. Also received speakers fees for lecture and medical advice from Merck Sharp & Dohme, no conflict of interest with the current work; L.Ball: No interest declared; M. Gama de Abreu: Ambu, GE Healthcare, ZOLL consulting fees, no conflict of interest with the current work; P.Pelosi: No interest declared; M.J.Schultz: No interest declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table 1.
Patient and surgery related characteristics. Table 2. Intraoperative venitlatory setting by group.
Additional file 2: Table S1.
Definition of postoperative pulmonary complications. Table S2. Definition of intraoperative complications. Table S3. Number of data available at each time point. Table S4. Patients demographics and surgery–related characteristics in the matched cohort for type of surgery. Table S5. Intraoperative and postoperative outcomes in matched cohort for type of surgery. Table S6. Mixed multivariable logistic regression in matched cohort for postoperative pulmonary complications. Figure S1. Time weighted average and coefficient of variation calculation. Figure S2. Summary plot of covariate balance for time-weighted ΔP before (red line) and after (blue line) conditioning for open surgery. Figure S3. Summary plot of covariate balance before (red line) and after (blue line) conditioning for closed surgery. A: time–weighted; B: Highest value; C: Lowest Value; D: Coefficient of variation. Figure S4. Residuals plot for postoperative pulmonary complications (PPCs) and intraoperative adverse events (AEs). A: PPCs in Open surgery; B: PPCs in closed surgery; C; AEs in open surgery; D: AEs in closed surgery.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mazzinari, G., Serpa Neto, A., Hemmes, S.N.T. et al. The Association of Intraoperative driving pressure with postoperative pulmonary complications in open versus closed abdominal surgery patients – a posthoc propensity score–weighted cohort analysis of the LAS VEGAS study. BMC Anesthesiol 21, 84 (2021). https://doi.org/10.1186/s12871-021-01268-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-021-01268-y