Abstract
Background
Dexamethasone is an antiemetic alternative to ondansetron. We aimed to compare the effects of dexamethasone and ondansetron in preventing postoperative nausea and vomiting (PONV) in patients undergoing laparoscopic surgery.
Methods
We searched PubMed, Embase, Medline and Cochrane Library (from inception to July 2014) for eligible studies. The primary outcome was the incidence of PONV during the first 24 h after surgery. The secondary outcomes included PONV in the early postoperative stage (0–6 h), PONV in the late postoperative stage (6–24 h), and the postoperative anti-emetics used at both stages. We calculated pooled risk ratios (RR) and 95 % CIs using random- and fixed-effects models.
Results
Seven trials involving 608 patients were included in this meta-analysis, which found that dexamethasone had a comparable effectiveness in preventing PONV (RR, 0.91; 95 % CI, 0.73-1.13; P = 0.39) with that of ondansetron within 24 h of laparoscopic surgery, with no evidence of heterogeneity among the studies (I2 = 0 %; P = 0.71). In the early postoperative stage (0–6 h), ondansetron was better at decreasing PONV than dexamethasone (RR, 1.71; 95 % CI, 1.05-2.77; P = 0.03), while in the late postoperative stage (6–24 h), dexamethasone was more effective in preventing PONV than ondansetron (RR, 0.51; 95 % CI, 0.27-0.93; P = 0.03). There was no significant difference in the postoperative anti-emetics used (RR, 0.90; 95 % CI, 0.67-1.19; P = 0.45).
Conclusions
Dexamethasone was as effective and as safe as ondansetron in preventing PONV. Dexamethasone should be encouraged as an alternative to ondansetron for preventing PONV in patients undergoing laparoscopic surgery.
Similar content being viewed by others
Background
Postoperative nausea and vomiting (PONV) is a common distressing symptom in patients undergoing laparoscopic surgery and can contribute to anxiety, dehydration, metabolic abnormality, wound disruption, delayed recovery and other issues [1]. The incidence of PONV varies from 20 to 80 %, and it is an economic and social burden. Ondansetron is a selective 5-HT3 receptor antagonist, that exhibits an anti-emetic action by antagonizing vomiting signals in the afferent pathway from the stomach or small intestine and solitary tract nucleus, and is effective at preventing PONV [2, 3], however the high cost of this drug has prevented it from being widely used. Dexamethasone, a corticosteroid, was first reported as an effective anti-emetic agent in patients undergoing cancer chemotherapy in 1981 [4, 5]. Wang et al. [6] confirmed that dexamethasone is most effective when it is administered at the induction rather than at the termination of anaesthesia. However, the mechanism underlying the anti-emetic effects of dexamethasone is still unknown. It may be involved in central inhibition of prostaglandin synthesis, or it may cause a decrease in serotonin turnover in the central nervous system [5, 7, 8].
Today, cost-benefit analyses have become an important factor when considering what drugs to use as prophylactic antiemetics. The cost-to-benefit ratio for the patient was much higher in the ondansetron group than in the dexamethasone group [9]. However, it has not been established whether dexamethasone is a cost-effective alternative to ondansetron in the prevention of PONV in patients undergoing laparoscopic surgery. It is highly necessary to conduct a meta-analysis to assess the results of the currently published studies on this topic. Therefore, we performed a systematic review to compare the efficacy of dexamethasone with that of ondansetron.
Methods
This systematic review was carried out according to the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) [10]. We prospectively registered our system review at PROSPERO. (Registration number: CRD420140013064).
