Abstract
Background
Positive end-expiratory pressure (PEEP) has been demonstrated to decrease ventilator-induced lung injury in patients under mechanical ventilation (MV) for acute respiratory failure. Recently, some studies have proposed some beneficial effects of PEEP in ventilated patients without lung injury. The influence of PEEP on respiratory mechanics in children is not well known. Our aim was to determine the effects on respiratory mechanics of setting PEEP at 5 cmH2O in anesthetized healthy children.
Methods
Patients younger than 15 years old without history of lung injury scheduled for elective surgery gave informed consent and were enrolled in the study. After usual care for general anesthesia, patients were placed on volume controlled MV. Two sets of respiratory mechanics studies were performed using inspiratory and expiratory breath hold, with PEEP 0 and 5 cmH2O. The maximum inspiratory and expiratory flow (QI and QE) as well as peak inspiratory pressure (PIP), plateau pressure (PPL) and total PEEP (tPEEP) were measured. Respiratory system compliance (CRS), inspiratory and expiratory resistances (RawI and RawE) and time constants (KTI and KTE) were calculated. Data were expressed as median and interquartile range (IQR). Wilcoxon sign test and Spearman’s analysis were used. Significance was set at P < 0.05.
Results
We included 30 patients, median age 39 (15–61.3) months old, 60% male. When PEEP increased, PIP increased from 12 (11,14) to 15.5 (14,18), and CRS increased from 0.9 (0.9,1.2) to 1.2 (0.9,1.4) mL·kg− 1·cmH2O− 1; additionally, when PEEP increased, driving pressure decreased from 6.8 (5.9,8.1) to 5.8 (4.7,7.1) cmH2O, and QE decreased from 13.8 (11.8,18.7) to 11.7 (9.1,13.5) L·min− 1 (all P < 0.01). There were no significant changes in resistance and QI.
Conclusions
Analysis of respiratory mechanics in anesthetized healthy children shows that PEEP at 5 cmH2O places the respiratory system in a better position in the P/V curve. A better understanding of lung mechanics may lead to changes in the traditional ventilatory approach, limiting injury associated with MV.
Similar content being viewed by others
Background
There are many detrimental effects of positive pressure mechanical ventilation (MV) to the lung parenchyma, giving shape to the entity we know as ventilator induced lung injury (VILI) [1, 2]. Despite that it was initially described for injured lungs [3], VILI has been recognized to affect patients with uninjured lungs, triggering many pathways of local and systemic inflammation [1,2,3,4]. Positive pressure MV can cause VILI even when applied for short periods of time, and the role of protective MV during anesthesia has become important for preventing postoperative complications [5,6,7,8,9,10]. Although the exact incidence of VILI during general anesthesia is unknown, the lungs of patients under general anesthesia are especially vulnerable to VILI, since anesthetic induction reduces the end expiratory lung volume (EELV) by 9–25% in adults and up to 46% in children [11,12,13,14,15]. Cyclic opening and collapsing of alveoli have been indicated as one of the primary mechanisms of VILI during anesthesia. Imaging studies have shown that general anesthesia induces atelectasis of dependent regions and that the use of PEEP prevents its formation [3, 5, 9, 10, 14]. Protective ventilation during general anesthesia [16, 17] to limit tidal volume (6–8 ml/kg) has been widely accepted and incorporated into the operating room (OR), but PEEP use is still not a common practice for patients undergoing general anesthesia [6,7,8,9,10, 17, 18].
In the absence of respiratory muscle activity, working pressure of the respiratory system is the pressure needed to overcome frictional forces, elastic forces and impedance. In this way, an improvement in CRS reflects lower elastic work pressures and, therefore, a pending situation of its more favorable dynamic pressure/volume curve (P/V curve) [19]. These observations also have been described in pediatric animal models [20, 21].
There is little evidence to guide MV during general anesthesia in children [18, 22, 23]. MV parameters are primarily dependent on anesthesiologist preferences and on the characteristics of anesthesia machines [24]. Facing this scenario, many scientific societies have pointed as priority areas of research to the pathophysiology, respiratory mechanics and practice of MV in children for improving outcomes and reducing secondary long-term lung injury [23].
