Abstract
Background
Unreduced gamete formation during meiosis plays a critical role in natural polyploidization. However, the unreduced gamete formation mechanisms in Triticum turgidum–Aegilops umbellulata triploid F1 hybrid crosses and the chromsome numbers and compostions in T. turgidum–Ae. umbellulata F2 still not known.
Results
In this study, 11 T.turgidum–Ae. umbellulata triploid F1 hybrid crosses were produced by distant hybridization. All of the triploid F1 hybrids had 21 chromosomes and two basic pathways of meiotic restitution, namely first-division restitution (FDR) and single-division meiosis (SDM). Only FDR was found in six of the 11 crosses, while both FDR and SDM occurred in the remaining five crosses. The chromosome numbers in the 127 selfed F2 seeds from the triploid F1 hybrid plants of 10 crosses (no F2 seeds for STU 16) varied from 35 to 43, and the proportions of euploid and aneuploid F2 plants were 49.61% and 50.39%, respectively. In the aneuploid F2 plants, the frequency of chromosome loss/gain varied among genomes. The chromosome loss of the U genome was the highest (26.77%) among the three genomes, followed by that of the B (22.83%) and A (11.81%) genomes, and the chromosome gain for the A, B, and U genomes was 3.94%, 3.94%, and 1.57%, respectively. Of the 21 chromosomes, 7U (16.54%), 5 A (3.94%), and 1B (9.45%) had the highest loss frequency among the U, A, and B genomes. In addition to chromosome loss, seven chromosomes, namely 1 A, 3 A, 5 A, 6 A, 1B, 1U, and 6U, were gained in the aneuploids.
Conclusion
In the aneuploid F2 plants, the frequency of chromosome loss/gain varied among genomes, chromsomes, and crosses. In addition to variations in chromosome numbers, three types of chromosome translocations including 3UL·2AS, 6UL·1AL, and 4US·6AL were identified in the F2 plants. Furthermore, polymorphic fluorescence in situ hybridization karyotypes for all the U chromosomes were also identified in the F2 plants when compared with the Ae. umbellulata parents. These results provide useful information for our understanding the naturally occurred T. turgidum–Ae. umbellulata amphidiploids.
Similar content being viewed by others
Introduction
Aegilops umbellulata (2n = 2x = 14, UU), as a wild relative of wheat, is a rich gene reservoir for the genetic improvement of wheat in several aspects [1, 2]. It possesses genes of resistance to biotic stresses, including stripe rust and leaf rust [3, 4], and abiotic stresses such as drought and salt tolerance [5]. For example, the leaf rust and stripe rust resistance genes Lr76 and Yr70 of Ae. umbellulata were successfully transferred into wheat [6]. These excellent genes could be introduced into wheat through direct or indirect distant hybridization, although some barriers such as hybrid sterility and abnormal pairing of chromosomes during meiosis still occur during these processes. To introduce the valuable traits/genes of Ae. umbellulata into common wheat via distant hybridization, it is necessary to overcome these hybridization barriers [7, 8]. Compared with direct hybridization with wild relative species, the use of amphidiploids between wheat and wild relative species as a bridge material can overcome these hybridization barriers to a certain extent. The development of amphidiploids depends on chromosome doubling, which can be done either by ionizing irradiation or clastogens induce chromosomal rearrangements, such as X-ray and colchicine, respectively, or via unreduced gametes [9].