Data sources and search strategy
PubMed, Embase, Medline and Cochrane Library databases were searched from inception to July, 2014 for relevant studies that investigated the differences in the anti-emetic effects of dexamethasone and ondansetron. The following search terms were used: Dexamethasone, Hexadecadrol, Methylfluorprednisolone, Decameth, “Foy Brand of Dexamethasone”, Decaspray, “Merck Brand of Dexamethasone”, Dexasone, “ICN Brand of Dexamethasone”, Dexpak, “ECR Brand of Dexamethasone”, Maxidex, “Alcon Brand of Dexamethasone”, Millicorten, Oradexon, Decaject, “Merz Brand 1 of Dexamethasone”, “Decaject L.A.”, “Decaject-L.A.”, “Merz Brand 2 of Dexamethasone”, Hexadrol, Ondansetron, “Ondansetron, (+,-)-Isomer”, “Ondansetron, (R)-Isomer”, “Ondansetron, (S)-Isomer”, “GR-38032F”, “GR 38032F”, “GR 38032F”, Zofran, “Ondansetron Hydrochloride”, “Hydrochloride, Ondansetron”, “Ondansetron Monohydrochloride Dihydrate”, “Dihydrate, Ondansetron Monohydrochloride”, “Monohydrochloride Dihydrate, Ondansetron”, “Ondansetron Monohydrochloride”, “Monohydrochloride, Ondansetron”, SN 307, “SN-307”, “Laparoscopy”, Laparoscopies, Peritoneoscopy, Peritoneoscopies, Celioscopy, Celioscopies, “Surgical Procedures, Laparoscopic”, “Laparoscopic Surgical Procedure”, “Surgical Procedure, Laparoscopic”, “Procedure, Laparoscopic Surgical”, “Procedures, Laparoscopic Surgical”, “Surgery, Laparoscopic”, “Laparoscopic Surgery”, “Laparoscopic Surgeries”, “Surgeries, Laparoscopic”, “Laparoscopic Surgical Procedures”, PONV, vomiting, emesis and nausea. A manual search of the reference sections of the included trials, published meta-analyses, and pertinent review articles was also conducted to identify additional relevant articles. If duplicated data were presented in several publications, only the most recent, largest or most complete study was included in this meta-analysis.
Study selection
The original studies included in this meta-analysis were based on PICOS (patient, intervention, comparison, outcomes and study design) as follows: (a) P: American Society of Anesthesiology (ASA) I/II grade adult patients undergoing laparoscopic surgery; (b) I and C: dexamethasone and ondansetron respectively; (c) O: reporting the incidence of PONV; (d) S: only randomized controlled trials (RCTs). Only articles published in English were included. Patients who had a history of nausea or vomiting or who had been given H2 blockers 48 h prior to the operation were excluded. Patients with the following characteristics were also excluded: history of motion sickness, facing kidney problems with a high level of BUN or Cr, history of allergy to the study drug, body mass index (BMI) > 35 and being pregnant or menstruating.
Data extraction
The characteristics of the patients (number of patients, ASA rating, age, gender, type of surgery and anaesthesia) and the trial designs (intervention, follow-up duration and reported outcomes) were also recorded. If the data mentioned above were unavailable in the article, the corresponding authors were called upon to obtain the missing information. All data were independently extracted using a standard data collection form by 2 reviewers (XXW and FRH), then, the collected data were checked and entered into Review Manager analysis software (RevMan) Version 5.3. All discrepancies were reviewed and a consensus was reached by discussion with a third author (DBP). The reasons that studies were excluded were recorded.
Assessment of study quality
A critical evaluation of the quality of the included studies was performed by two reviewers (XXW and HJG) using a 5-point Jadad scale [11]. The main categories consisted of the following five items: “Was the study described as randomized?”, “Was the method used to obtain the sequence of randomization described and appropriate (random numbers, computer-generated, etc.)?”, “Was the study described as double-blind?”, “Was the method of double-blinding described and appropriate (identical placebo, active placebo, dummy, etc.)?”, and “Was there a description of withdrawals and drop-outs?”. Scores of four and five indicated a high methodological quality.
Assessment of risk of bias
Two reviewers (XXW and QZ) independently evaluated the risk of bias according to the recommendations of the Cochrane Collaboration [12]. The principal categories consisted of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. Each domain was measured as “high risk”, “low risk”, or “unclear risk”. Namely, items with sufficient and correct information were judgment was “low risk”, and items that were reported incorrectly were judgment as “high risk”. If the information of the item was insufficient or unsanctioned, they were judged as “unclear risk”. An “unclear risk” judgement was applied if the item was reported, but the risk of bias was unknown. Discrepancies were resolved by a senior reviewer (DBP).