With these facts in mind, we sought to compare respiratory mechanics of setting PEEP at 5 cmH2O compared to ZEEP (PEEP set at 0 cmH2O) in anesthetized children without acute pulmonary pathology undergoing elective surgery. We hypothesized that usual respiratory mechanics parameters, measured with a commercial mechanical ventilator, would identify a more protective ventilation with addition of PEEP 5 cmH2O. Special emphasis was placed on evaluation on respiratory work pressure, flow patterns, resistance and inspiratory and expiratory time constants.
Methods
Study design and setting
This prospective study was conducted at the Surgical Block of Centro Hospitalario Pereira Rossell located in Montevideo, Uruguay. The local ethic committee approved the study and informed consent was obtained for each patient before entering the OR.
Study population
Between February 1, 2015 and April 30, 2016, children younger than 15 years old without preexisting lung injury scheduled for elective surgery were screened for the study. For definitive selection, patients categorized as ASA I or II, requiring orotracheal intubation for the surgery according to the anesthesiologist’s criteria, were considered. Patients were excluded if they had any acute condition before or during anesthesia (e.g., laryngospasm, bronchoconstriction, pneumothorax), thoracic surgery, thoracic or airway malformation and chronic lung disease with oxygen dependence. Additionally, patients with endotracheal tube air leak > 20% of tidal volume (VT) were excluded due to possible interference with data acquisition.
Data collection
We registered demographics and clinical information at admission. All procedures before respiratory system mechanics measurements were done according to institutional protocol and anesthesiologist preferences, including anesthesia induction, orotracheal intubation and initial ventilatory settings on the anesthesia machine.
Study protocol
Patients were ventilated on a Galileo Gold® ventilator (Hamilton, Bonaduz, Switzerland) on volume control mode after verification of correct positioning of the endotracheal tube. Baseline settings were as follows: VT = 6–8 mL·kg− 1, PEEP = 5 cmH2O, fraction of inspired oxygen (FiO2) was adjusted to a target pulse oximetry greater than 95%, inspiratory: expiratory ratio = 1:2, and respiratory rate (RR) was adjusted to achieve an end-tidal carbon dioxide (ETCO2) 40 ± 5 mmHg. Tracheal tube leak compensation was deactivated through the measurements.
Respiratory mechanics measurements
Two sets of measurements were performed, at ZEEP and PEEP 5 cmH2O, separated by 5 min of stability, following local protocol for respiratory mechanics measurements. This protocol is summarized in Additional file 1 [19]. All measurements were made in pre-incision surgical time.
Ventilator parameters [peak inspiratory pressure (PIP), plateau pressure (PPL), extrinsic (set) PEEP (PEEP), total PEEP (tPEEP), intrinsic PEEP (iPEEP = tPEEP - PEEP), driving pressure (∆P = PPL–tPEEP), mean airway pressure expiratory (Paw), VT, inspiratory time (IT), respiratory rate (RR), maximum inspiratory and expiratory flow (QI and QE, L·min− 1)] were assessed. An inspiratory hold followed by an expiratory hold was performed following the protocol described in Additional file 1. Flow and pressure parameters in these quasi-static conditions at the Y piece (proximal flow sensor) were recorded in an ad hoc Microsoft Excel 2010 (Microsoft®, NY, USA) database to calculate respiratory system compliance (CRS, mL·cmH2O− 1·kg− 1), inspiratory and expiratory airway resistance (RawI and RawE, cmH2O·L− 1·s− 1), and inspiratory and expiratory time constants (KTI and KTE, s) according to formulas described in Additional file 2.
Data analysis
Data are expressed as median and interquartile range (IQR). A previous study found that changing PEEP from 0 to 12 cmH20 resulted in a decrease in dynamic compliance (Cdyn) in 9.4 ml/cmH2O with SD 6.8 [25]. Given the fact that our protocol included a moderate modification of PEEP, from 0 to 5 ml/cmH2O, we expected a smaller change of Cdyn, 2/3 of previously described [25]. A sample size of 30 patients is needed to determine a variation of Cdyn by 6 ml/cmH2O, using chi-square test and assuming an α of 0.05 and a power of 90%. Normality was assessed with the Anderson–Darling test. Wilcoxon sign test was performed for comparisons between respiratory mechanics assessments. Spearman’s analysis was used to examine correlations between changes in respiratory mechanics and age and ideal body weight. Differences were considered significant if p < 0.05. All statistical analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Figures were plotted with GraphPad Prism version 5.0c for Mac (GraphPad Software, La Jolla, CA, USA).