Polyploid plants mainly occur through somatic chromosome doubling or through the union of two unreduced gamete formation. Both autopolyploids (e.g., potato) and allopolyploids (e.g., wheat) can be produced via the unreduced gamete pathway [10, 11]. The unreduced gametes can promote the formation of polyploid species and can also produce some intermediate materials for transferring heterologous genetic material. For example, the union of two unreduced gametes in the double haploid F1 hybrids (ABD, 2n = 3x = 21) between tetraploid wheat and Ae. tauschii produced synthetic wheat by spontaneous chromosome doubling (AABBDD, 2n = 6x = 42) [12]. The formation of unreduced gametes is mainly modulated by unreduced gamete genes [13]. Quantitative trait loci (QTLs) that contribute to unreduced gamete formation have been mapped on chromosomes 1 A, 3 A, 3 B, and 4 B of tetraploid wheat [14, 15]. Two types of unreduced gamete formation mechanisms, namely first-division restitution (FDR) and single-division meiosis (SDM), have been discovered in monocotyledonous wheat hybrids [11]. In the FDR, the first meiotic division is abnormal and generates a restitution nucleus, and the second division is normal and produces only dyads [16]. In the SDM, a single equational division of sister chromatids takes place at meiotic anaphase I generates dyads before the restitution nucleus is formed, and leads to the absence of the second meiosis [17]. It has been reported that the chromosome numbers of hybrids between tetraploid or hexaploid wheat and some Aegilops species could be naturally doubled to form amphidiploids through FDR and SDM, such as T. turgidum–Ae. tauschii [9], T. turgidum–Ae. longissimia [18], T. aestivum–Ae. triuncialis [19, 20], and T. turgidum–Ae. comosa hybrids [21]. The T. turgidum–Ae. umbellulata amphidiploids can be formed by doubling the chromosomes of haploid hybrids by colchicine treatment or unreduced gametes [7, 22,23,24] but the unreduced gamete formation process of the spontaneous doubling pathway and the chromosomal changes in the selfed progeny of T. turgidum–Ae. umbellulata hybrids are still unknown.
To study the meiosis processes as well as unreduced gamete formation in T. turgidum–Ae. umbellulata triploid F1 hybrids and to analyze the chromosomal changes in the selfed progeny after natural chromosome doubling, two tetraploid wheats, namely T. turgidum ssp. durum var. Langdon and ssp. dicoccum PI 94,668, were crossed with 10 Ae. umbellulata accessions to obtain 11 T. turgidum–Ae. umbellulata F1 hybrids. The results provide basic information for understanding unreduced gamete formation in T. turgidum–Ae. umbellulata hybrids and could also aid the generation of T. turgidum–Ae. umbellulata amphidiploids for potential use in transferring desirable traits/genes from Ae. umbellulata into wheat.
Results
Generation of T. turgidum–Ae. umbellulata F1hybrid seeds and identification of their chromosomes by in situ hybridization
A total of 30 T. turgidum–Ae. umbellulata triploid F1 plants were obtained. The seed-setting rate of the F1 plants among the 11 crosses ranged from 3.1% (STU 10) to 20.0% (STU 7), with a mean of 8.7% (Table 1). The germination rates of these F1 hybrid seeds ranged from 33.3% (STU 12 and STU 16) to 100.0% (STU 2, STU 8-STU 11, and STU 14) (Table 1). The chromosome numbers in the root tip cells of all the F1 hybrid plants were 2n = 3x = 21, and fluorescence in situ hybridization (FISH) and genomic in situ hybridization (GISH) verified that each root tip cell had a complete set of A, B, and U chromosomes from the diploid Ae. umbellulata and T. turgidum parents (Figure S1).
Meiosis in the T. turgidum–Ae. umbellulata F1 hybrids
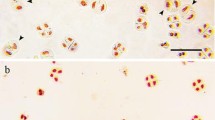
The unreduced gamete formation and chromosome pairing during meiosis at the booting stage in 30 T. turgidum–Ae. umbellulata triploid F1 plants were investigated (Table 1). All 11 triploid F1 hybrid crosses could produce unreduced gametes, either only by FDR or by both FDR and SDM (Table 1). Five crosses (STU 2, STU 7, STU 11, STU 13, and STU 14) produced gametes by both FDR and SDM (Fig. 1A, Figure S2A–D and I–L), and the remaining six combinations (STU 8–STU 10, STU 12, and STU 15 and STU 16) only produced gametes via FDR (Fig. 1B, Figure S2E–H, and M–N). Additionally, some chromosome-specific behaviors during meiosis in the pollen mother cells (PMCs) of the 11 F1 hybrid crosses were also observed, such as lagging chromosomes (Figure S3C–E), chromosome bridges Figure S3F), micronuclei (Figure S3G), and multipolar division (Figure S3H).
