Abstract
Saline-sodic stress can limit the absorption of available zinc in rice, subsequently impacting the normal photosynthesis and carbohydrate metabolism of rice plants. To investigate the impact of exogenous zinc application on photosynthesis and carbohydrate metabolism in rice grown in saline-sodic soil, this study simulated saline-sodic stress conditions using two rice varieties, 'Changbai 9' and 'Tonghe 899', as experimental materials. Rice seedlings at 4 weeks of age underwent various treatments including control (CT), 2 μmol·L−1 zinc treatment alone (Z), 50 mmol·L−1 saline-sodic treatment (S), and 50 mmol·L−1 saline-sodic treatment with 2 μmol·L−1 zinc (Z + S). We utilized JIP-test to analyze the variations in excitation fluorescence and MR820 signal in rice leaves resulting from zinc supplementation under saline-sodic stress, and examined the impact of zinc supplementation on carbohydrate metabolism in both rice leaves and roots under saline-sodic stress. Research shows that zinc increased the chloroplast pigment content, specific energy flow, quantum yield, and performance of active PSII reaction centers (PIABS), as well as the oxidation (VOX) and reduction rate (Vred) of PSI in rice leaves under saline-sodic stress. Additionally, it decreased the relative variable fluorescence (WK and VJ) and quantum energy dissipation yield (φDO) of the rice. Meanwhile, zinc application can reduce the content of soluble sugars and starch in rice leaves and increasing the starch content in the roots. Therefore, the addition of zinc promotes electron and energy transfer in the rice photosystem under saline-sodic stress. It enhances rice carbohydrate metabolism, improving the rice plants’ ability to withstand saline-sodic stress and ultimately promoting rice growth and development.
Similar content being viewed by others
Introduction
Soil salinization is a significant abiotic stress that affects global agriculture and impacts plant growth and development [1]. In China, the extent of saline-alkali soil covers approximately 99.13 million hm2. The Songnen Plain(42°30–51°20 N and 121°40–128°30 E) alone has an area of 3.73 million hm2 of saline-sodic land, making it one of the world's three major areas with this type of soil [2]. The soil in this region contains a significant amount of soluble salt, with NaHCO3 and Na2CO3 being the main salt components. The pH values of the soil in this area are mostly higher than 8.5. The Songnen Plain is one of China's major grain-producing regions; however, its crop yields are severely limited due to the presence of saline-sodic soils. The high concentration of salt ions in saline-sodic soil not only reduces the osmotic potential of the soil, leading to osmotic stress in crops, but also disrupts the delicate ion balance within crop cells as salt ions like Na+ and Cl− enter, resulting in ion stress [3]. Additionally, the high pH levels facilitate salt ion absorption by crops, but hinder the absorption of essential nutrient ions such as zinc, iron, calcium, and magnesium, thereby impeding their growth and development [4]. Consequently, alleviating saline-sodic stress, improving crop nutrient absorption, and promoting crop growth and development are significant challenges in agriculture within this region.
Zinc, a micronutrient crucial for crop growth and development, acts as a cofactor for numerous enzymes involved in key physiological and biochemical processes like photosynthesis and hormone synthesis [5]. Zinc in the soil is primarily absorbed by the plant in its Zn2+ state. The root cells absorb Zn2+ through both passive and active transport via the cell membrane [6]. The uptake and transport of zinc in plants can be influenced by external factors, including saline-sodic conditions. Research indicates a strong correlation between soil saline-sodic concentration, and zinc availability [7]. It has been observed that as the pH levels rise, the amount of zinc accessible to wheat plants decreases notably, particularly in soils rich inwith high bicarbonate content [8]. However, zinc deficiency has significant implications for crop growth and photosynthesis. Research indicates that insufficient zinc levels can result in a decrease in chloroplast count, structural damage to chloroplasts, reduced chlorophyll levels, and diminished photosynthetic activity [9]. Inadequate zinc supply can also lower the maximum quantum efficiency of PSII, disrupt plant chloroplast ultrastructure, decrease carbonic anhydrase activity, and impair photosynthesis [10, 11]. Moreover, zinc deficiency hampers corn leaf photosynthesis, reduces soluble sugar content in corn leaves, and ultimately impacts yield formation [12]. Rice is one of the main food crops in the Songnen Plain, Zinc deficiency, resulting from saline-sodic soil, can impede rice growth and photosynthesis, leading to reduced rice yield. Hence, it is vital to investigate the potential of zinc supplementation through foreign aid in increasing zinc levels in saline-sodic soil, alleviating saline-sodic stress, and ultimately enhancing rice growth and photosynthesis.
Photosynthesis is the fundamental process behind plant growth and development, as it supplies the necessary materials and energy for their progress [13]. Chlorophyll is a key light-absorbing molecule that provides valuable information about the structure, conformation, and function of the photosynthetic apparatus through its fluorescence [14]. In recent years, rapid chlorophyll a fluorescence assays have gained popularity due to their non-destructive, sensitive, fast, and easy operation [15]. These assays provide detailed information on the status and function of PSII reaction centers, light-trapping antenna complexes, and PSII donor/acceptor side [16]. They have been widely used to analyze crop heat stress [17], saline-sodic stress [18, 19], and other abiotic stress responses [20]. Research has shown that abiotic stress can damage the photosynthetic structure of crop leaves, leading to decreased photophase activity and electron transfer efficiency [21]. This disruption in the balance of reactive oxygen species (ROS) can have a negative impact on crop growth [22]. It has also been found that environmental stress can significantly degrade the D1 protein in plant leaves, causing damage to both the donor and recipient sides of PSII [23]. Furthermore, stress conditions can hinder the uptake of CO2 by crops, while a limited supply of CO2 can obstruct the transport, distribution, and utilization of photosynthates. Research indicates that under saline-sodic stress, the combination of ionic toxicity and osmotic stress can cause stomatal closure in rice leaves, thereby affecting the absorption of carbon dioxide [24]. Studies also have demonstrated that saline-sodic stress can impede the uptake of carbon dioxide by influencing crop PSII, cytochrome (Cyt b6/f) complexes, and electron transfer processes, consequently affecting the production of photosynthetic products [25]. Saline-sodic stress limits light energy in plants, hindering assimilate production and leading to an accumulation of soluble sugars and starch in the source leaves. This accumulation results in feedback inhibition of photosynthesis [26]. Furthermore, saline-sodic stress decreases the carbon content in carbon sink tissues, leading to a reduction in energy supply that impacts crop growth and development [27]. Therefore, enhancing chlorophyll fluorescence parameters in rice under saline-sodic stress and balancing assimilate source-sink conversion are effective strategies for promoting rice growth.
Multiple recent studies have highlighted the significant role of zinc in enhancing plant growth and photosynthesis [28, 29]. However, there is a scarcity of research on the impact of zinc on photosynthetic fluorescence and carbohydrate metabolism in saline-sodic rice. Therefore, this study utilized a NaCl: Na2SO4: Na2CO3: NaHCO3 of 1:9:1:9 to mimic saline-sodic stress conditions [30]. By employing the JIP test in conjunction with chlorophyll fluorescence and the 820 nm technique, the study investigated the effects of zinc supplementation on photosynthetic electron transport chain and non-structural carbohydrate metabolism in rice leaves and roots. Our hypothesis: The addition of exogenous zinc could improve photosynthetic fluorescence parameters in rice under saline-sodic conditions, regulate assimilate source conversion, and ultimately bolster the growth and development of rice.