Results
Thirty patients were included in the study. Sixty percent were male, the median age was 39 months (15–61.3), and weight was 15 kg (10.6–21).
Sixty percent of patients received inhaled anesthesia and 40% mixed inhalation and intravenous anesthesia. Abdominal surgery was the most frequent (20 cases), mostly hernioplasty. Malformation of the digestive tract was the most frequent comorbidity, present in 7 patients. Table 1 shows clinical characteristics of included patients.
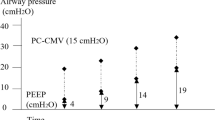
There were no complications related to the protocol. Table 2 shows ventilatory parameters and respiratory system mechanics for the study population with ZEEP and PEEP of 5 cmH2O. After setting PEEP at 5 cmH2O we observed an increase in PIP, PPL and Paw, with a concomitant decrease in iPEEP and ∆P. Figure 1 shows individual changes on ∆P throughout the study. No modifications were done on VT, and thus, changes on ∆P were coupled to variation on CRS.
There was a moderate correlation between age and ideal body weight and changes in QE and changes in KTE. Additional file 3 shows the correlation between changes in respiratory parameters and age and ideal body weight. No other correlations with age and weight were found.
Discussion
In this study, we measured pulmonary mechanics in anesthetized healthy children on MV for elective surgery at ZEEP and PEEP 5 cmH2O using a commercially available mechanical ventilator. Setting PEEP at 5 cmH2O resulted in a significant decrease in ∆P and iPEEP, thereby improving CRS. Changes observed in respiratory mechanics after setting PEEP at 5 cmH2O are summarized in Fig. 2.
Pressure – Volume loop (P/V curve) sketch summarizing findings of the study: Anesthetized children with ZEEP (panel a) and after setting PEEP at 5 cmH2O (panel b). PIP, PPL and Paw increased after addition of PEEP of 5 cmH2O (PIP’, PPL’,Paw’), but dP decreased (dP'). Given the same VT, CRS, represented as the angle of the diagonal line between PEEP and PPL, increased (CRS’). All of them are sign of a more efficient working pressures of the respiratory system better position in P/V curve. On (panel b) respiratory mechanics with ZEEP was added in gray tone as reference
We also found a decrease in QE when PEEP was applied. All these findings are indirect signs of lungs in a better position in dynamic P/V curve, suggesting that the addition of a low level of PEEP improves respiratory mechanics.
The effect of PEEP on patients with ARDS was described more than a decade ago, promoting recruitment of non-aerated lung volume and increasing EELV. More recently, many investigators have shown that PEEP should be incorporated to lung protective strategy during the perioperative period in patients at risk for ARDS as well as previously healthy patients [6, 8,9,10]. In this setting, hypoxemia was not associated with the reduction in EELV, and thus, hypoxemia is thought to be a poor predictor of potential injurious MV. Similar findings have been described in children. Serafini et al. described densities in dependent regions of both lungs on CT scan of 10 infants after induction of anesthesia. They observed reopening of the collapsed lung with addition of 5 cmH2O of PEEP for 5 min [14]. Kadini et al., in a small study of 8 anesthetized children (range between 2.5 and 6.5 years old), described that 5 cmH2O resulted in a significant increase of VT; thus, CRS improved [24].
Our results show that the addition of PEEP sets the respiratory system in a better position of the P/V curve, probably related to the reduction of the atelectasis in lung dependent zones after induction of anesthesia and MV with ZEEP. An increase in EELV, maintaining the same VT, denotes a reduction in global strain and, potentially, VILI [26].