Fluorescence in situ hybridization (FISH) (A1–E1; A2–E2) and genome in situ hybridization (GISH) (A3–E3) of Triticum turgidum–Aegilops umbellulata euploids and pseudoeuploids. A1–A3. Euploids; B1–E3. Pseudoeuploids (B1–C3. 1B trisomic and 7U monosomic; D1–D3. 2U, 3U, 4U, 5U, 7U monosomic, 5 A tetrasomic, and 6 A pentasomic; E1–E3. 2 U, 3 U, 4 U, 5 U, 6 U, 7 U monosomic, 5 A tetrasomic, 6 A pentasomic, and one unknown)
The chromosome configurations at metaphase MI in the triploid F1 hybrids were analyzed (Table 1). The chromosome numbers in the PMCs of these F1 hybrid plants were 21. The number of univalent, rod bivalents (II), ring bivalents (II), and trivalents (III) (Figure S3A, B) among the 11 crosses ranged from 14.47 ± 1.03 to 19.71 ± 0.11, 0.59 ± 0.04 to 3.17 ± 0.61, 0 to 0.06 ± 0.05, and 0 to 0.09 ± 0.01, respectively. STU 8 had a significantly higher number of ring bivalents than the other 10 combinations, whereas STU 14 had significantly higher (P < 0.05) univalent (STU 14 vs. other crosses: > 19.5 vs. < 18.0) and lower (P < 0.05) rod bivalents (STU 14 vs. other crosses: < 0.6 vs. > 1.4) than the other 10 crosses. STU 16 had the highest number of chromosome chiasmata and rod bivalents among the 11 F1 hybrid crosses (Table 1).
The T. turgidum–Ae. umbellulata triploid F1 hybrid plants produced 0–128 seeds by selfing, which may be due to the formation of unreduced gametes, resulting in doubling success and fruiting of triploid F1 plants. The selfed seed-set rate of the 11 hybrid crosses ranged from 0 (STU 16) to 13.0% (STU 14), with a mean of 4.7%. The selfed seed-set rates of the T. turgidum–Ae. umbellulata F1 hybrid plants were significantly positively correlated with the number of univalents (r = 0.71*, P < 0.05) and significantly negatively associated with the number of rod II (r = − 0.37*, P < 0.05) and chiasmata (r = − 0.73*, P < 0.05).
Chromosome numbers and compositions in the selfed seeds from triploid F1 hybrid plants
The chromosome numbers in the root tip cells of the selfed F2 seeds from the plants of 10 triploid F1 hybrid crosses (except for STU 16 for which there were no F2 seeds) were studied (Table 2). The chromosome numbers of most F2 plants in the 10 crosses ranged from 39 to 43, except for the two F2 plants of STU 9, which had 35 chromosomes. Among the 127 F2 plants from 10 crosses, the chromosome numbers 2n = 42 had the highest frequency of 54.3%, followed by 2n = 41 (30.7%), and the other chromosome numbers were less than 10%. Of the 10 crosses, the F2 seeds from STU 2 and STU 15 were all 2n = 42, which may be due to the limited sample numbers. In addition, two (STU 11 and STU 14), four (STU 7, STU 8, STU 9, and STU 10), and two crosses (STU 12 and STU 13) had the frequency of 2n = 42 chromosomes higher than, lower than, and equal to that of the non-42 chromosomes, respectively.
The chromosome compositions of the F2 seeds from the selfed F1 hybrid plants were investigated by FISH and GISH (Table S1). The results showed that the frequency of euploid T. turgidum–Ae. umbellulata (2n = 42, had three sets of complete A, B, and U chromosomes) (Fig. 2A1–A3) was 49.61% in comparison with 50.39% for aneuploids (2n ≠ 42) and pseudoeuploids (2n = 42 but some chromosomes lost or gained) (Figure S4). According to the composition of the A, B, and U chromosomes, the pseudoeuploids could be divided into three types (I, II, and III). All three pseudoeuploids lost some U chromosomes but gained some A or B chromosomes. Type I gained one 1 B chromosome but lost one 7 U chromosome (Fig. 2B1–C3), whereas type II and type III gained two 5 A and three 6 A chromosomes but differed in the absence (Fig. 2D1–D3) and presence of unknown chromosomes (Fig. 2E1–E3). Both the types II and III lost one U chromosome each for 2 U, 3 U, 4 U, 5 U, and 7 U but type III also lost one 6U chromosome.
