Abstract
Background
Adequate cerebral perfusion pressure with quick and smooth emergence from anesthesia is a major concern of the neuroanesthesiologist. Anesthesia techniques that minimize anesthetic requirements and their effects may be beneficial. Esmolol, a short acting hyperselective β-adrenergic blocker is effective in blunting adrenergic response to several perioperative stimuli and so it might interfere in the effect of the anesthetic drugs on the brain. This study was designed to investigate the effect of esmolol on the consumption of propofol and sevoflurane in patients undergoing craniotomy.
Method
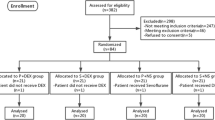
Forty-two patients that underwent craniotomy for aneurysm clipping or tumour dissection were randomly divided in two groups (four subgroups). Anesthesia was induced with propofol, fentanyl and a single dose of cis-atracurium, followed by continuous infusion of remifentanil and either propofol or sevoflurane. Patients in the esmolol group received 500 mcg/kg of esmolol bolus 10 min before induction of anesthesia, followed by additional 200 mcg/kg/min of esmolol. Monitoring of the depth of anesthesia was also performed using the Bispectral Index-BIS and cardiac output. The inspired concentration of sevoflurane and the infusion rate of propofol were adjusted in order to maintain a BIS value between 40–50. Intraoperative emergence was detected by the elevation of BIS value, HR or MAP.
Results
The initial and the intraoperative doses of propofol and sevoflurane were 18–50 mcg/kg/min and 0.2–0.5 MAC respectively in the esmolol group, whereas in the control group they where 100–150 mcg/kg/ and 0.9–2.0 MAC respectively (p = 0.000 for both groups). All procedures were anesthesiologically uneventful with no episodes of intraoperative emerge.
Conclusions
Esmolol is effective not only in attenuating intraoperative hemodynamic changes related to sympathetic overdrive but also in minimizing significant propofol and sevoflurane requirements without compromising the hemodynamic status.
ClinicalTrials.gov Identifier: NCT02455440. Registered 26 May 2015.
Similar content being viewed by others
Introduction
Neurosurgical procedures result in a significant release of adrenaline requiring large amounts of anesthetics and analgesics. However all agents used in neurosurgery should have minimal effect on brain function, not interfere with intraoperative neuromonitoring, and allow for a quick and smooth extubation and recovery. Rapid emergence from anesthesia after tumor dissection or aneurysm clipping is essential for early detection of possible neurological impairment due to hematoma formation, edema or vasospasm and ischemia. In addition, early recovery and extubation is associated with fewer metabolic and cardiovascular changes than a 2-hour delayed recovery [1].
Anesthesia with either propofol or sevoflurane combined with a short acting opioid, like remifentanil, is the preferred pharmacological approach in neuroanesthesia. Propofol and sevoflurane have a quite similar recovery time and neuroprotective effect but still controversy exists regarding the best choice of anesthetics for the neurosurgical patient [2, 3]. Although propofol is an appropriate component of total intravenous neuroanesthesia, in prolonged neurosurgical cases there is always the risk of delayed awakening. On the other hand, the use of sevoflurane in neurosurgery is still under consideration due to its ability to provoke vasodilation, cerebral hyperemia and increase intracranial pressure (ICP) even in the subanesthetic dose of 0.4 MAC (minimum alveolar concentration) [1, 4–6]. Investigators have reported significantly high doses of propofol (75–200 mcg/kg/min) and sevoflurane (2.5 MAC) intraoperatively during craniotomy maintaining a BIS (Bispectral Index) value of 40–50, which might delay the recovery time [3, 7–11]. These facts limit the use of both these anesthetic agents.