∆P has taken high priority after the recent pooled data analysis of adult patients with ARDS that showed a significant relationship with mortality [27]. ∆P represents the pressure required for the movement of inspiratory flow and depends on the lung and chest wall viscoelastic resistance [28]. Recently, Neto et al. in a meta-analysis of 2250 patients under general anesthesia found that high ∆P was the only MV parameter associated with postoperative complications [29]. Even more, they found that changes in PEEP that resulted in an increase in ∆P were associated with more postoperative pulmonary complications. It is not surprising that in these studies, ∆P was the best predictor of unfavorable outcomes. We believe that the best performance of this parameter is because it integrates the set tidal volume and the patient’s compliance, thus giving a more precise idea of the individual conditions of each patient.
In our patients, we observed a 14.8% (CI95% 9.3,20.3%) reduction of ∆P when PEEP of 5 cmH2O was applied. The range of improvement was very wide and only one patient had a significant increase in ∆P (greater than 10%). These results are in accordance with a study by Wirth et al. They elegantly showed, with electric impedance tomography in 30 anesthetized children, that changing PEEP from 2 cmH2O to 5 cmH2O homogenizes regional lung ventilation [30]. One of our patients had a significant increase of ∆P. This a very good example that ventilatory settings need to be tailored individually, because adding 5 cmH20 of PEEP probably led to overdistension in this patient. We maintained the set VT after applying PEEP of 5 cmH2O; thus, the reduction of ∆P was dependent on the improvement in CRS. The improvement in CRS and EELV may be seen as minor, but we believe that even these small changes may have significant consequences during and after surgery, reducing lung inflammation, alteration of gas exchange, among others. Experimental and clinical data have shown that injurious ventilatory parameters can generate detrimental effects, even when applied for short periods of time [1, 2, 31, 32].
The prolongation of KTI and KTE with PEEP of 5 cmH2O probably reflects mathematical coupling of changes on CRS without modifications on airway resistance. In the same way, the observed lower expiratory flow is related to the lower ∆P. These parameters are indirect markers of improvement on respiratory system mechanics and more protective ventilation.
Our study has some limitations. Respiratory mechanics measurements were done before the surgery, after a short period of applied PEEP; thus, we cannot extrapolate these findings to other surgical timings, e.g., during pneumoperitoneum, when the effect of PEEP preventing lung collapse could be even higher. Due to small size of patients, we did not measure pleural pressure, so we could not determine the contribution of the chest wall to CRS. Included patients were heterogeneous and age range is wide, so in the absence of normal reference data, we cannot generalize these results to all pediatric patients (i.e., younger patients have higher chest compliance, being at higher risk for derecruitment of the lung on ZEEP). Finally, we have to acknowledge that setting of MV parameters can directly modify the component of the equation of motion (i.e., QI, RR, I:E ratio), but we tried to standardize the ventilatory setting during measurements. Despite these limitations, we consider our observation of the effect of PEEP in anesthetized children under mechanical ventilation important in terms of a pathophysiological approach to reduce VILI.
Conclusion
Setting PEEP at 5 cmH2O in children during general anesthesia improved elastic working pressure of the respiratory system, decreasing driving pressure and intrinsic PEEP. These findings are indirect signs of lungs in a better position in pressure/volume curve, suggesting that the addition of a low level of PEEP improves respiratory mechanics. These findings may be measured with usual mechanical ventilators and anesthesia machines, analyzing respiratory system mechanics after an inspiratory and expiratory hold. Future studies in infants are needed to address respiratory mechanics during anesthesia, ideally in a specific age group and pathologies with high-risk postoperative complications. A better understanding of respiratory system mechanics in children during general anesthesia may lead to a better titration of mechanical ventilation, preventing VILI.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- CRS :
-
Compliance respiratory system
- CT:
-
Computed tomography
- EELV:
-
End expiratory lung volume
- iPEEP:
-
Intrinsic positive pressure at the end of exhalation
- KTE :
-
Expiratory time constant
- KTI :
-
Inspiratory time constant
- MV:
-
Mechanical ventilation
- Paw:
-
Airway pressure
- PEEP:
-
Positive pressure at the end of exhalation
- PIP:
-
Peak inspiratory pressure
- PPL :
-
Plateau pressure
- QE :
-
Maximum expiratory flow
- QI :
-
Maximum inspiratory flow
- RawE:
-
Resistance expiratory airway
- RawI:
-
Resistance inspiratory airway
- RR:
-
Respiratory rate
- tPEEP:
-
Total positive pressure at the end of exhalation
- VILI:
-
Ventilator induced lung injury
- VT :
-
Tidal volume
- ZEEP:
-
Positive pressure at the end of exhalation set at 0 cmH2O
- ΔP:
-
Driving pressure
References
Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–36.