The chromosome composition of aneuploids included 13 types: the loss of some A chromosomes but not gain (Figure S5 A1–B3) or gain of some unknown chromosomes (Figure S5 C1–C3), the loss of some B chromosomes but not gain (Figure S5 D1–E3) or gain of some unknown chromosomes (Figure S5 F1–F3), the loss of some U chromosomes (Figure S5 G1–I3), the simultaneous loss of some A and U chromosomes but not gain (Figure S5 J1–K3) or gain of some other A chromosomes (Figure S5 L1–L3), the simultaneous loss of some B and U chromosomes but not gain (Figure S5 M1–N3) or gain of some other B chromosomes (Figure S5 O1–O3), the simultaneous loss of some A and B chromosomes but not gain (Figure S5 P1–P3) or gain of some A chromosomes (Figure S5 Q1–Q3), the simultaneous loss of some A, B, and U chromosomes but gain of some other A and U chromosomes (Figure S5 R1–R3), and gain of U chromosomes (Figure S5 S1–S3), respectively.
According to FISH and GISH, chromosome deletions were detected in the A, B, and U chromosomes of the six crosses (STU 7–STU 9, STU 11, and STU 13–STU 14) (Table S1). To explain the chromosome deletions in these crosses, we made the chromsome observations for some crosses. As an example, meiosis in the PMCs of the STU 9 triploid hybrid F1 was observed. The results showed that lagging chromosomes including U (Fig. 3A, B) and A and B chromosomes (Fig. 3B) were found during meiosis. The lagging chromosomes were lost during meiosis, resulting in aneuploids. During meiosis, no association of the U chromosome with the A and B chromosomes was observed, which may be caused by the limited number of crosses and cells observed or perhaps there is no homoeologous relationship.
Genome in situ hybridization (GISH) of the pollen mother cells (PMCs) of STU 9 (Langdon/PI 227436) triploid F1 hybrids during meiosis. The DNA probe and block in the GISH figures were Aegilops umbellulata PI 227436 (green) and Langdon, respectively. The U and AB lagging chromosomes are indicated by red (A, B) and white arrows (B), respectively
In the aneuploids of T. turgidum–Ae. umbellulata, the frequency of chromosome loss varied among genomes, with U being the highest (26.77%), followed by B (22.83%) and A (11.81%) (Figure S6A). The frequency of chromosome gain among the A, B, and U genomes was 3.94%, 3.94%, and 1.57%, respectively (Figure S6A). The frequency of chromosome loss also changed among chromosomes. Of the 21 chromosomes, 7 U had the highest frequency of chromosome loss (16.54%), followed by 1 B (9.45%), and the other five chromosomes (2 A, 7 A, 2 B, 4 B, and 5 B, each with 0.79%) had the lowest loss frequency (Figure S6B). Among the seven A chromosomes, 5 A had the highest loss frequency (3.94%), followed by 1 A, 3 A, and 6 A (each with 2.36%), and the loss frequency of the other three A chromosomes was lower than 2.00%. Among the seven B chromosomes, 1 B had the highest loss frequency (9.45%), followed by 3 B (7.09%), and the loss frequency of the other five B chromosomes was less than 3%. Among the seven U chromosomes, 7 U had the highest loss frequency (16.54%), followed by 3 U and 4 U (7.87%), and the loss frequency of the other four U chromosomes was less than 6.00%. The frequency of chromosome gain also varied among chromosomes. Among the 21 chromosomes, only seven chromosomes, namely, 1 A, 3 A, 5 A, 6 A, 1 B, 1 U, and 6 U, were gained in aneuploids. Of these, 1 B had the highest gain frequency (3.94%), followed by 6 A (2.36%), and the gain frequency of the other five chromosomes was less than 2.00% (Figure S6B). The chromosome loss/gain in the A, B, and U chromosomes varied among crosses (Table S1). Three crosses each showed no loss/gain in chromosomes A and U (STU 2, STU 12, and STU 15) and B (STU 2, STU 10, and STU 15). STU 13 (1 A, 3 A, 4 A, 5 A, and 6 A), STU 7 (1 B, 2 B, 3 B, and 7 B) and STU14 (1 B, 3 B, 6 B, and 7 B), and STU 9 (1–7 U) were the crosses with more chromosome loss/gain in the A, B, and U genomes, respectively.