Esmolol is an ultra-short acting, cardioselective β1-adrenergic receptor antagonist that is effective in blunting sympathetic overdrive to several perioperative stimuli, including laryngoscopy with intubation, intraoperative events, emergence, and extubation and can be used as an alternative to opioids for maintaining hemodynamic and BIS stability during general anesthesia [12]. Esmolol enhances the analgesic effect of opioids but has no analgesic effect when administered alone [13, 14]. As a β1-adrenergic blocking agent, esmolol is not a cerebral vasodilator and has no significant effect on ICP [15, 16]. Studies evaluating the interaction between β-adrenoceptor antagonists and anesthetics on BIS have concluded that esmolol minimizes the dose of propofol required for intubation [12, 17]. Our study investigates the effect of esmolol on intraoperative requirements of propofol and sevoflurane in patients undergoing craniotomy.
Methods
The local research ethics committee (Scientific and ethics committee of G.Papanikolaou Hospital) approved the study and written informed consent was obtained from all patients. We included in the study ASA I-II patients, aged from 18 until 75 years, scheduled for resection of brain tumor or aneurysm clipping under general anesthesia. Exclusion criteria included the presence of one or more of the following: ASA > II, Body Mass Index (BMI) > 30, indication for rapid sequence induction, contraindication to β-blocker administration, atrial fibrillation, Glasgow Coma Scale (GCS) < 15, history of drug abuse, preoperative aphasia, neurologic deficit or preoperatively foreseen delayed extubation.
The patients were randomly divided in two groups; the esmolol group and the control group. Patients in the control group were further divided in two subgroups: control-propofol and control-sevoflurane. Similarly, patients in the esmolol group were further divided in two subgroups: esmolol-propofol and esmolol-sevoflurane. The method used for randomization was simple randomization, similar to repeated fair coin-tossing. This method was used also for randomly allocating TIVA vs sevoflurane too.
Before induction in anesthesia standard monitoring (electrocardiogram of five leads-ECG, noninvasive arterial blood pressure and pulse oximeter probe) was placed. Peripheral vein access was established and an isotonic crystalloid solution 10 ml/kg before induction and 5 ml/kg/h intraoperatively was infused. All patients had a 20G catheter inserted in the radial artery for invasive monitoring of blood pressure and cardiac output (CO) using arterial pulse contour analysis. Cardiac output was calculated using the FloTrac™ sensor kit connected to the arterial line and to the Vigileo™ monitor programmed with the 3.02 version (Edwards Lifesciences 2009, Thessaloniki, GR) of the software for this device. Patient data (age, gender, body weight, and height) were entered and after checking the arterial line waveform fidelity, the system was zeroed and CO measurement was initiated.
A BIS sensor (Aspect Medical System, Inc, GR) was placed in the frontal area with consideration for the location of the surgical incision. Regional brain hemoglobin oxygen saturation (rSO2) was also monitored using near-infrared spectroscopy. Immediately before induction of anesthesia, radiolucent Adult Somasensor oximetry strips were applied bilaterally to the forehead at standard positions relative to the midline. Consideration was also given to the site of craniotomy. The somasensors were connected to an INVOS™ cerebral oximeter, Model 4100 (Somanetics Corp, Covidient, Greece), which provides real-time numerical rSO2 values for bilateral frontal cortex. An initial baseline measurement of rSO2 was performed bilaterally by taking the mean of readings of the last 10 min before intubation. Intraoperative normothermia was actively maintained with a forced airwarming blanket.