Statistical analysis
RR with 95 % CI was used as a common measure of the difference in the efficacy of dexamethasone and ondansetron for prophylaxis of postoperative nausea and vomiting across studies. The power analyses of the individual studies (Power and Precision V4) and meta-analysis were all conducted by Review Manager 5.3. I2 was used to estimate statistical heterogeneity. When I2 <50 %, heterogeneity was considered present, and the fixed-effects model was applied. Otherwise, the randomized-effects model and sensitivity analysis were applied. Publication bias was evaluated by Egger’s test. A P value <0.05 was considered statistically significant.
The quality of evidence was evaluated by two reviewers (HWD and AGZ) using the GRADE system (GRADEprofiler 3.6.1). RCTs were considered to have high-quality evidence. However, this assessment could be downgraded for five reasons: risk of bias, inconsistency, indirectness, imprecision and publication bias. Finally, there were four levels of evidence quality, namely high, moderate, low and very low.
Results
Identification of eligible studies
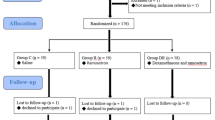
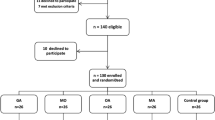
A total of 184 potentially relevant abstracts were identified. After duplicates were removed, one hundred and forty six unique abstracts remained. After reviewing the abstracts, only ten publications met the inclusion criteria. Three of them were excluded because of incomplete data. Finally, the remaining seven studies [1, 13–18] were included in the present meta-analysis. The search strategy and study selection are presented in the flow diagram of Fig. 1.
Study characteristics
The characteristics of all included studies are shown in Table 1. All studies were published between 2003 and 2013. The sample sizes of the studies ranged from 40 to 160 (total 608). Drug preparations were administered intravenously before surgery. All included studies were randomized controlled trials and all reported PONV and the rescue anti-emetics used. No significant side effects of dexamethasone or ondansetron were observed in these studies. The definition of PONV was only clearly stated in two studies (Gautan B et al. and Ashwani khimar et al.).
Meta-analyses of primary outcomes
Postoperative nausea and vomiting (0–24 h)
The results are shown in Fig. 2 and Table 1. PONV within 24 h was studied in seven trials [1, 13–18]. Overall, the rates of PONV in the dexamethasone and ondansetron groups were 33.3 and 36.7 %, respectively. Dexamethasone was not associated with a significant reduction in the incidence of PONV (RR, 0.91; 95 % CI, 0.73-1.13; P = 0.39), but no evidence of heterogeneity was observed among the remaining studies (I2 = 0 %; P = 0.71).
Postoperative nausea and vomiting at different stages
Furthermore, we conducted a subgroup meta-analysis based on the postoperative stage to explore the efficacy of dexamethasone for the prevention of PONV compared with that of ondansetron. Dexamethasone was not superior to ondansetron in preventing PONV in the early postoperative stage (0–6 h) (RR, 1.22, 95 % CI 0.87-1.73; P = 0.25), but there was significant heterogeneity in these data among the studies (I2 = 65 %; P = 0.02). After the exclusion of two trials that did not have any occurrence of PONV in the late postoperative stage (6–24 h), dexamethasone was found to be superior to ondansetron in preventing PONV in the late postoperative stage (RR, 0.51, 95 % CI, 0.27-0.93; P = 0.03), and the heterogeneity among the studies was more moderate for these data than for the data for the early postoperative stage (I2 = 37 %; P = 0.20) (Fig. 3). Subsequently, a sensitivity analysis was carried out to explore the potential source of heterogeneity in the early postoperative stage. As shown in Fig. 4, the results of Nita D’souza et al. [1] were completely out of the range of those of the other studies and this probably contributed to the observed heterogeneity. After excluding this study, the results suggested that compared with dexamethasone, ondansetron was associated with a decreased of incidence PONV (RR 1.71, 95 % CI, 1.05-2.77; P = 0.03).