In addition to chromosome loss/gain, the selfed F2 seeds from the triploid hybrid F1 plants of T. turgidum–Ae. umbellulata were involved in three types of chromosome translocations between U and A chromosomes (Fig. 4A), which included 3UL·2AS (STU 11-1-8 and STU 12-3-1), 6UL·1AL (STU 13-1-1, STU 13-1-2 and STU 13-1-5), and 4US·6AL (STU 14-4-7).
In addition to the variations in chromosome structure and numbers described above, polymorphic U chromosomes were also detected in the amphidiploids from different crosses (Fig. 4B). For example, STU 7 had two types of 1 U, 2 U, 4 U, and 7 U, three types of 5 U, and four types of 6 U. STU 14 had two types of 3 U, 4 U, 5 U, and 6 U and three types of 2 U and 7 U. Three crosses (STU 8, STU 10, and STU 12) showed polymorphism in only one U chromosome, involving 6 U (two types), 2 U (three types), and 5 U (two types), respectively. STU 11 had three and two polymorphic types in 6 U and 7 U, respectively. The remaining three crosses (STU 2, STU 9, and STU 15) showed no polymorphism in all U chromosomes.
Discussion
The role of unreduced gametes in wheat distant hybridization
Unreduced gametes have been discovered in many haploids of Triticeae [13, 25, 26], such as hexaploid/tetraploid wheat with rye, and they are an important means for the origin of Triticeae [27, 28]. It was estimated that the average frequency of unreduced gametes in hybrid was 50 times greater than non-hybrid populations [29] and approximately 0.1–2.0% of gametes in a non-hybrid plant population were expected to be unreduced [30]. In the F1 hybrids of tetraploid wheat with Ae. tauschii, Ae. longissima, and Ae. comosa, both FDR and SDM mechanisms are involved in unreduced gamete formation [9, 18, 21]. Only FDR was found in the F1 hybrids of wheat–Ae. triuncialis, as well as in some F1 hybrids of T. turgidum–Ae. markgrafii and T. turgidum–Ae. tauschii [14, 19, 21]. Homologous chromosome pairing can prevent the formation of unreduced gametes. For example, no unreduced gamete formation was found in the haploid hybrid F1 of wheat–Ae. cylindrica (ABDDC) and tetraploid wheat with tetraploid Ae. tauschii (2n = 4x = 28, DDDD) due to homologous pairing between the D genomes [20, 31]. The present results showed that unreduced gametes were produced in triploid F1 hybrids of tetraploid wheat ssp. durum var. Langdon and ssp. dicoccon PI 94668 with Ae. umbellulata, providing a theoretical basis for the formation of doubled haploids by the natural doubling of triploid T. turgidum–Ae. umbellulata hybrids. Both FDR and SDM pathways of unreduced gamete formation have been found in the triploid hybrids of Langdon with Ae. comosa [21]. In this study, only FDR and both FDR and SDM were found in the triploid F1 hybrids of durum wheat Langdon and Ae. umbellulata, but only FDR occurred in the two triploid F1 hybrids of PI 94668 and Ae. umbellulata, which may be due to the limited number of crosses that were used. In the FDR, no homologous chromosomes were paired (univalent formation) and separated during meiosis I or occurred at a very low frequency, whereas the two sister chromatids of homologous chromosomes moved to opposite poles during the second division [11]. Interspecific hybridization is an alternative and prospective strategy to introduce valuable traits from wild relative species into new cultivars but usually produce mostly sterile offspring due to the barriers of chromosome pairing during meiosis [12]. The FDR gamete formation had the advantage of transferring parental heterozygosity and maintain epistatic interactions other than cross-over fragments, and the formation of the 2n-gametes can be used to develop sexual polyploids with more genetic vigor, better yield and more resistant to biotic and abiotic stresses [32].