Esmolol group patients received 500 mcg/kg of esmolol 10 min before the induction in anesthesia and for a period of 4 min followed by continuous infusion of 200 mcg/kg/min. General anesthesia was induced with propofol (2 mg/kg) and fentanyl (2mcg/kg). Intubation of the trachea was facilitated by cis-atracurium (0.15 mg/kg) and ventilation was adjusted to an end-tidal-carbon dioxide (EtCO2) tension of 30–35 mmHg in 50 % O2/air. Anesthesia was maintained using remifentanil (0.2 mcg/kg/min) and either propofol or sevoflurane. The inspired concentration of sevoflurane and the infusion rate of propofol were adjusted in order to maintain BIS values between 40–50. Intraoperative emergence was detected by elevation of BIS value, HR or MAP. After extubation, patients were asked if they had experience episodes of recall. The use of neuromuscular block was avoided after intubation. At dura closure all patients received paracetamol 1gr. Immediately before the discontinuation of remifentanil, all patients received 1 mcg/kg of fentanyl. Propofol or sevoflurane and remifentanil were discontinued after skin closure while the administration of esmolol was continued for an additional 30 min following extubation. The dose of esmolol in the esmolol group was reduced in cases of bradycardia (heart rate-HR < 40) and in cases where mean arterial pressure-MAP < 50 mmHg. Patients in the control group, who did not receive esmolol, were transferred to the intensive care unit (ICU) where they were extubated in the following hours. This decision was made because it was considered safer for the patients not to be extubated immediately after the end of the procedure in order to avoid any increase of blood pressure, considering that they had not received β-blocker for sympathetic stimulus control.
Study endpoints
Parameters recorded were operative time, duration of anesthesia (time from induction till discontinuing of remifentanil-propofol/sevoflurane), orientation time and extubation time. Orientation time was defined as the time from discontinuation of all anesthetic agents until the time the patient opened his eyes and performed specific movements. Extubation time was defined as the time from drug discontinuation to extubation. The time to reach an Aldrete score of at least 9 after tracheal extubation was also recorded. The Aldrete scoring system is widely accepted in order to assess the physical status of patients recovering from anesthesia based on a score of five criteria (mobility-able to move 4 extremities on command, respiration-able to breathe deeply and cough, oxygenation-O2 saturation on room air > 92 %, cardiovascular stability-MAP stable and consciousness-fully awake) [18].
Statistics
Data were expressed as mean ± SD. Differences in categorical data were evaluated using the student t test. A beta error level of 20 % or statistical power of 80 % and a α-level of 0.05 was used to calculate the sample size of this study. A value of p < 0.05 was considered to represent statistical significance.
Results
A total of 42 patients (male/female: 23/19, mean age 53.3 ± 17.24) were enrolled. There were no significant differences between the two groups in regard to their demographics, duration of anesthesia or type of surgery (Table 1). All the patients enrolled completed the study.
In the control-propofol group (11 patients), propofol infusion started at rates ranging from 100 to150 μcg/kg/min (134 ± 12.9mcg/Kg/min) and the infusion rate for each patient remained quite stable throughout the procedure. Conversely, in the esmolol-propofol group (11 patients), propofol was initiated at 40mcg/kg/min in all patients and was reduced to 18-35 μcg/kg/min (25.83 ± 9.32 mcg/kg/min) during the procedure (p = 0.000) [Fig. 1]. In the control-sevoflurane group (10 patients), sevoflurane’s MAC was initiated at 0.9 to 2 MAC (1.49 ± 0.288) and remained quite stable throughout the procedure with no significant fluctuation in any patient. Conversely, in the esmolol-sevoflurane group (10 patients) the initial MAC of sevoflurane was 0.5 MAC for all patients and was adjusted at 0.2–0.3 MAC (0.31 ± 0.13) during the operation (p = 0.000) [Fig. 2]. It should be noted that BIS values were comparable between the control and the esmolol group considering that BIS values cannot be absolutely stable. We adjusted propofol infusion rate and inspired concentration of sevoflurane in order to maintain a BIS between 40–50 throughout the procedure.