Material and methods
Experimental design
This study utilized two rice (O.sativa-ssp.japonica) cultivars: 'Changbai 9' (Changbai No. 9 was developed through a breeding program at the Rice Research Institute of Jilin Academy of Agricultural Sciences in Jilin Province. It was created using Jijing 60 as the female parent and Northeast 125 as the male parent. Initially known as Ji 89–45) and 'Tonghe 899' (Tonghe 899 was developed through a breeding program at Tonghua Academy of Agricultural Sciences, using Y348 as the maternal parent and Ji 01–125/Tonghe 830 as the paternal parent. Initially known as Tonghe 10–8019), with the latter being more sensitive to saline-sodic stress. The test materials consisted of uniformly disinfected rice seeds, treated with a 5% sodium hypochlorite solution for 10 min. The seeds were rinsed with deionized water, germinated in the dark at 30 °C for 48 h, followed by growth in light at the same temperature for another 48 h. Subsequently, the germinated seedlings were moved to a 1/2 nutrient solution for 3 days, before being transferred to a full nutrient solution for a period of 3 weeks. For the upcoming treatments, select rice seedlings that exhibit consistent growth. These treatments include the control group (CT), the zinc alone treatment (Z), the saline-sodic treatment (S), and the saline-sodic zinc treatment (Z + S). The seedlings were subjected to saline-sodic and zinc treatment for 7 days before being sampled for determination. The hydroponic experiment was conducted in a controlled environment room with artificial climate conditions. The photoperiod was set at 14 h of light (28 °C) and 10 h of darkness (22 °C). Additionally, the relative humidity was maintained at approximately 70%. The nutrient solution used in this study was prepared based on the Hoagland nutrient solution preparation method. The composition Hoagland full-concentration nutrient solution is detailed in Table 1. To ensure optimal nutrient availability, the solution was refreshed every three days. In this study, a simulated saline-sodic stress was created using a NaCl: Na2SO4: Na2CO3: NaHCO3 ratio of 1:9:1:9, with a saline-sodic concentration of 50 mmol L−1, pH of 8.5, and conductivity of 3673 µs cm−1 [30]. ZnSO4·7H2O serves as the primary source of zinc, with an optimal concentration of 2 μmol L−1. This concentration was determined through previous screening experiments. To maintain a pH of 5.5 in the nutrient solution, we adjusted with 0.2 mol L−1 H2SO4 or 1 mol L−1 KOH every two days.
Sampling and determination
Dry weight and leaf water content
The determination of leaf relative water content (RWC) was conducted according to Machado and Paulsen [31]. After subjecting rice seedlings to salt sodium and zinc treatments for 7 days, fully unfolded leaves were chosen. The leaves were briefly rinsed with deionized water and weighed (FW). Subsequently, the leaves were immersed in a container of clean water and sealed for 24 h. After removing the leaves from the water, the surface moisture was wiped off and they were weighed again (SW). Finally, the blades were dried at 80 °C until a constant weight was achieved, and the weight was recorded as DW. This entire process was repeated five times for each treatment. The relative water content (RWC) was calculated using the following formula:
Determination of chlorophyll content
The method for determining pigment is based on the Gao study [32]. To extract the pigment, a fully unfolded rice leaf is taken, weighing 0.5 g, and placed into a mixture of 15 ml acetone and ethanol (v:v = 1:1). The mixture containing rice leaves is then kept in the dark at 25℃ for 24 h. Subsequently, due to the absorption peaks of chlorophyll a, chlorophyll b and carotenoids at 645 nm and 663 nm and 470 nm. The absorbance values of chlorophyll a, chlorophyll b, and carotenoids at 645 nm and 663 nm and 470 nm were determined using an ultraviolet spectrophotometer (UV-2600, Shimadsu, Japan). The content of chlorophyll a, chlorophyll b, and carotenoids was calculated using the following formula: Subsequently, the absorbance values at 470 nm, 645 nm, and 663 nm are measured using an ultraviolet spectrophotometer (UV-2600, Shimadzu, Japan) to determine the pigment content. The calculation formula used is as follows:
Determination of gas exchange parameters
The net photosynthetic rate (Pn), stomatal conductance (Gs), and intercellular carbon dioxide concentration of the first fully unfolded leaf of rice plants were measured under various treatments using the portable photosynthetic measurement system Li-6400 (Li-Cor Inc, USA). These measurements were taken between 9:00–11:00 am, seven days after saline-sodic and zinc treatments were applied. For each treatment, 3 rice seedlings with uniform growth were selected for measurement to ensure accuracy. The leaf chamber's temperature was maintained at approximately 26 °C, and the light intensity was set at 800 μmol·m−2 s−1. Additionally, the CO2 concentration was kept at 400 μmol mol−1, and the relative humidity ranged between 60 to 70% during the measurements.
Chlorophyll a fluorescence transient and 820 nm reflection
After subjecting the rice leaves to 7 days of saline-sodic stress and zinc treatment, their chlorophyll fluorescence was measured. For each treatment, 9 rice seedlings with uniform growth were selected for measurement to ensure accuracy. The leaves with uniform growth in each treatment were dark-adapted for 30 min. An M-PEA fluorometer (Hansatech, UK) was utilized to measure the Chl a fluorescence rise kinetics OJIP curve under 1 s pulsed continuous red light (3000 μmol (photons) m−2 s−1). The fluorescence data were recorded at varying sampling rates: from 0.01 to 0.3 ms, data recorded every 10 μs; from 0.3 to 3 ms, data recorded every 100 μs; from 3 to 30 ms, data recorded every 1 ms; from 30 to 300 ms, data recorded every 10 ms; from 300 to 1000 ms, data recorded every 100 ms [33]. Subsequently, the data were analyzed using the JIP test. Fast fluorescence curve OJIP calculations were also conducted: OP normalized Vt = (Ft − FO)/(FM − FO), OJ normalized WOJ = (Ft − FO)/(FJ − FO), OI normalized WOI = (Ft − FO)/(FI − FO). The definitions and calculation formulas for each parameter can be found in Table 2.
Meanwhile using M-PEA (Hansatech, UK), we conducted a red pulse of 3000 μmol (photon) m−2 s−1 for 2 s to generate a modulated reflection curve at 820 nm and analyzed the rise kinetics. In our measurement setup, we applied a sequence of light pulses including a 1-s red pulse (3000 μmol m−2 s−1), a 10-s far-red pulse, and another 1-s red pulse (3000 μmol m−2 s−1) to trigger electron transfer. The reflectance measured around 820 nm corresponds to the redox status of PC+ and P700+ indicating the activity of PSI. The ratio MR/MR0, where MR0 represents the signal value at the start of the actinic illumination (0.7 ms, the first reliable MR measurement), is used to track the oxidation of PSI carriers leading to the formation of PC+ and P700+ (Vox), as well as the reduction of PC+ and P700+ (Vred). [34]. Parameters like MRfast/MR0, MRslow/MR0, VOX, and Vred are further detailed in Table 2 for reference.
Determination of soluble sugars, starches and related metabolic enzymes
After subjecting the rice plants to 7 days of salt, sodium, and zinc treatment, we carefully selected six plants with similar growth for further analysis. To begin, we collected the leaves and roots from three rice seedlings and subjected them to a heat treatment at 105 °C for 30 min. Following this, the samples from each section were dried and weighed at 80 °C. Subsequently, the samples were crushed and passed through a 100-mesh sieve before being saved for testing. We determined the sucrose and fructose content using the resorcinol colorimetric method [35]. Additionally, we determined the starch content in both the rice leaves and roots using anthrone colorimetry [31]. The remaining three rice seedlings' leaves and roots were frozen in liquid nitrogen and stored at -80 °C for enzyme activity analysis. Specifically, we analyzed the activity of invertase (acidic and neutral), sucrose synthetase, sucrose phosphate synthetase, ADP-glucose pyrophosphorylase, and amylase. The enzyme activity analysis was carried out using kits provided by the company (Shanghai Enzyme Linked Biotechnology Co., LTD., China.)