The selfed seed-set rate of F1 hybrid plants is an important index for measuring the frequency of unreduced gamete formation [9, 31, 33]. During the meiosis of PMCs of tetraploid wheat-Ae. tauschii hybrids, the ratio of dyads and the frequency of unreduced gametes are positively correlated with the seed-setting rate of selfed F1 hybrid plants [14]. Compared with the selfed seed-set rate of F1 hybrid plants of tetraploid wheat–Ae. tauschii (mean: 5.83%, range: 0–18.57%) [12] and T. turgidum–Ae. comosa (mean: 5.44%, range: 1.96–11.02%) [21], the selfed seed-set rate of the present T. turgidum–Ae. umbellulata F1 hybrid plants (mean: 4.7%, range: 0–13.0%) was lower than both [12, 21], although it was higher than that of the F1 hybrid plants of T. turgidum–Ae. umbellulata in our previous report (mean: 1.22%, range: 0.09–4.65%) [7]. The low seed-setting rate of these F1 hybrids may be ascribed to the lower number of univalents (14.47–19.71 vs. 20.26–20.87) and higher number of Rod II bivalents (0.59–3.17 vs. 0.05–0.34) and chiasmata (0.66–3.31 vs. 0.07–0.38) compared with those of the T. turgidum–Ae. comosa F1 hybrids [21]. However, the higher seed-setting rate of the present F1 hybrids of T. turgidum–Ae. umbellulata compared with that of T. turgidum–Ae. markgrafii F1 hybrids could be explained by a higher number of univalents and a lower number of Rod II bivalents and chiasmata in the T. turgidum–Ae. umbellulata hybrids [21]. The low frequency of unreduced gamete formation with fewer univalents, leading to a low seed-setting rate, was also detected in T. durum–Ae. longissima hybrids [18, 28].
Chromosome variation of F1 hybrids and their offsprings of wheat and related species
Polyploid plants are highly tolerant to aneuploidy in comparsion with the diploid species. In most allopolyploid plants, homologous chromosome mismatch and the formation of multivalents are the main causes of aneuploidy and chromosome structure variation [34,35,36]. Theoretically, the Ph1 gene can promote homologous chromosome pairing and inhibit homoeologous chromosome pairing between common wheat and synthetic wheat, and the meiosis of their offspring should be very stable, synthetic wheat and its offspring have a high frequency of chromosome number variation [14, 37]. Chromosome number variation is the main cause of aneuploids, which is common in nascent synthetic wheat and even in higher generations, and the frequency changes greatly among generations, ranging from 20 to 100% [37]. The newly synthetic wheat containing the active Ph1 gene was also associated with meiotic abnormality and aneuploidy in a parent-dependent manner [38]. The chromosome numbers in synthetic wheat range from 39 to 45, and the frequency of euploids (2n = 42) is 51.4% [39]. The chromosome numbers (35–43) and the frequency of euploids (49.61%) in the current nascent synthetic T. turgidum–Ae. umbellulata species are close to those of synthetic hexaploid wheat [39]. The frequency of aneuploids in synthetic wheat SHW-L1 was 48.6%, though it decreased to 12.5% in a recombinant inbred line containing SHW-L1 blood [39] and was reduced to 1.3% and 3.0%, respectively, in the new wheat varieties ‘Shumai 969’ and ‘Shumai 830’ derived from SHW-L1 [40]. These results suggest that hybridization and multiple generations of self-crossing could significantly enhance the chromosome stability of synthetic wheat species, including T. turgidum–Ae. umbellulata and its offspring. In addition to variations in chromosome number, chromosome structure variation, such as translocation, duplication, deletion, and inversion, has also been discovered in synthetic wheat [41, 42]. In the selfed F2 seeds from triploid hybrid plants of T. turgidum–Ae. umbellulata, we observed a wide range of chromosome number variation and three types of chromosome translocations involving 3 U and 2 A, 6 U and 1 A, and 4 U and 6 A, as well as different types of polymorphic U chromosomes (Fig. 4A). These results suggested that alterations in chromosome number and structure variation still exist in the newly synthetic wheat species T. turgidum–Ae. umbellulata. A high frequency of aneuploid plants is an indicatior of chromosomal instability. In the primary synthetic wheat, wide variation of chromosome numbers between plants was colsely related to the frequency of univalent during meiosis [39]. Univalents separate irregularly during meiosis and their derivative chromosomes usually not equally delivered to offspring nuclei or lost in the formation of micronuclei [39]. Univalents in wheat subject to chromosome breakage and generate chromosome fragments and the possibility of breakage-fusion causing translocation chromosomes [43].