Intraoperative fluctuations of propofol in the control (top of the picture) and the esmolol group (bottom of the picture). Each patient is indicated with a different color. Initial infusion rate of propofol is higher in the control group compared to the esmolol group. Also, infusion rate of propofol at each time point was much lower for the esmolol group than for the control group. Mean value of propofol in control group was 134 ± 12.9mcg/Kg/min, where in the esmolol group was 25.83 ± 9.32 mcg/kg/min during the procedure
Intraoperative fluctuations of sevoflurane in the control (top of the picture) and the esmolol group (bottom of the picture). Each patient is indicated with a different color. Initial MAC of sevoflurane is higher in the control group compared to the esmolol group. Also MAC of sevoflurane at each time point was much lower for the esmolol group than the control group. Mean value of sevoflurane in control group was 1.49 ± 0.288 MAC MAC, where in the esmolol group was 0.31 ± 0.13MAC during the procedure
All patients in the esmolol group were successfully extubated with excellent neurological outcomes, coinciding with the data obtained from the intraoperative clinical data of the patient, the regional brain saturation monitoring and the macroscopic estimation of the condition of the cerebral tissue. In one patient in the esmolol group there was extensive bleeding during tumor dissection resulting in an hemoglobin drop from 12.3 to 9.8 mg/dl without however any significant change in cardiac output. However, the value of the right rSO2 revealed desaturation 26 %, even though the hemoglobin at that moment was not very low (Fig. 3). The patient was transfused with two packs of red blood cell (600ml) and the value of rSO2 returned to normal. The patient was successfully extubated 7 min after the end of the operation.
In the esmolol group, orientation and extubation times were 8.81 ± 4.69 min and 14.4 ± 6.84 min respectively for the propofol subgroup and 7.57 ± 2.82 min and 14.1 ± 6.99 min respectively for the sevoflurane subgroup. There were no significant differences between the two subgroups in orientation times (p = 0.553) and extubation times (p = 0.949). However, there was a trend towards sevoflurane achieving a better and more rapid emerge, although orientation times did not significantly differ between the propofol and sevoflurane group. The Aldrete score criteria were reached before extubation, so the actual time of the Aldrete score of at least 9 was 0 min for all patients in the esmolol group.
All patients in the esmolol group were fully conscious with no hypoxia after extubation. No patient experienced hypertension at the time of extubation or during the early postoperative period after the discontinuation of esmolol. Hypertension defined as an increase in SBP > 20 % of the baseline.
There was only one case of postoperative shivering. There was not noticed any episode of intraoperative emerge, that could be attributed to low dose of sevoflurane and propofol. This was in agreement also with the values of BIS. Moreover, none of the patients reported any episode of recall after when they were asked at the first postoperative day.
The esmolol regimen used in the present study did not have any severe hemodynamic effect during induction or maintenance of anesthesia. Predosing with esmolol 10 min before induction in anesthesia did not affect the BIS index value. There was no episode of bradycardia or persistent hypotension requiring intervention. Cardiac output did not decrease significantly even though MAP and HR were decreased. The esmolol group showed lower overall HR than the control group. Heart rate during tracheal intubation, skin incision, and tracheal extubation fluctuated less in the esmolol group. The MAP and HR variations during anesthesia are depicted in Figs. 4 and 5, respectively. There was no significant difference in HR and MAP between the control and esmolol group before induction in anesthesia. However after administration of esmolol, MAP and HR was significant different; from beginning of infusion until the first postoperative period, mean MAP at the control group was 83.8 ± 4.46mmHg when for the same period at the esmolol group was 78.5 ± 4.7mmHg (p = 0.003). Also, for the same period, mean HR at the control group was 72.2 ± 4.99/min when at the esmolol group was 65.2 ± 4.45/min (p = 0.000).
Discussion
One of the major challenges of neuroanesthesia is the ability to provide effective intraoperative analgesia and anesthesia, perioperative hemodynamic stability and smooth and early extubation. Our study showed that esmolol significantly decreased the consumption of anesthetics (propofol and sevoflurane) for patients undergoing elective craniotomy [7–11]. This reduction in anesthetics is clinically important because it achieves early orientation, less recovery time, and a smooth extubation without any hypertension during extubation. Moreover the administration of esmolol allows for the use of any anesthetic agent in “subanesthetic” doses (<0.5 MAC). Administration of esmolol before induction in anesthesia with propofol did not affect BIS suggesting that esmolol does not modify BIS during general anesthesia. On the one hand many authors argue that this is the result of the lack of any substantial sympathetic activation before intubation [19, 20]. On the other hand patients suffering from a cerebral tumor or even an unruptured aneurysm, have their sympathetic system already stimulated [21].