Meta-analyses of secondary outcomes (0–24 h)
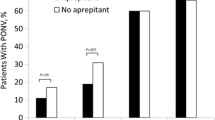
Figure 5 describes the postoperative anti-emetics used within 24 h. The results of these studies suggested no difference in the overall postoperative anti-emetics between the dexamethasone and ondansetron groups (RR, 0.90, 95 % CI, 0.67-1.19; P = 0.45). Additionally, no heterogeneity in any of the secondary outcomes was observed (I2 = 0 %; P = 0.88).
Quality assessment and quality of evidence
This systematic review included 7 RCTs: the baseline characteristics of patients were reported by all trials. Only one trial mentioned the method of randomization (Fig. 6).
This meta-analysis examined four outcomes: PONV (0–24 h, 0–6 h and 6–24 h) and postoperative anti-emetics (0–24 h). The quality of the evidence for each outcome is presented in Table 2.
Power analysis
Although statistical results were presented in some studies, a portion of the primary data was unavailable. The available data were reassessed by a power analysis with an α level of 0.05 (Table 3). The power of the individual studies ranged from 5.1 to 64 %. The power of the meta-analysis with respect to PONV (0–24 h, 0–6 h and 6–24 h) and postoperative anti-emetics (0–24 h) was 12.2, 17.9, 64 and 9.1 %, respectively (Table 3).
Publication bias
Egger’s test did not reveal any significant difference with respect to all outcomes. This result indicated that no publication bias existed (t = 0.66, P = 0.537).
Discussion
This is the first meta-analysis to compare the efficacy of dexamethasone with that of ondansetron in preventing PONV after laparoscopic surgery. The pooled meta-analysis of 7 RCTs using a fixed-effects model suggested that there were no significant differences between dexamethasone and ondansetron in regards to the incidence of PONV or postoperative anti-emetics used during the first 24 h after laparoscopic surgery. Ondansetron was more effective at decreasing PONV in the early postoperative stage (0–6 h), while dexamethasone was more effective at decreasing PONV in the late postoperative stage (6–24 h).
Glucocorticoids bind to intracellular glucocorticoid receptors, and exert their effects via gene transcription [19]. As changes to both gene expression and protein synthesis take time, most effects of corticosteroids are not instantaneous, rather, they only become apparent after several hours. Therefore, glucocorticoids usually take 1–2 h to have biologic effects, and this also depends on the route of administration [20]. This may explain why dexamethasone was found to significantly decrease PONV in the late postoperative stage (6–24 h) rather than in the early postoperative stage (0–6 h) in our data analysis. Interestingly, Thomas and Jones [21] found a failure of prophylaxis during the first 3 h after laparoscopic surgery in 28.3 % of patients who had received dexamethasone compared to with 22 % of patients who had received ondansetron. The late onset and prolonged antiemetic efficacy of dexamethasone may be attributed to its prolonged biological half-life (36–72 h) [22]. Accordingly, the timing of dexamethasone administration is important. It is necessary to administrate dexamethasone 1–2 h preoperatively [23], especially for short surgical procedures. The findings of this meta-analysis depend on the quality of the included primary trials. Despite the declaration of randomization design, only one trial reported sequence generation, which might decrease the level of evidence of this meta-analysis.
Based on the GRADE system, the qualities of outcomes were all “moderate”, and evidence quality was degraded owing to this imprecision. Only two studies clearly defined PONV.
To assess the probability of correctly rejecting the null hypothesis, a power analysis was performed. If H1 is true, the power is 1-β. To guard against Type I error, α is typically set to 0.05, and to guard against Type II error, β is set to 0.20 [24–26]. Thus, a power of more than 80 % is necessary to reject the null hypothesis. In our meta-analysis, the power was <80 %. Therefore, this meta-analysis did not provide enough evidence on the effects of the study drugs and more high level studies are required.