The Aegilops tauschii D genome acts as a key genome in the wheat–Aegilops complex group. The phenotypic characteristics encoded by the D genome have hardly changed during evolution, whereas those of the coexisting genomes usually change significantly [44]. In the three genomes (A, B, and D) of synthetic wheat, the frequency of chromosome loss or gain is not completely consistent, with B being the highest, followed by A, with the last added D genome being the most stable. The frequency of chromosome loss and gain in each genome differs among chromosomes, which may be related to the self-stability of the D genome of Ae. tauschii, and once combined with other genomes by allopolyploidy, it might cause instability in other genomes [37]. In the synthetic T. turgidum–Ae. umbellulata species, the frequency of chromosome loss was the highest for the U genome, followed by the B genome, whereas the A genome was the most stable. To overcome the chromosome instability of synthetic T. turgidum–Ae. umbellulata, hybridization and multiple generations of self-crossing could be applied in order to retain less genomic content from the synthetic. As an example, the chromosome stability of nascent wheat was largely elevated by increasing the genetic background of common wheat through such way [39].
Materials and methods
Plant materials
A total of 30 T. turgidum–Ae. umbellulata triploid F1 hybrid plants from the 11 crosses, which were named STU 2 and STU 7–STU 16 (Table S2), were produced in our lab by T. turgidum (2n = 4x = 28, AABB) subsp. durum var. Langdon crossed with nine Ae. umbellulata accessions (2n = 2x = 14, UU) and T. turgidum subsp. dicoccum PI 94668 crossed with two Ae. umbellulata accessions. All the Ae. umbellulata accessions and T. turgidum ssp. dicoccum accession PI 94668 were kindly supplied by USDA-AGRS germplasm bank (https://www.ars-grin.gov/). The voucher specimens for Ae. umbellulata (Deposition number 201,400,199) and T. turgidum ssp. dicoccum (201,404,987) that were identified by Prof. Yang Junliang in our Institute and that of T. turgidum ssp. durum (NAS00533826) was idenfied by Prof. Guo Benzhao in Northwest Plateau Biology Institute, Chinese Academy of Sciences can be found at Chinese virtual herbaium (https://www.cvh.ac.cn/).
Production of triploid F1 hybrids
Eleven triploid F1 hybrids were produced using T. turgidum ssp. as the female parent and Ae. umbellulata as the male parent following reference [45]. During the generation of F1 hybrid seeds, no embryo rescue or hormone treatment was applied. The F1 hybrid seeds were germinated in petri dishes, and the chromosome numbers in the root tip cells were cytologically checked to retain those with 21 chromosomes. Triploid plants with 2n = 3x = 21 (Figure S1) were transplanted into the field of Wenjiang experimental farm of Chengdu in Sichuan, China for further analysis. No chemical reagent was used for doubling the chromosomes of these triploid F1 plants, and therefore, their chromosomes were doubled via spontaneous unreduced gametes.