In the current study, the mechanism by which esmolol decreased anesthetic requirements is unknown. Miller et al. [22] suggested that drugs that affect central catecholamine release might alter anesthetic requirements. Our results clearly indicate that β-adrenoceptor antagonists not only block cardiovascular stress responses after noxious stimulation, but also increase the antinociceptive component of anesthesia. Esmolol, unlike propranolol [23], has no analgesic activity, is believed to have minimal sedative effects and given its low lipid solubility does not cross the blood–brain barrier in significant amounts [20, 24].
One explanation for the action of esmolol on central antinociceptive mechanism is its propensity to block β-adrenoceptors. The hemodynamic effects of esmolol are thought to be mediated by the blockage of peripheral, β-adrenergic receptors [25]. B-adrenergic receptors are present in various parts of the reticular activating system, particularly the medial septal region of the basal forebrain. Administration of β-adrenergic receptor agonists in this region elicits enhancement of behavioral and EEG indices of waking in animals [26]. The low-potency, low-lipid solubility and rapid metabolism within the blood stream do not exclude a central site for esmolol action [27].
Many previous studies have focused on the relationship between perioperative use of β-blocker with opioids. However, very few have addressed the interaction between β-blockers and anesthetics agents. Wilson et al, using esmolol in patients before intubation found that the propofol requirements for induction of anesthesia were reduced by 25 %. Based on those findings he suggested that there might be a correlation between reduced requirements of propofol and low CO attributed to the use of esmolol. However, in this study there was no CO monitoring during esmolol infusion [28]. In another study on patients undergoing non-neurosurgical procedures, it was shown that high doses of esmolol (250mcg/kg/min) reduced propofol requirements by 26 %. The authors proposed that, since there was no evidence of altered propofol pharmacokinetics, esmolol interacted with the opioid component in an unspecified manner [29].
Esmolol is thought to suppress the EEG, with a decrease in BIS and an increase in the burst suppression ratio during propofol/alfentanil anesthesia [23]. However this is in contrast with a recent study reporting that the blood concentration of propofol necessary to preventing response to command was unaltered by esmolol. According to Orme et al esmolol may alter drug metabolism and distribution via effects on hepatic blood flow and subsequent drug clearance [20]. In addition, redistribution of propofol from the central compartment may be limited and the decreased consumption of anesthetics may be due to decreased CO and consequently decreased hepatic blood flow altering the pharmacokinetic of propofol [24]. However, this is not in agreement with our findings that CO was not changed during infusion of esmolol. Concurrent with our results is also the one by Chia et al. [30] reporting that MAP was not different between groups that received esmolol and those that did not receive. Thus it seems unlikely that the reduction in CO is responsible for the low MAC of sevoflurane and propofol. Moreover, esmolol is metabolized by red blood cells esterases and not by the liver.
Adequate cerebral blood flow needs adequate global hemodynamics including blood pressure and CO. The major concern about esmolol is the possible bradycardia and low MAP that may occur following its administration. Regional cerebral oxygen saturation and CO monitoring by INVOS™ and FloTrac™ closely monitor cerebral perfusion and oxygenation respectively in patients who receive esmolol. Our results indicate that esmolol infusion did not produce significant changes in HR or CO, indicating that esmolol even in the dose of 200mcg/kg/min, is a safe component of balanced anesthesia during craniotomies.
Another issue that may arise is that the low dose of anesthetics agents used intraoperative might lead to a higher cerebral metabolic ratio of oxygen (CMRO2). However the values of rSO2 did not show any reduction that could correlate with inadequate level of anesthesia.
Certain limitations of this study have to be recognized although we consider that they did not alter the accuracy of our results. The study population was relatively small, and we did not use target controlled infusion (TCI) for calculating the dose of propofol and remifentanil.