Statistical analyses
All data were collected and analyzed using Microsoft Excel 2019 software. Subsequently, the data were analyzed using the SPSS statistical package version 22 (IBM Corp., Armonk, NY, USA). Descriptive statistics were employed to test the mean value and standard error of measurement parameters. One-way analysis of variance (ANOVA) was conducted in this study, and Duncan's multiple comparison method was utilized. The observed differences in comparisons were found to be statistically significant (p < 0.05). The results are presented as standard error (SE). The charts were generated using Origin 2021 software.
Results
Plant growth
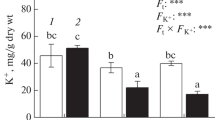
It can be seen from Fig. 1 that under non-stress treatment, adding zinc had no significant effect on the aboveground dry weight of the two varieties, root dry weight and leaf relative water content of 'Changbai 9', but significantly increased the root dry weight and relative water content of 'Tonghe 899'. In comparison to CT treatment, the application of saline-sodic stress resulted in a significant decrease in the above-ground and root dry weight, as well as the relative leaf water content of both rice varieties. Conversely, the addition of zinc had a positive impact on the biomass and relative water content of the two rice varieties.
Effect of zinc on dry weight of leaves (A) and roots (B) and relative water content of leaves (C) of rice seedlings under saline-sodic stress. Different letters represent the significant differences between treatments at p ≤ 0.05. Values are mean ± SE (n = 3). CT: no saline-sodic and no zinc treatment; Z: zinc treatment; S: saline-sodic treatment; Z + S: saline-sodic and zinc treatment. CB: Changbai 9; TH: Tonghe 899
Photosynthetic pigment and gas exchange parameters
Figure 2 illustrates that in non-stress conditions, the addition of zinc notably enhances chlorophyll, carotenoid content, net photosynthetic rate, intercellular carbon dioxide, and stomatal conductance in 'Changbai 9' of the two varieties. However, the effect of zinc on 'Tonghe 899' There was no significant effect on stomatal conductance. When exposed to saline-sodic stress, both varieties experienced a significant reduction in chlorophyll, carotenoid content, and gas exchange parameters. The external application of zinc can mitigate saline-sodic stress and substantially increase pigment content and gas exchange parameters in rice leaves. Therefore, adding zinc under saline-sodic stress conditions aids in the synthesis of chloroplast pigments in rice leaves, diminishes chlorophyll and carotenoid degradation, and enhances gas exchange parameters.
Effect of zinc on chlorophyll a (A), chlorophyll b (B), and carotenoid (C) contents, as well as on the net photosynthetic rate (D), intercellular carbon dioxide concentration (E), and stomatal conductance (F) of rice leaves under conditions of saline-sodic stress. Different letters represent the significant differences between treatments at p ≤ 0.05. Values are mean ± SE (n = 3). CT: no saline-sodic and no zinc treatment; Z: zinc treatment; S: saline-sodic treatment; Z + S: saline-sodic and zinc treatment. CB: Changbai 9; TH: Tonghe 899
The relative variable fluorescence V t, the O—J phase and The O–I phase
Figure 3 (A, D) displays the OJIP (chlorophyll fluorescence induction kinetics) curve of rice. In the absence of stress, the J and I points in the OJIP curves of both rice cultivars were reduced by zinc. Conversely, saline-sodic stress heightened the J and I points in the OJIP curves of both rice varieties. However, the application of zinc mitigated the saline-sodic stress and decreased the J and I points in the OJIP curves. When exposed to saline-sodic stress, both varieties displayed a shift in fluorescence rising kinetics from OJIP to OKJIP. The ΔVt (Fig. 3a,d) measurement of 'Tonghe 899' was lower under the same treatment, indicating that it was more susceptible to salinity than 'Changbai 9'. The application of zinc resulted in a decrease of K, J, and I points under saline-sodic stress, with significant changes observed in K and J points.
Effect of zinc on the Vt (A, D), O-J (B, E), and O-I (C, F) phases of variable chlorophyll a fluorescence in two rice varieties 'Changbai 9' (A, B, C) and 'Tonghe 899' (D, E, F) under saline-sodic stress. Values are mean ± SE (n = 9). CT: no saline-sodic and no zinc treatment; Z: zinc treatment; S: saline-sodic treatment; Z + S: saline-sodic and zinc treatment
The analysis of Fig. 3B,E involves the double standardization of K-step O-J phases. The K step, a new intermediate step occurring at 300 μs, is often attributed to limitations in electron transfer on the donor side of PSII. This study observed that the O-J curve of rice leaves treated with saline-sodic was higher than that of leaves treated with saline-sodic and zinc combination. This suggests that saline-sodic treatment impairs the activity of the oxygen extraction complex (OEC), while zinc application enhances the activity of the oxygen extraction complex (OEC). Furthermore, the 'Tonghe 899' variety exhibited a more pronounced effect compared to the 'Changbai 9' variety (Fig. 3B,E). Phase O-I analysis revealed a consistent trend between the two rice varieties, as depicted in Fig. 3C,F. The amplitude of the I-P phase in the WOI ≥ 1 component indicates the magnitude of the terminal electron acceptor pool on the PSI acceptor side. A smaller amplitude signifies a smaller terminal electron acceptor pool on the PSI acceptor side. The findings demonstrated that saline-sodic stress reduced the size of the receptor pool, whereas exogenous zinc increased its size.
The JIP parameters estimating the quantum yields, efficiencies and Probabilities
To assess the level of K-step variation in the OJIP curve, we determined the normalized relative variable fluorescence (WK) of K-step. As depicted in Fig. 4A,B, the WK value of 'Changbai 9' and 'Tonghe 899' decreased by 17.82% and 13.66%, when subjected to saline-sodic and zinc treatment compared to saline-sodic treatment. Additionally, VJ values were calculated to evaluate the connectivity and receptor-side properties of PSII components. Saline-sodic and zinc treatment resulted in decreased VJ values. In comparison to the S treatment, the VJ values of 'Changbai 9' and 'Tonghe 899' showed a significant increase of 26.27% and 23.45% respectively under the Z + S treatment.
Effects of zinc on quantum yield and specific energy flux of PSII reaction center of two rice varieties 'Changbai 9' (A) and 'Tonghe 899' (B), and energy conservation performance index (C, D) of PSII in two rice varieties (Changbai 9, Tonghe 899) under saline-sodic stress. Fig. E presents the correlation analysis of PIABS, VJ, WK, φDo, φPo, ψEo, φEo, φRo, δRo, ABS/RC, DI0/RC and TR0/RC. * indicates significant correlation at P < 0.05, ** indicates significant correlation at P < 0.01. φPo, maximum quantum yield for primary photochemistry; ψEo, probability that an electron moves further than QA; φEo, quantum yield for electron transport (ET); φDo, quantum yield (at t = 0) of energy dissipation; φDo, quantum yield (at t = 0) of energy dissipation; φRo, quantum yield for reduction of the end electron acceptors at the PSI acceptor side (RE); δRo, probability that an electron is transported from the reduced intersystem electron acceptors to the final electron acceptors of PSI (RE); ABS/RC, Absorbed photon flux per active PSII; TR0/RC, Trapped energy flux per active PSII; DI0/RC, Dissipated energy (as heat and fluorescence) flux per active PSII; ET0/RC, Electron flux from QA‒ to the PQ pool per active PSII; RE0/RC, Electron flux from QA‒ to the final electron acceptors of PSI per active PSII. Values are mean ± SE (n = 9). CT: no saline-sodic and no zinc treatment; Z: zinc treatment; S: saline-sodic treatment; Z + S: saline-sodic and zinc treatment
We also assessed the quantum yield and various relevant parameters of rice seedlings exposed to saline-sodic stress. The results depicted in Fig. 4A, B indicate that under saline-sodic stress conditions, the energy dissipation quantum yield (t = 0) φDo increases, while the quantum yield per unit ABS (φPo) decreases. Furthermore, the introduction of zinc leads to an enhancement in the quantum yield values of φPo, φEo, φRo, δRo, and ψEo per unit ABS, along with a reduction in the energy dissipation quantum yield φDo. The results presented in Fig. 4C,D demonstrate that the PIABS index of the two rice varieties increased significantly with the addition of zinc when no stress was present. However, saline-sodic stress led to a notable reduction in the PIABS index of both varieties. Fortunately, the application of zinc helped in restoring the PIABS activity index in both varieties.