Donoso A, Cruces P. Daño pulmonar inducido por ventilación mecánica y estrategia ventilatoria convencional protectora. Rev Chil Pediatr. 2007;78:241–52.
Bein T, Grasso S, Moerer O, Quintel M, Guerin C, Deja M, et al. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med. 2016;42:699–711.
Imai Y, Kawano T, Miyasaka K, Takata M, Imai T, Okuyama K. Inflammatory chemical mediators during conventional ventilation and during high frequency oscillatory ventilation. Am J Respir Crit Care Med. 1994;150:1550–4.
Tusman G, Böhm SH, Warner DO, Sprung J. Atelectasis and perioperative pulmonary complications in high-risk patients. Curr Opin Anaesthesiol. 2012;25:1–10.
Vargas M, Sutherasan Y, Gregoretti C, Pelosi P. PEEP role in ICU and operating room: from pathophysiology to clinical practice. ScientificWorldJournal. 2014;2014:852356.
Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, et al. IMPROVE study group. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–37.
Wolthuis EK, Choi G, Dessing MC, Bresser P, Lutter R, Dzoljic M, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology. 2008;108:46–54.
Barbosa FT, Castro AA. de Sousa-Rodrigues CF. positive end-expiratory pressure (PEEP) during anaesthesia for prevention of mortality and postoperative pulmonary complications. Cochrane Database Syst Rev. 2014;6:CD007922.
Severgnini P, Selmo G, Lanza C, Chiesa A, Frigerio A, Bacuzzi A, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology. 2013;118:1307–21.
Wahba RW. Perioperative functional residual capacity. Can J Anaesth. 1991;38:384–400.
Fletcher ME, Stack C, Ewart M, Davies CJ, Ridley S, Hatch DJ, et al. Respiratory compliance during sedation, anesthesia, and paralysis in infants and young children. J Appl Physiol. 1985;70:1977–82.
Dobbinson TL, Nisbet HI, Pelton DA, Levison H. Functional residual capacity (FRC) and compliance in anaesthetized paralysed children. II. Clinical results. Can Anaesth Soc J. 1973;20:322–33.
Serafini G, Cornara G, Cavalloro F, Mori A, Dore R, Marraro G, et al. Pulmonary atelectasis during paediatric anaesthesia: CT scan evaluation and effect of positive end-expiratory pressure (PEEP). Paediatr Anaesth. 1999;9:225–8.
Trachsel D, Svendsen J, Erb TO. von Ungern-Sternberg BS. Effects of anaesthesia on paediatric lung function. Br J Anaesth. 2016;117:151–63.
Feldman JM. Optimal ventilation of the anesthetized pediatric patient. Anesth Analg. 2015;120:165–75.
Wanderer JP, Ehrenfeld JM, Epstein RH, Kor DJ, Bartz RR, Fernandez-Bustamante A, et al. Temporal trends and current practice patterns for intraoperative ventilation at U.S. academic medical centers: a retrospective study. BMC Anesthesiol. 2015;15:40.
Kneyber MC. Intraoperative mechanical ventilation for the pediatric patient. Best Pract Res Clin Anaesthesiol. 2015;29:371–9.
Erranz B, Díaz F, Donoso A, Salomón T, Carvajal C, Torres MF, et al. Decreased lung compliance increases preload dynamic tests in a pediatric acute lung injury model. Rev Chil Pediatr. 2015;86:404–9.
Díaz F, Erranz B, Donoso A, Carvajal C, Salomón T, Torres M, Cruces P. Surfactant deactivation in a pediatric model induces hypovolemia and fluid shift to the extravascular lung compartment. Paediatr Anaesth. 2013;23:250–7.