Conclusion
Our data revealed that continuous esmolol infusion could significantly decrease anesthetic requirements during balanced anesthesia with propofol or sevoflurane and remifentanil, resulting in successful and uneventful emergence, with no significant changes in heart rate or cardiac output. B-adrenergic antagonists may represent a novel class of drugs that can modify anesthetic requirements in patients undergoing craniotomy and be component of balanced anesthesia.
References
Bruder N, Stordeur JM, Ravussin P, Valli M, Dufour H, Bruguerolle B, et al. Metabolic and hemodynamic changes during recovery and tracheal extubation in neurosurgical patients: immediate versus delayed recovery. Anesth Analg. 1999;89:674–8.
Hans P, Bonhomme V. Why we still use intravenous drugs as the basic regimen for neurosurgical anaesthesia. Curr Opin Anaesthesiol. 2006;19:498–503.
Bilotta F, Doronzio A, Cuzzone V, Caramia R, Rosa G. Early postoperative cognitive recovery and gas exchange patterns after balanced anesthesia with sevoflurane or desflurane in overweight and obese patients undergoing craniotomies. J Neurosurg Anesthesiol. 2009;21:207–13.
Fraga M, Rama-Maceiras P, Rodiño S, Aymerich H, Pose P, Belda J. The effects of isoflurane and desflurane on intracranial pressure, cerebral perfusion pressure, and cerebral arteriovenous oxygen content difference in normocapnic patients with supratentorial brain tumors. Anesthesiology. 2003;98:1085–90.
Ogawa Y, Iwasaki K, Shibata S, Kato J, Ogawa S, Oi Y. The effect of sevoflurane on dynamic cerebral blood flow autoregulation assessed by spectral and transfer function analysis. Anesth Analg. 2006;102:552–9.
Kolbitsch C, Lorenz IH, Hormann C, Schocke M, Kremser C, Zschiegner F, et al. A subanesthetic concentration of sevoflurane increases regional cerebral blood flow and regional cerebral blood volume and decreases regional mean transit time and regional cerebrovascular resistance in volunteers. Anesth Analg. 2000;91:156–62.
Türe H, Sayin M, Karlikaya G, Bingol CA, Aykac B, Türe U. The analgesic effect of gabapentin as a prophylactic anticonvulsant drug on postcraniotomy pain: a prospective randomized study. Anesth Analg. 2009;109:1625–31.
Bilotta F, Caramia R, Paoloni FP, Favaro R, Araimo F, Pinto G, et al. Early postoperative cognitive recovery after remifentanil–propofol or sufentanil–propofol anaesthesia for supratentorial craniotomy: a randomized trial. Eur J Anaesthesiol. 2007;24:122–27.
Bundgaard H, von Oettingen G, Larsen KM, Landsfeldt U, Jensen KA, Nielsen E, et al. Effects of sevoflurane on intracranial pressure, cerebral blood flow and cerebral metabolism. A dose-response study in patients subjected to craniotomy for cerebral tumours. Acta Anaesthesiol Scand. 1998;42:621–7.
Magni G, Baisi F, La Rosa I, Imperiale C, Fabbrini V, Pennacchiotti ML, et al. No difference in emergence time and early cognitive function between sevoflurane-fentanyl and propofol-remifentanil in patients undergoing craniotomy for supratentorial intracranial surgery. J Neurosurg Anesthesiol. 2005;17:134–8.
Magni G, La Rosa I, Gimignani S, Melillo G, Imperiale C, Rosa G. Early postoperative complications after intracranial surgery: comparison between total intravenous and balanced anesthesia. J Neurosurg Anesthesiol. 2007;19:229–34.
Coloma M, Chiu JW, White PF, Armbruster SC. The use of esmolol as an alternative to remifentanil during desflurane anesthesia for fast-track outpatient gynecologic laparoscopic surgery. Anesth Analg. 2001;92:352–7.
Yang H, Fayad A. Are beta-blockers anesthestics? Can J Anaesth. 2003;50:627–30.