Under saline-sodic stress conditions, ABS/RC, DI0/RC, and TR0/RC increased (Fig. 4A,B), indicating that the unit reaction center absorbed more energy primarily for capture and heat dissipation, with less energy being transmitted downstream. Interestingly, the introduction of zinc led to a significant decrease in ABS/RC, DI0/RC, and TR0/RC, resulting in a more balanced energy flux. The positive impact of zinc on energy absorption, transfer, and transmission in the reaction center under saline-sodic stress is evident.
According to the findings presented in Fig. 4E, there is a positive correlation between PIABS and φPo, ψEo, φEo, φRo, and δRo. On the other hand, PIABS is negatively correlated with VJ, WK, φDo, ABS/RC, DI0/RC, and TR0/RC. Therefore, zinc effectively enhances the connectivity and receptor-side properties of PSII components, which is beneficial for improving the quantum yield and efficiency of rice under saline-sodic stress. This results in a reduction of the energy dissipation ratio and improvement in photosynthetic fluorescence performance indicators.
The reflection at 820 nm
MR/MR0 values were determined using 820 nm red light reflection technology and double standardization. In this study (Fig. 5A,D), it was observed that exogenous zinc supplementation reduced the minimum MR/MR0 values of the two rice varieties under non-stress treatment. The minimum MR/MR0 value of the rice varieties was higher than that of the control group (CT) under saline-sodic stress, but zinc application decreased the minimum MR/MR0 value. This indicates that saline-sodic stress slow down the oxidation rate of P700 and PC, while the addition of foreign zinc accelerates the oxidation rate of P700 and PC. ΔMRfast/MR0 and ΔMRslow/MR0 represent the amplitude of variation from MR0 to MRmin and MRmin to MRmax(Fig. 5B,E), respectively, for the determination of Vox and Vred. Vox and Vred are defined as the slopes of two different phases in a curve. Vox represents the slope from MR0 to the rapid descent stage of MRmin, and Vred represents the slope from the slow ascent stage of MRmin to MRmax. The results of Fig. 5C,F show that, there was no significant change in the VOX and Vred values of the two rice varieties after adding zinc under no stress condition. Under saline-sodic stress, the VOX and Vred values of the two rice varieties decreased significantly. However, the addition of zinc exogenously led to a significant increase in VOX and Vred values even under saline-sodic stress. The results demonstrated that saline-sodic stress inhibited the rates of oxidation and reduction of P700 and PC. However, the application of zinc alleviated the reduction in the rates of reoxidation and reduction of P700 and PC.
Normalized modulated 820 nm reflectance kinetics (MR0 = MR0.7 ms) of zinc on 'Changbai 9' rice variety(A, B) and 'Tonghe 899' rice variety(D, E) under saline-sodic stress. (B, E) the amplitudes of the fast phase (ΔMRfast/MR0) and the slow phase (ΔMRslow/MR0). (C, F) Vox, the slope of the fast descending phase (MR0 to MRmin) and Vred, the slope of the slow ascending phase (MRmin to MRmax). Values are mean ± SE (n = 9). CT: no saline-sodic and no zinc treatment; Z: zinc treatment; S: saline-sodic treatment; Z + S: saline-sodic and zinc treatment. CB: Changbai 9; TH: Tonghe 899
The contents of carbohydrate
The results presented in Fig. 6 indicate that zinc did not have a significant impact on fructose and starch levels in the leaves and roots of the two varieties under normal conditions. However, saline-sodic stress did lead to significant changes in sucrose, fructose, and starch contents in the leaves of both varieties. Interestingly, the addition of zinc helped alleviate this effect, resulting in a decrease in sucrose, fructose, and starch levels. Specifically, compared to the S + Z treatment, the sucrose content of 'Changbai 9' and 'Tonghe 899' decreased by 33.7% and 46.4%, respectively. Furthermore, under the S + Z treatment, the starch content of 'Changbai 9' and 'Tonghe 899' decreased by 17.7% and 8.2%, respectively. It is worth noting that the ratio of sucrose to starch in both varieties increased under saline-sodic stress, but zinc application significantly reduced this proportion. Furthermore under saline-sodic stress, there was a significant increase in the content of sucrose and fructose in the roots of the two rice varieties, while the starch content decreased. The addition of zinc resulted in a decrease in sucrose and fructose content and the sucrose to starch ratio in the root of 'Changbai 9'. However, zinc led to a significant increase in the starch content of the root system of 'Changbai 9'. In addition, the root systems of 'Tonghe 899' responded differently to zinc, with an increase in sucrose and fructose content and the sucrose to starch ratio, but a significant decrease in starch content for 'Tonghe 899'. Comparatively, the addition of zinc (S + Z) increased the starch content of 'Changbai 9' and 'Tonghe 899' by 17.6% and 14.2% respectively, when compared to the S treatment.
Effects of zinc on sucrose (A, D), fructose (B, E) and starch contents (C, F) and ratio of sucrose to starch contents (c, f) in rice leaves (A, B, C, c) and roots (D, E, F, f) under saline-sodic stress. Different letters represent the significant differences between treatments at p ≤ 0.05. Values are mean ± SE (n = 3). CT: no saline-sodic and no zinc treatment; Z: zinc treatment; S: saline-sodic treatment; Z + S: saline-sodic and zinc treatment. CB: Changbai 9; TH: Tonghe 899
The carbohydrate metabolism enzyme activity
Figure 7A shows the effect of zinc on carbohydrate-metabolizing enzymes in the leaves of two rice varieties. Sucrose phosphate synthase is the primary enzyme responsible for catalyzing sucrose synthesis within the cytoplasm. Conversely, sucrose degradation is predominantly facilitated by sucrose synthase and invertase. While sucrose synthase has the ability to both synthesize and break down sucrose, it typically functions primarily in the decomposition of sucrose. ADP-glucose pyrophosphorylase and amylase are crucial enzymes involved in starch synthesis and degradation, respectively. It is evident that the invertase activity of both varieties was significantly increased with zinc supplementation under non-stress treatment. However, the sucrose phosphate synthetase activity was observed to decrease. When subjected to saline-sodic stress, the activities of sucrose synthetase, sucrose phosphate synthetase, ADP-glucose pyrophosphorylase, and amylase in the leaves of both cultivars were notably higher compared to CT. However, the acid invertase activity of 'Changbai 9' and the neutral invertase activity of 'Tonghe 899' were observed to decrease. The invertase activities of both cultivars subjected to saline-sodic stress were significantly increased with the addition of zinc. However, the activities of sucrose phosphate synthetase, ADP-glucose pyrophosphorylase, and amylase were decreased.