In our meta-analysis, the patients enrolled were quite homogeneous. The studies all had Jadad scores of ≥ 4 and were of high quality. For all studies, a fixed-effects model and test of heterogeneity between trials resulted in an I2 value of (0.0 %) and a P of value (0.71), indicating no heterogeneity. The participants in all studies were well matched (e.g., sex, age, ASA grade, administration time, method of surgery/anaesthesia, et al.). However, several limitations of this meta-analysis should be taken into account. First, there was no gold standard for the definition of PONV, resulting in possible overestimation or underestimation of the true effect of dexamethasone administration compared with that of ondansetron. Furthermore, this meta-analysis was based on studies published in the English language, which may have generated bias. Next, the sample sizes of the studied individual trials were small or moderate. The over difference in the incidence of PONV (0–24 h) was not different between the dexamethasone and ondansetron groups, which may be due to the small sample size and lack of evidence. Finally, we selected published studies, and many studies were not registered on clinical trial data-bases. Data from unpublished literature could be missing, which would lead to bias. However, the Egger’s test result suggested that no publication bias existed.
Nonetheless, our study provides useful evidence for future studies on PONV. Different drugs (dexamethasone and ondansetron) have different working times and half-lives, so attention should be paid to the time-effect relationship of these drugs. Further studies may also focus on the safety of dexamethasone, as long-term corticosteroid administration causes side effects such as wound healing delays, infection, and adrenal suppression. Moreover, in our study, the drugs were only compared when they were used in patients undergoing laparoscopic surgery, so other medical situations in which these drugs are used should also be studied.
Conclusion
In summary, dexamethasone was equally effective and as safe as ondansetron in preventing PONV. However, in the late postoperative stage (6–24 h), dexamethasone probably has an advantage over ondansetron. Considering the limitations of this study, our findings should be considered with caution, and large-scale studies are needed to confirm our findings.
Abbreviations
- RR:
-
Risk ratios
- PONV:
-
Postoperative nausea and vomiting
- PICOS:
-
Patient, intervention, comparison, outcome and study design
- ASA:
-
American Society of Anesthesiology
- RCTs:
-
Randomized controlled trials
References
D’souza N, Swanmi M, Bhaqwat S. Comparative study of dexamethasone and ondansetron for prophylaxis of postoperative nausea and vomiting in laparoscopic gynecologic surgery. Int J Gynaecol Obstet. 2011;113:124–7.
Pearman MH. Single dose intravenous ondansetron in the prevention of postoperative nausea and vomiting. Anaesthesia. 1994;49(Suppl):11–5.
Hirayama T, Ishii F, Yaqo K, Oqata H. Evaluation of the effective drugs for the prevention of nausea and vomiting induced by morphine used for postoperative pain: a quantitative systematic review. Yakuqaku Zasshi. 2001;121(2):179–85.
Aapro MS, Alberts DS. Dexamethasone as an antiemetic in patients treated with cisplatin. N Engl J Med. 1981;305:520.
Lopez-olaondo L, Carrascosa F, Pueyo FJ, Monedero P, Busto N, Saez A. Combination of ondansetron and dexamethasone in the prophylaxis of postoperative nausea and vomiting. Br J Anaesth. 1996;76:835–40.
Wang JJ, Ho ST, Tzeng JI, Tang CS. The effect of timing of dexamethasone administration on its efficacy as a prophylactic antiemetic for postoperative nausea and vomiting. Anesth Analg. 2000;91:136–9.
Fredrickson M, Hursti T, Furst CJ, Steineck G, Borjeson S, Wikblom M, et al. Nausea in cancer chemotherapy is inversely related to urinary cortisol excretion. Br J Cancer. 1992;65:779–80.
Aapro MS, Plezia PM, Alberts DS, Graham V, Jones SE, Surwit EA, et al. Double-blind cross-over study of the anti-emetic efficacy of high dose dexamethasone versus high dose metoclopramide. J Clin Oncol. 1984;2:466–71.
Subramaniam B, Madan R, Sadhasivam S, Sennaraj B, Tamilselvan P, Rajeshwari S, et al. Dexamethasone is a cost-effective alternative to ondansetron in preventing PONV after paediatric strabismus repair. Br J Anaesth. 2001;86(1):84–9.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaqhan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928.