Collard V, Mistraletti G, Taqi A, Asenjo JF, Feldman LS, Fried GM, et al. Intraoperative esmolol infusion in the absence of opioids spares postoperative fentanyl in patients undergoing ambulatory laparoscopic cholecystectomy. Anesth Analg. 2007;105:1255–62.
Grillo P, Bruder N, Auquier P, Pellissier D, Gouin F. Esmolol blunts the cerebral blood flow velocity increase during emergence from anesthesia in neurosurgical patients. Anesth Analg. 2003;96:1145–9.
Dubois M, Caputy A, MacCosbe P, Lea D, Duma C. Cerebral blood flow measurements during blood pressure control with intravenous labetalol following craniotomy. J Neurosurg Anesthesiol. 1992;4:176–81.
Zaugg M, Tagliente T, Lucchinetti E, Jacobs E, Krol M, Bodian C, et al. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology. 1999;91:1674–86.
Aldrete JA. The postanesthesia recovery score revised. J Clin Anesth. 1995;7:89–91.
Menigaux C, Guignard B, Adam F, Sessler DI, Joly V, Chauvin M. Esmolol prevents movement and attenuates the BIS response to orotracheal intubation. Br J Anaesth. 2002;89:857–62.
Orme R, Leslie K, Umranikar A, Ugoni A. Esmolol and anesthetic requirement for loss of responsiveness during propofol anesthesia. Anesth Analg. 2002;94:112–16.
Bloomfield EL, Porembka DT, Ebrahim ZY, Grimes-Rice M, Secic M, Little JR, et al. Analysis of catecholamine and vasoactive peptide release in intracranial arterial venous malformations. J Neurosurg Anesthesiol. 1996;8:101–10.
Miller RD, Way WL, Eger 2nd EI. The effects of alpha-methyldopa, reserpine, guanethidine, and iproniazid on minimum alveolar anesthetic requirement (MAC). Anesthesiology. 1968;29:1153–8.
Johansen JW. Esmolol promotes electroencephalographic burst suppression during propofol/alfentanil anesthesia. Anesth Analg. 2001;93:1526–31.
Wood AJ, Feely J. Pharmacokinetic drug interactions with propranolol. Clin Pharmacokinetic. 1983;8:253–62.
Davidson EM, Doursout MF, Szmuk P, Chelly JE. Antinociceptive and cardiovascular properties of esmolol following formalin injection in rats. Can J Anaesth. 2001;48(1):59–64.
Berridge CW, Foote SL. Enhancement of behavioral and electroencephalographic indices of waking following stimulation of noradrenergic b-receptors within the medial septal region of the basal forebrain. J Neurosci. 1996;16:6999–7009.
Angaran DM, Schultz NJ, Tschida VH. Esmolol hydrochloride: An ultrashort-acting, beta-adrenergic blocking agent. Clin Pharm. 1986;5:288–303.
Wilson ES, McKinlay S, Crawford JM, Robb HM. The influence of esmolol on the dose of propofol required for induction of anaesthesia. Anaesthesia. 2004;59:122–6.
Johansen JW, Flaishon R, Sebel PS. Esmolol reduces anesthetic requirement for skin incision during propofol/nitrous oxide/morphine anesthesia. Anesthesiology. 1997;86:364–71.
Chia YY, Chan NH, Ko NH, Liu K. Role of b-blockade in anaesthesia and postoperative pain management after hysterectomy. Br J Anaesth. 2004;93:799–805.
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Dr. I. Asouhidou states that she has not any conflict of interest. Dr. A. Trikoupi was head of the committee that approved this study. However, she states that this did not impact upon whether the study received approval or not.
Authors’ contributions
AI designed the study, collected the data and drafted the paper. TA had scientific supervision of the study. Both authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Asouhidou, I., Trikoupi, A. Esmolol reduces anesthetic requirements thereby facilitating early extubation; a prospective controlled study in patients undergoing intracranial surgery. BMC Anesthesiol 15, 172 (2015). https://doi.org/10.1186/s12871-015-0154-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-015-0154-1