Effect of zinc on carbohydrate metabolism enzyme activity of rice leaves (A) and roots (B) under saline-sodic stress. Different letters represent the significant differences between treatments at p ≤ 0.05. Values are mean ± SE (n = 3). CT: no saline-sodic and no zinc treatment; Z: zinc treatment; S: saline-sodic treatment; Z + S: saline-sodic and zinc treatment. CB: Changbai 9; TH: Tonghe 899
Figure 7B shows the effect of zinc on carbohydrate-metabolizing enzymes in the roots of two rice varieties. the addition of zinc from an external source considerably decreased the activity of sucrose synthase in both rice varieties when not subjected to stress. when exposed to saline-sodic stress, the roots of the two rice varieties exhibited a significant increase in the activities of invertase, sucrose synthetase, sucrose phosphate synthetase, and amylase. When exposed to saline-sodic stress, the addition of zinc from an external source led to a significant increase in the activities of starch synthetase in the root system of 'Changbai 9'. However, the activities of invertase, sucrose synthetase, and sucrose phosphate synthetase decreased. Meanwhile, the addition of zinc also resulted in an increase in the activities of invertase and sucrose phosphate synthetase, but a decrease in the activities of sucrose synthetase and amylase in the root system of 'Tonghe 899'.
Discussion
Effects of zinc on growth, chlorophyll content and gas exchange parameters of rice under saline-sodic stress
Biomass serves as a reliable indicator of how plants react to abiotic stress environments, such as salinity [36]. Various abiotic stresses, including salinity, can impede crop growth by restricting photosynthesis [37,38,39]. Research indicates that saline-sodic stress leverages light energy to trigger the excessive production of reactive oxygen species, leading to chlorophyll breakdown and further constraining photosynthesis and rice development [40]. Additionally, saline-sodic stress disrupts rice photosynthesis by compromising the integrity of the plasma membrane [41]. Consistent with previous findings, our study demonstrates that saline-sodic stress significantly impairs net photosynthesis, stomatal conductance, and intercellular carbon dioxide concentration in rice leaves (Fig. 2D,E,F). The application of zinc has been shown to mitigate saline-sodic stress, resulting in a notable improvement in the aforementioned parameters of rice leaves (Fig. 2). This highlights the significance of zinc in enhancing rice resilience under saline-sodic stress. Zinc not only alleviates saline-sodic stress but also enhances the integrity of the plasma membrane [41]. It also helps maintain high concentrations of potassium in protective cells, improves stomatal conductance of rice leaves, and promotes photosynthesis of rice in saline-sodic land [42]. Additionally, zinc, as a vital component of chloroplasts, plays a key role in the formation and function of chlorophyll [43]. Our study further validates these findings, showing that zinc application benefits pigment synthesis in rice leaves under saline-sodic stress (Fig. 2A,B,C), facilitating rice photosynthesis. Consequently, zinc can enhance rice's tolerance to saline-sodic stress, improve photosynthesis, and ultimately promote rice growth (Fig. 1).
Effects of zinc on chlorophyll fluorescence in rice under saline-sodic stress
Chlorophyll fluorescence is a widely used method to assess plant photosynthetic performance [44, 45]. This research employed rapid chlorophyll fluorescence kinetics technology and JIP-text analysis to investigate the impact of zinc on chlorophyll fluorescence in two rice varieties under saline-sodic stress conditions. Previous studies have indicated that saline-sodic stress leads to a decrease in the efficiency of the PSII reaction center and significant changes in chlorophyll fluorescence parameters [25], aligning with the findings of this study. Figure 3A,B illustrates that the J-step and I-step in the OJIP curves of both rice varieties increased under saline-sodic stress. However, the application of zinc in this study resulted in a reduction of both J-step and I-step in the OJIP standard curve for both varieties, with a more pronounced change observed in the J-step. This may be that under saline-sodic stress, the destruction of the PSII reaction center D1 protein may lead to a decrease in electron flow from the plastoquinone pool of PSII and an imbalance in the reoxidation of the plastoquinone pool due to PSI activity. Studies have shown that zinc plays a crucial role in energy transport, protein synthesis, protecting protein structure, and maintaining cell membrane integrity [46]. Therefore, exogenous zinc application has been found to repair damage caused by saline-sodic stress to the D1 protein, facilitating electron transfer from QA to the secondary quinone receptor QB and aiding in the maintenance of quinone pool reduction equilibrium between the two photosystems [47]. Additionally, Li et al. discovered that the degradation of PSII protein under saline-sodic stress conditions is a consequence of excessive reactive oxygen species accumulation, particularly under saline-sodic stress [48]. However, the application of zinc has been shown to alleviate saline-sodic stress, decrease reactive oxygen species accumulation [41], and reduce PSII protein degradation, leading to improved chlorophyll fluorescence in rice leaves. Furthermore, this study observed a distinct intermediate 'K' step at around 300 ms under saline-sodic stress in both varieties, with the 'Tonghe 899' saline-sodic tolerant variety exhibiting a more pronounced magnitude of this step. Research indicates that the emergence of the 'K' step may be attributed to the delay in Oxygen-Evolving Complex (OEC) transferring electrons to oxidized chlorophyll during saline-sodic stress, leading to an electron transfer imbalance between PSII donors and acceptors. However, the introduction of external zinc has been observed to diminish the 'K' step, suggesting that zinc has the potential to mitigate saline-sodic stress, safeguard the OEC, and reinstate the equilibrium in electron transfer between PSII donors and acceptors.
PIABS serves as a valuable standard for evaluating crop stress damage due to its high sensitivity and ability to detect various stress conditions [49]. The study demonstrated that saline-sodic led to a decrease in PIABS content in rice leaves, while zinc treatment increased it. PIABS composed of three components: RC/ABS, φPo, and ψEo [18]. Consistent with Demetriou findings, our study revealed a significant positive correlation between PIABS and φPo and ψEo, as well as a significant negative correlation with ABS/RC (Fig. 4). Figure 4A,B illustrated that both rice varieties exhibited a decrease in φPo and an increase in φDo under saline-sodic stress. Li similarly observed a decrease in photochemical reaction efficiency (φPo) under saline-sodic stress conditions. This decrease resulted in an increase in energy dissipation, including heat, fluorescence, and energy transfer to other systems [50]. The application of zinc reduces energy dissipation, increases φPo, and decreases φDo. This could be attributed to zinc's role in the electron transport chain, where higher zinc levels improve electron acceptor efficiency and facilitate electron transport between PSI and PSII [51, 52]. Under zinc deficiency conditions, energy is dissipated as heat, leading to φDo production. Furthermore, ψEo reflects the likelihood of electrons moving beyond QA−. Our study revealed that saline-sodic stress decreased the excitation pressure of PSII (ψEo), whereas zinc increased it. Furthermore, PSII exhibited similar changes in quantum yields for ψEo and φEo, as illustrated in Fig. 4D. These alterations could be associated with variations in the VJ (Fig. 4A,B). It's worth noting that VJ has a significant negative correlation with ψEo and φEo. An increase in VJ was linked to damage on both sides of the PSII donor and recipient under saline-sodic stress. And the rise in VJ was attributed to a decrease in QA and plastoquinone (PQ) [45]. Moreover, Fig. 4A,B illustrates that reductions in φPo, φEo, and φRo under saline-sodic stress suggest that absorbed light energy is primarily utilized for capture (TR0/RC) and dissipation (DI0/RC), resulting in a decline in the probability of PSI final electron acceptor (δRo). Exogenous zinc application can enhance electron acceptor efficiency in the electron transport chain, facilitating electron transfer between PSI and PSII, thereby boosting the likelihood of electron transfer and PSI final electron acceptor (δRo).