Feldman JM. Optimal ventilation of the anesthetized pediatric patient. Anesth Analg. 2015;120:165–75.
Peterson-Carmichael S, Seddon PC, Cheifetz IM, Frerichs I, Hall GL, Hammer J, et al. ATS/ERS working group on infant and young ChildrenPulmonary function testing. An official American Thoracic Society/EuropeanRespiratory society workshop report: evaluation of respiratory mechanics andFunction in the pediatric and neonatal intensive care units. Ann Am Thorac Soc. 2016;13:S1–11.
Kaditis AG, Motoyama EK, Zin W, Maekawa N, Nishio I, Imai T, et al. The effect of lung expansion and positive end-expiratory pressure on respiratory mechanics in anesthetized children. Anesth Analg. 2008;106:775–85.
Cruces P, González-Dambrauskas S, Quilodrán J, Valenzuela J, Martínez J, Rivero N, et al. Respiratory mechanics in infants with severe bronchiolitis on controlled mechanical ventilation. BMC Pulm Med. 2017;17:129.
Jonson B, Richard JC, Straus C, Mancebo J, Lemaire F, Brochard L. Pressure-volume curves and compliance in acute lung injury: evidence of recruitment above the lower inflection point. Am J Respir Crit Care Med. 1999;159:1172–8.
Protti A, Maraffi T, Milesi M, Votta E, Santini A, Pugni P, et al. Role of strain rate in the pathogenesis of ventilator-induced lung edema. Crit Care Med. 2016;44:e838–45.
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–55.
Lucangelo U, Bernabé F, Blanch L. Respiratory mechanics derived from signals in the ventilator circuit. Respir Care. 2005;50:55–65.
Neto AS, Hemmes SN, Barbas CS, Beiderlinden M, Fernandez-Bustamante A, Futier E, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med. 2016;4:272–80.
Wirth S, Artner L, Broß T, Lozano-Zahonero S, Spaeth J, Schumann S, et al. Intratidal recruitment/derecruitment persists at low and moderate positive end-expiratory pressure in paediatric patients. Respir Physiol Neurobiol. 2016;234:9–13.
Rausch SMK, Haberthur D, Stampanoni M, Schittny JC, Wall WA. Local strain distribution in real three-dimensional alveolar geometries. Ann Biomed Eng. 2011;39:2835–43.
Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis. 2003;1974:556–65.
Acknowledgements
‘Not applicable’.
Funding
This work was supported by CONICYT #1160631 (Dr. Cruces) and CONICYT.
#11160463 (Dr. Diaz) grants. These grants were involved in design of the study, collection of data, writing the manuscript and manuscript language edition services.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Raw data generated cannot be publicly available due to concerns of privacy of research participants, but may be available from the corresponding author FD on request.
Author information
Authors and Affiliations
Contributions
Study design: PC, SG, FC, JM, RH, FD; Measurements of respiratory mechanics: SG, FC, JM, RH; Statistic analysis: PC, BE, FD, SG; Writing the manuscript: PC, SG, BE, FD; Review of the manuscript: All authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional Review Board at Centro Hospitalario Pereira Rossell approved the study (#2852015).
Written informed consent to participate was obtained from the parents/guardians after preanesthetic interview by the PI of the study.
Consent for publication
Written consent was obtained from caregivers of included patients.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Respiratory mechanics measurements. Panel A shows Respiratory mechanics measurement protocol. Panel B shows and illustration of Airway Pressure versus time and flow versus time curves during inspiratory and expiratory breathhold. The components of work of breathing, elastic and threshold are represented. (JPG 2451 kb)
Additional file 2:
Formulas for estimation of lung mechanics in quasi - static conditions. (DOCX 15 kb)
Additional file 3:
Correlations between changes in respiratory parameters and age and ideal body weight. (DOCX 15 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cruces, P., González-Dambrauskas, S., Cristiani, F. et al. Positive end-expiratory pressure improves elastic working pressure in anesthetized children. BMC Anesthesiol 18, 151 (2018). https://doi.org/10.1186/s12871-018-0611-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-018-0611-8