Erhan Y, Erhan E, Aydede H, Yumus O, Yentur A. Ondansetron, granisetron, and dexamethasone compared for the prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 2008;22:1487–92.
Alghanem SM, Massad IM, Rashed EM, Abu-Ali HM, Daradkeh SS. Optimization of anesthesia antiemetic measures versus combination therapy using dexamethasone or ondansetron for the prevention of postoperative nausea and vomiting. Surg Endosc. 2010;24(2):353–8.
Yuksek MS, Alici HA, Erden AF, Cesur M. Comparison of prophylactic anti-emetic effects of ondansetron and Dexamethasone in women undergoing day-case gynaecological laparoscopic surgery. J Int Med Res. 2003;31:481–8.
Gautam B, Shrestha BR, Lama P, Rai S. Antiemetic prophylaxis against postoperative nausea and vomiting with ondansetron-dexamethasone combination compared to ondansetron or dexamethasone alone for patients undergoing laparoscopic cholecystectomy. Kathmandu Univ Med J. 2008;6(23):319–28.
Ashwani K, Madhusudan P, Pandove PK, Sharda VK. A randomized, placebo controlled study evaluating preventing role of ondansetron, dexamethasone and ondansetron plus dexamethasone for postoperative nausea and vomiting (PONV) in patients undergoing laparoscopic cholecystectomy. JIMSA. 2013;26(4):217–8.
Mohammad E, Hamid R, Mehdi R, Mahdi M, Abdolreza P. Effect of ondansetron and dexamethasone on post-operative nauseas and vomiting in patients undergoing laparoscopic cholecystectomy. J Minim Invasive Surg Sci. 2013;2(2):138–43.
Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond). 1998;94:557–72.
Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89.
Thomas R, Jones N. Prospective randomized, double-blind comparative study of dexamethasone, ondansetron and ondansetron plus dexamethasone as prophylactic antiemetic therapy in patients undergoing day-case gynaecological surgery. Br J Anaesth. 2001;87(4):588–9.
Schimmer BP, Parker KL. Adrenocorticotropic hormone-adrenocortical steroids and their synthetic analogs: inhibitors of the synthesis and actions of adrenocortical hormones. In: Gilman G, editor. The Pharmacological Basis of Therapeutics. 9th ed. New York: McGraw Hill; 1996. p. 1459–85.
Bisgaard T, Klarskov B, Kehlet H, Rosenberg J. Preoperative dexamethasone improves surgical outcome after laparoscopic cholecystectomy. Ann Surg. 2003;238(5):651–60.
Wang D, Zhai JX, Zhang LM, Liu DW. Null genotype of GSTT1 contributes to increased Parkinson’s disease risk in Caucasians: evidence from a meta-analysis. Mol Biol Rep. 2014;41(11):7423–30.
Mizuguchi T, Kawamoto M, Meguro M, Shibata T, Nakamura Y, Kimura Y, et al. Laparoscopic hepatectomy: a systematic review, meta-analysis, and power analysis. Surg Today. 2011;41(1):39–47.
Jia Z, Chen C, Wu Y, Ding F, Tian X, Li W et al. No difference in clinical outcomes after total knee arthroplasty between patellar eversion and non‑eversion. Knee Surg Sports Traumatol Arthrosc. 2014. [Epub ahead of print].
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XXW and DBP conceived the study, participated in the study design, collected the data, and drafted the manuscript. XXW, DBP and QZ participated in the study design, collected the data, performed the statistical analysis and contributed to drafting the manuscript. HWD, AGZ, FRH and HJG helped to perform the statistical analysis and revised the manuscript critically to ensure all important intellectual content was present. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, XX., Zhou, Q., Pan, DB. et al. Dexamethasone versus ondansetron in the prevention of postoperative nausea and vomiting in patients undergoing laparoscopic surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 15, 118 (2015). https://doi.org/10.1186/s12871-015-0100-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-015-0100-2