The kinetics of photoinduced 820 nm reflection (MR/MR0) can be utilized to identify the buildup of P700 in the PSI reaction center [30], as well as the subsequent rereduction of PC+and P700+ by electrons that were initially captured by P680 [53]. This study demonstrated that saline-sodic stress significantly impacted the oxidation–reduction rate of PC and P700, whereas the addition of external zinc enhanced the oxidation–reduction rate of PC and P700 (Fig. 5A,D). Additionally, to further assess the redox rates of PC and P700 using the MR820 signal, we calculated the values of ΔMRfast/MR0 and ΔMRslow/MR0 (Fig. 5B,E) as well as the values of Vox and Vred (Fig. 5C,F). The study revealed that saline-sodic stress decreased the ratios of △MRfast/MR0 and △MRslow/MR0, along with the values of Vox and Vred. Conversely, the application of zinc led to an increase in the ratios of △MRfast/MR0 and △MRslow/MR0, as well as the values of Vox and Vred. These findings suggest that zinc supplementation positively impacts the oxidation of PC and P700, or the re-reduction of PC+ and P700+. This effect may be attributed to zinc's ability to enhance electron flow through PSI, thereby accelerating the redox rates of PC and P700 (Fig. 3C,F), the maximum amplitude of WOI ≥ 1 is indicative of the terminal electron acceptor pool size on the PSI receptor side. Zinc administration has been shown to increase the size of this pool. Additionally, the study also show that exogenous zinc application enhances electron acceptor efficiency (δRo) in the electron transport chain (Fig. 4A,B). This improvement facilitates electron transfer between PSI and PSII, leading to enhanced efficiency of PC and P700 oxidation–reduction rates.
As shown in Fig. 8, saline-sodic stress negatively impacts chlorophyll synthesis and the electron transport chain between PSI and PSII in rice leaves. Zinc application mitigates damage to the oxygen release complex from saline-sodic stress, enhances performance of PSII donor/acceptor sides and the redox rate of PSI, repairs the photosynthetic electron transport chain, and improves the transfer of QA to QB. These effects contribute to a more balanced energy distribution, ultimately promoting photosynthesis in rice leaves in saline-sodic soil rice areas.
Schematic diagram illustrating the composition of Z-shaped electron transport membrane proteins in the photosynthetic electron transport chain. The protein complex includes PSII, cytochrome b6f complex, PSI, and ATP synthase. Electrons are initially released from water by the oxygen-releasing complex (OEC), then transferred to quinone molecules QA and QB, and further to PSI via quinone and cyanin. Ultimately, these electrons are utilized for ATP synthesis facilitated by ATP synthase. Under saline-sodic stress, the OEC is significantly impaired, leading to inhibition of the electron acceptor and electron donor of PSII and PSI. Nonetheless, the addition of zinc supplement mitigates the saline-sodic stress, diminishes the damage to the OEC, enhances the electron acceptor and electron donor of PSII and PSI, and facilitates electron transfer. Consequently, the photosynthetic efficiency of rice leaves in saline-sodic soil is enhanced. Fdx, Ferredoxin. FNR, Ferredoxin NADP+ reductase
Effects of zinc on carbohydrate metabolism in rice under saline-sodic stress
Photosynthesis is a process that supplies plants with a sufficient carbon source, which in turn promotes the growth and development of crops. However, under saline-sodic stress, plants experience significant inhibition in their ability to absorb and utilize light energy, resulting in impaired photosynthetic capacity [54]. This study discovered that zinc did not have a significant impact on the sucrose, fructose, and starch levels in rice leaves under non-stress conditions. However, when exposed to saline-sodic stress, there was a notable increase in the carbohydrate content in the leaves. Saline-sodic stress impacts the production, transport, distribution, and utilization of sucrose, leading to the buildup of soluble sugars and starches in the source leaves, aligning with Richter's previous research [54]. The application of zinc was found to alleviate these effects by decreasing the levels of sucrose, fructose, and starch in the leaves. This, in conjunction with zinc, could mitigate saline-sodic stress, lower the presence of reactive oxygen species in the source leaves, and enhance the generation of triose phosphate during photosynthesis, thereby facilitating the carbon cycle. On the other hand, sucrose phosphate synthetase serves as the primary enzyme responsible for catalyzing sucrose synthesis in the plant cytoplasm. Additionally, ADP-glucose pyrophosphorylase and amylase are crucial enzymes that play a key role in starch synthesis and degradation, respectively [55]. In this study, in the absence of stress, the addition of exogenous zinc led to a notable increase in invertase activity, while sucrose phosphate synthase activity experienced a significant decrease. Under saline-sodic stress, sucrose phosphate synthetase activity, which promotes sucrose synthesis, increases, while the activity of acidic invertase and neutral invertase, which promote sucrose decomposition, decreases. It is important to note that sucrose synthase activity, which catalyzes sucrose decomposition, increases under saline-sodic stress. Despite this increase, the sucrose content in the leaves did not decrease, possibly because sucrose is being synthesized at a faster rate than it is being broken down. As shown in Fig. 9, sucrose content was significantly positively correlated with sucrose phosphate synthase activity, further supporting this view. Simultaneously, the study revealed that rice leaves experienced a significant increase in starch content under saline-sodic stress, while the application of zinc resulted in a decrease in starch content. This decrease was attributed to the reduced activity of ADP-glucose pyrophosphorylase. Furthermore, the introduction of zinc led to a notable decrease in the sucrose/starch ratio in rice leaves when subjected to saline-sodic stress. This suggests that zinc supplementation could enhance the conversion of sucrose into starch within rice leaves, ultimately aiding in mitigating the transport disruption of photocontracted products caused by the saline-sodic stress [56].
To ensure normal growth in the presence of saline-sodic stress, plants must effectively regulate the transportation and distribution of carbohydrates, as well as their utilization in the reservoir organs [56]. In the context of saline-sodic stress, the roots of plants are the first and most significantly impacted components [57]. This stressor affects the size of the carbohydrate pool by disrupting the transport and distribution of these important nutrients [56]. The study determined that zinc does not have a substantial impact on the levels of sucrose, fructose, and starch in rice roots under normal conditions. However, Saline-sodic stress had a notable impact on the sucrose content in the root system of 'Changbai 9', leading to an increase. However, the application of zinc decreased the sucrose content, possibly due to the rise in invertase activity and reduction in sucrose phosphate synthetase activity. Although sucrose synthetase activity decreased, the accumulation of sucrose indicated that sucrose decomposition exceeded sucrose synthesis. The sucrose content of 'Tonghe 899' root was found to increase significantly under saline-sodic stress, and this was further augmented by the application of zinc. In this study, saline-sodic stress was found to significantly reduce the starch content in the roots of two rice varieties. However, the application of zinc was able to increase the root starch content in both varieties. This effect can be attributed to the higher rate of starch synthesis in rice roots compared to starch decomposition, thereby facilitating starch accumulation. Additionally, the utilization of zinc aids in the transportation of assimilated products from the source leaf to the underground section, thereby promoting the conversion of sucrose into starch within the root system. When plants are exposed to saline-sodic stress, an increase in root starch content can be advantageous. This increase not only helps plants resist stress but also provides more energy storage for them during times of stress [19]. It helps to maintain normal growth ability. Furthermore, the addition of zinc helps to promote the transport of photosynthates from source leaves to roots (reservoir) in rice, particularly under saline-sodic stress. This leads to an increase in the starch content found in rice roots.
In summary, saline-sodic stress significantly impacts the transport and distribution of photosynthetic products in rice leaves, leading to their accumulation in the source leaves and consequent feedback inhibition of photosynthesis. The addition of zinc, however, mitigates the accumulation of soluble sugar and starch in leaves, facilitating the transport of assimilation products to underground parts. This not only enhances rice photosynthesis but also boosts the plant's resilience saline-sodic stress.
In short, zinc deficiency is a well-known issue in saline-sodic rice fields, but the application of exogenous zinc effectively addresses this issue. Figure 10 illustrates that introducing exogenous zinc in saline-sodic rice areas not only resolves the lack of available zinc but also mitigates the damage inflicted by saline-sodic stress on rice plants. This intervention not only aids in pigment synthesis in rice leaves but also enhances electron transfer in the photosynthetic system, improves carbohydrate metabolism, ultimately influencing the growth and development of rice plants in saline-sodic rice fields.
Conclusion
Photosynthesis is a fundamental process in plant growth and development. Saline-sodic stress can have a detrimental impact on photosynthesis in rice, leading to decreased yield. This study found that zinc had a positive influence on the growth of two rice varieties under saline-sodic stress, with 'Tonghe 899' showing a more pronounced response. When exposed to saline-sodic stress, the application of exogenous zinc enhances the pigment content in rice leaves, improves the performance of the PSII donor/acceptor side (WK and VJ), and increases the redox rate of PSI (Vox and Vred). It also repairs the photosynthetic electron transport chain and promotes the balance of energy distribution. Moreover, zinc application not only decreases the accumulation of soluble sugar and starch in rice leaves in saline-sodic regions, reducing the feedback inhibition of photosynthesis, but also facilitates the transport of assimilation products to the underground parts of the plant. This provides more energy for root growth under saline-sodic stress, enhancing the saline-sodic resistance of rice and promoting the overall growth and development of the plants.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author.
Abbreviations
- ROS :
-
Reactive oxygen species
- RWC :
-
Relative water content
- Pn :
-
Net photosynthetic rate
- Gs :
-
Stomatal conductance
- PQ :
-
Plastoquinone
- OEC :
-
Oxygen extraction complex
- Fdx :
-
Ferredoxin
- FNR :
-
Ferredoxin NADP+ reductase
References
Tedeschi A. Irrigated agriculture on saline soils: a perspective. Agronomy. 2020;10(11):1630.
Yang F, An F, Ma H, Wang Z, Zhou X, Liu Z. Variations on Soil Salinity and Sodicity and Its Driving Factors Analysis under Microtopography in Different Hydrological Conditions. Water. 2016;8(6):227.
Chi CM, Zhao CW, Sun XJ, Wang ZC. Reclamation of saline-sodic soil properties and improvement of rice (Oriza sativa L.) growth and yield using desulfurized gypsum in the west of Songnen Plain, northeast China. Geoderma. 2012;187:24-30.
Guo H, Huang Z, Li M, Hou Z. Growth, ionic homeostasis, and physiological responses of cotton under different salt and alkali stresses. Sci Rep 2020;10(1):21844.
Nazir Q, Wang X, Hussain A, Ditta A, Aimen A, Saleem I, Naveed M, Aziz T, Mustafa A, Panpluem N. Variation in Growth, Physiology, Yield, and Quality of Wheat under the Application of Different Zinc Coated Formulations. Appl Sci. 2021;11(11):4797.
Arnold T, Kirk GJD, Wissuwa M, Frei M, Zhao F-J, Mason TFD, Weiss DJ. Evidence for the mechanisms of zinc uptake by rice using isotope fractionation. Plant Cell Environ. 2009;33(3):370–81.
Misra AK. Evaluation of Zinc Supplying Power of Soils for Rice. Journal of the Indian Society of Soil Science. 1985;33(4):pp. 826-830-1985 v.1933 no.19840.
Guo Z, Guo X, Wang J, Wang D. Occlusive effect of soil aggregates on increased soil DTPA-extractable zinc under low soil pH causedby long-term fertilization. Plant Soil Environ. 2013;59(11):524–9.
Zhang J, Wang S, Song S, Xu F, Pan Y, Wang H. Transcriptomic and proteomic analyses reveal new insight into chlorophyll synthesis and chloroplast structure of maize leaves under zinc deficiency stress. J Proteom. 2019;199:123–34.
Koroidov S, Shevela D, Shutova T, Samuelsson G, Messinger J. Mobile hydrogen carbonate acts as proton acceptor in photosynthetic water oxidation. In: Proceedings of the National Academy of Sciences of the United States of America. 2014;111(17):6299–304 .
Boye M, Adjou MA, Dulaquais G, Tréguer P. Trace metal limitations (Co, Zn) increase PIC/POC ratio in coccolithophore Emiliania huxleyi. Marine Chemistry. 2017;192:22–31.
Li ZT, Yang KJ, Wang YF, Cai XX, Zhang SY. Effect of zinc on C, N metabolism of different varied maize (Zea mays L.) genotype seedlings. Plant Physiology Communications. 2010;46:664–670.
He Y, Duan W, Xue B, Cong X, Sun P, Hou X, Liang Y-K. OsαCA1 Affects Photosynthesis, Yield Potential, and Water Use Efficiency in Rice. Int J Mol Sci 2023;24(6):5560.
Zhang M, Shan Y, Kochian L, Strasser RJ, Chen G: Photochemical properties in flag leaves of a super-high-yielding hybrid rice and a traditional hybrid rice (Oryza sativa L.) probed by chlorophyll a fluorescence transient. Photosynthesis Research 2015, 126(2):275–284.
Ogawa T, Sonoike K. Screening of mutants using chlorophyll fluorescence. J Plant Res. 2021;134(4):653–64.
Smythers AL, Crislip JR, Slone DR, Flinn BB, Chaffins JE, Camp KA, McFeeley EW, Kolling DRJ: Excess manganese increases photosynthetic activity via enhanced reducing center and antenna plasticity in Chlorella vulgaris. Scientific Reports. 2023;13(1):11301.
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci. 2013;14(5):9643–84.
Demetriou G, Neonaki C, Navakoudis E, Kotzabasis K. Salt stress impact on the molecular structure and function of the photosynthetic apparatus–the protective role of polyamines. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2007;1767(4):272–80.
Roccotiello E, Manfredi A, Drava G, Minganti V, Giorgio Mariotti M, Berta G, Cornara L. Zinc tolerance and accumulation in the ferns polypodium cambricum L. and Pteris vittata L. Ecotoxicol Environ Safety. 2010;73(6):1264–71.
Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA, Cetner MD, Łukasik I, Goltsev V, Ladle RJ. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant. 2016;38(4):102.
Qu Y, Sakoda K, Fukayama H, Kondo E, Suzuki Y, Makino A, Terashima I, Yamori W. Overexpression of both Rubisco and Rubisco activase rescues rice photosynthesis and biomass under heat stress. Plant Cell Environ 2021;44(7):2308-2320.
Hu Z, Fan J, Chen K, Amombo E, Chen L, Fu J. Effects of ethylene on photosystem II and antioxidant enzyme activity in Bermuda grass under low temperature. Photosynth Res. 2015;128:59–72.
Hu L, Xiang L, Li S, Zou Z, Hu XH. Beneficial role of spermidine in chlorophyll metabolism and D1 protein content in tomato seedlings under salinity-alkalinity stress. Physiol Plant. 2016;156(4):468–77.
Yang F, Liang Z-W, Wang Z-C, Chen Y. Relationship Between Diurnal Changes of Net Photosynthetic Rate and Influencing Factors in Rice under Saline Sodic Stress. Rice Sci. 2008;15(2):119–24.
Huihui Z, Xin L, Yan-hui C, Yue W, Ma-bo L, Rong-yi Y, Nan X, Guang-yu S. A study on the effects of salinity and pH on PSII function in mulberry seedling leaves under saline–alkali mixed stress. Trees 2020;34:693-706.
Kumar A, Kumar A, Lata C, Kumar S. Eco-physiological responses of Aeluropus lagopoides (grass halophyte) and Suaeda nudiflora (non-grass halophyte) under individual and interactive sodic and salt stress. S Afr J Bot. 2016;105:36–44.
Khelil A, Menu T, Ricard B. Adaptive response to salt involving carbohydrate metabolism in leaves of a salt-sensitive tomato cultivar. Plant Physiol Biochem 2007;45(8):551-9.
Ahmed R, Zia-Ur-Rehman M, Sabir M, Usman M, Rizwan M, Ahmad Z, Alharby HF, Al-Zahrani HS, Alsamadany H, Aldhebiani AY, et al. Differential response of nano zinc sulphate with other conventional sources of Zn in mitigating salinity stress in rice grown on saline-sodic soil. Chemosphere. 2023;327:138479.
Basit A, Hussain S, Abid M, Zafar-ul-Hye M, Ahmed N. Zinc and potassium priming of maize (Zea mays L.) seeds for salt-affected soils. J Plant Nutr. 2020;44(1):130–41.
Gao D, Ran C, Zhang Y, Wang X, Lu S, Geng Y, Guo L, Shao X. Effect of different concentrations of foliar iron fertilizer on chlorophyll fluorescence characteristics of iron-deficient rice seedlings under saline sodic conditions. Plant Physiol Biochem. 2022;185:112–22.
Machado S, Paulsen GM. Combined effects of drought and high temperature on water relations of wheat and sorghum. Plant Soil. 2001;233:179–87.
Hussain S, Huang J, Zhu C, Zhu L, Cao X, Hussain S, Ashraf M, Khaskheli MA, Kong Y, Jin Q, et al. Pyridoxal 5′-phosphate enhances the growth and morpho-physiological characteristics of rice cultivars by mitigating the ethylene accumulation under salinity stress. Plant Physiol Biochem. 2020;154:782–95.
Guo Y, Lu Y, Goltsev V, Strasser RJ, Kalaji HM, Wang H, Wang X, Chen S, Qiang S. Comparative effect of tenuazonic acid, diuron, bentazone, dibromothymoquinone and methyl viologen on the kinetics of Chl a fluorescence rise OJIP and the MR820 signal. Plant Physiol Biochem 2020;156:39-48.
Todorenko D, Timofeev N, Kovalenko I, Kukarskikh G, Matorin D, Antal T. Chromium effects on photosynthetic electron transport in pea (Pisum sativum L.). Planta 2019;251(1):11.
Dong CJ, Wang XL, Shang QM. Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Scientia Horticulturae. 2011;129(4):629-36.
Wang Y, Nii N: Changes in chlorophyll, ribulose bisphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration inAmaranthus tricolorleaves during salt stress. Jo Hortic Sci Biotechnol 2000;75(6):623-7.
Srivastava PC, Bhatt M, Pachauri SP, Tyagi AK. Effect of zinc application methods on apparent utilization efficiency of zinc and phosphorus fertilizers under basmati rice–wheat rotation. Arch Agron Soil Sci. 2013;60(1):33–48.
Weisany W, Sohrabi Y, Heidari G, Siosemardeh A, Badakhshan H. Effects of Zinc Application on Growth, Absorption and Distribution of Mineral Nutrients Under Salinity Stress in Soybean (Glycine MaxL.). J Plant Nutri 2014;37(14):2255-69.
Ashraf MY, Iqbal N, Ashraf M, Akhter J. Modulation of Physiological and Biochemical Metabolites in Salt Stressed Rice by Foliar Application of Zinc. J Plant Nutr 2014;37(3):447-57.
Sudhalakshmi C, Krishnasamy R, Rajarajan A, Meena S: Genotypic Divergence in Expression of Zinc-Deficiency Symptoms of Rice (Oryza sativaL.) in Sand Culture. Commun Soil Sci Plant Anal 2014;45(22):2932-48.
Fatemi H, Carvajal M, Rios JJ. Foliar Application of Zn Alleviates Salt Stress Symptoms of Pak Choi Plants by Activating Water Relations and Glucosinolate Synthesis. Agronomy. 2020;10(10):1528.
Sharma PN, Tripathi A, Bisht SS. Zinc Requirement for Stomatal Opening in Cauliflower. Plant Physiol 1995;107(3):751-756.
Petrovic J, Nikolic G, Markovic D. In vitro complexes of copper and zinc with chlorophyll. J Serb Chem Soc 2006;71(5):501-12.
Percival GC, Henderson A: An assessment of the freezing tolerance of urban trees using chlorophyll fluorescence. J Hortic Sci Biotechnol 2003;78(2):254-60.
Kalaji HM, Oukarroum A, Alexandrov V, Kouzmanova M, Brestic M, Zivcak M, Samborska IA, Cetner MD, Allakhverdiev SI, Goltsev V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol Biochem 2014;81:16-25.
Aravind P, Prasad MNV. Zinc protects chloroplasts and associated photochemical functions in cadmium exposed Ceratophyllum demersum L., a freshwater macrophyte. Plant Science 2004;166(5):1321-7.
Bertamini M, Nedunchezhian N, Borghi B. Effect of Iron Deficiency Induced Changes on Photosynthetic Pigments, Ribulose-1,5-Bisphosphate Carboxylase, and Photosystem Activities in Field Grown Grapevine (Vitis Vinifera L. cv. Pinot Noir) Leaves. Photosynthetica. 2001;39(1):59–65 .
Li YT, Xu WW, Ren BZ, Zhao B, Zhang J, Liu P, Zhang ZS: High temperature reduces photosynthesis in maize leaves by damaging chloroplast ultrastructure and photosystem II. J Agron Crop Sci 2020;206(5):548-64.
Larkum AWD. Chlorophyll a Fluorescence A Signature of Photosynthesis. Phycologia. 2006;45(4):478–9.
Li X, Zhang L: Endophytic infection alleviates Pb(2+) stress effects on photosystem II functioning of Oryza sativa leaves. J Hazard Mater 2015;295:79-85.
Roosta HR, Estaji A, Niknam F. Effect of iron, zinc and manganese shortage-induced change on photosynthetic pigments, some osmoregulators and chlorophyll fluorescence parameters in lettuce. Photosynthetica 2018;56:606-15.
Strasser RJ, Tsimilli-Michael M, Qiang S, Goltsev V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2010;1797(6-7):1313–326.
Gao J, Li P, Ma F, Goltsev V. Photosynthetic performance during leaf expansion in Malus micromalus probed by chlorophyll a fluorescence and modulated 820nm reflection. J Photochem Photobiol B. 2014;137:144–50.
Richter JA, Erban A, Kopka J, Zörb C. Metabolic contribution to salt stress in two maize hybrids with contrasting resistance. Plant Science. 2015;233:107-15.
Wang H, Sui X, Guo J, Wang Z, Cheng J, Ma S, Li X, Zhang Z. Antisense suppression of cucumber (Cucumis sativus L.) sucrose synthase 3 (CsSUS3) reduces hypoxic stress tolerance. Plant Cell Environ. 2013;37(3):795–810.
Balibrea ME, Dell'Amico J, Bolarin MC, Perez-Alfocea F: Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiologia Plantarum. 2000;110(4):503-11.
Li T, Heuvelink E, Marcelis LFM. Quantifying the source-sink balance and carbohydrate content in three tomato cultivars. Front Plant Sci. 2015;6:416.
Acknowledgements
We thank the anonymous referees for their comments and suggestions that led to the improvement of this manuscript.
Funding
We gratefully acknowledge funding support by Science and technology training program of Jilin Province (20230508001RC) and (20230202031NC), National key research and development program (2022YFD1500505), Open Project of the Key Laboratory of Germplasm Innovation and Physiological Ecology of Coldland Grain Crops, Ministry of Education (CXSTOP202202).
Author information
Authors and Affiliations
Contributions
Kun Dang: Conceptualization, Methodology, Software, Data curation, Writing-original draft preparation. Jinmeng Mu, Hao Tian, Dapeng Gao, Hongxiang Zhou: Conceptualization, Methodology, Visualization, Investigation. Yanqiu Geng, Liying Guo, Qiang Zhang: Reviewing and Editing, Supervision. Xiwen Shao: Reviewing and Editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. We have obtained permission to collect plant material and seedlings.
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dang, K., Mu, J., Tian, H. et al. Zinc regulation of chlorophyll fluorescence and carbohydrate metabolism in saline-sodic stressed rice seedlings. BMC Plant Biol 24, 464 (2024). https://doi.org/10.1186/s12870-024-05170-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05170-w