Cytological observations
Chromosome numbers in the root tip cells and PMCs at meiotic metaphase I were determined following reference [9]. A total of 150 cells were observed in each plant of every cross. The chromosome configurations including the number of univalent (I), ring and rod bivalents (II), trivalents (III), and quadrivalents (IV) were recorded at the metaphase of the first meiosis. The number of chimastata for the univalent, rod bivalent, and ring bivalent and trivalent was calcaulated as 0, 1, and 2, respectively. The chromosome numbers of 30 F1 triploid plants and the selfed seeds (eight seeds were randomly selected for those crosses generate more than eight seeds, and all for those produce less than five seeds) were determined. All the triploid hybrid seeds from the 11 crosses and one seed each from the selfed seeds of each F1 plant were analyzed for chromosome constitution by in situ hybridization. Slides were prepared for FISH and GISH [46, 47]. Four oligonucleotide probes, namely, Oligo-pTa-535 (pTa535), Oligo-pSc119.2 (pSC119.2), Oligo-pTa71 (pTa71) [48], and (AAC)5 [49], were labeled by 6-carboxyfluorescein (6-FAM) or 6-carboxytetramethylrhodamine (Tamra) and synthesized by Sangon Biotech in Shanghai, China. These four probes could distinguish all the A, B, and U chromosomes in T. turgidum–Ae. umbellulata hybrids. The probe combinations pSc l19.2, (AAC)5, and pTa71 could distinguish all the U chromosomes, whereas the probes pSc119.2 and pTa535 could differentiate all the A and B chromosomes [7]. The genomic DNA used for GISH was isolated from the young leaves of the Ae. umbellulata accessions PI 227436 and PI 554395 and the tetraploid wheat lines Langdon and PI 94668 using a modified cetyltrimethylammonium bromide (CTAB) method [50]. The genomic DNA of Ae. umbellulata was used as a probe and labeled by nick translation with Chroma Tide Alexa Fluor 488-5-dUTP (Invitrogen, USA; no. C11397, green coloration). The genomic DNA of tetraploid wheats (Langdon, or PI 94668) was used as a blocker. The following steps, including hybridization, image capture and treatment, and re-hybridization, were the same as described by [51].
Statistical analyses
The data were statistically analyzed using Excel 2019 (Microsoft Corp., Redmond, WA, USA) and SPSS 27 (IBM Corp., Armonk, NY, USA). One-way analysis of variance was performed for the number of chiasmata and chromosome configuration and the least significant difference was applied for Post-hoc test of significant difference. The chromosome signals in multi-channel were combined with DP Manager (Olympus, Tokyo, Japan), and chromosome extraction and image processing were carried out with Adobe Photoshop 12.0.3 (Adobe Systems Incorporated, San Jose, CA, USA).
Conclusions
The unreduced gamete formation pathways in 30 triploid F1 hybrid plants of T. turgidum–Ae. umbellulata were studied herein. FDR alone and both FDR and SDM were found in different crosses. The chromosome numbers in the F2 plants derived from the selfed seeds of the triploid F1 hybrid plants varied from 35 to 43, and the frequencies of euploids and aneuploids in these F2 plants were 49.61% and 50.39%, respectively. In the aneuploid F2 plants, all 21 chromosomes and seven of the 21 chromosomes (1 A, 3 A, 5 A, 6 A, 1 B, 1 U, and 6 U) were involved in chromosome loss and gain, respectively. U had a higher frequency of chromosome loss (26.77%) and a lower frequency of chromosome gain than the B (loss/gain: 22.83%/3.94%) and A (loss/gain: 11.81%/3.94%) genomes, respectively. Furthermore, three types of chromosome translocations related to 3 U and 2 A, 6 U and 1 A, and 4 U and 6 A were also identified in the F2 plants. In addition to variations in chromosome number and structure, polymorphic FISH karyotypes were also found in the F2 plants for all the U chromosomes. These results are valuable for producing naturally occurring T. turgidum–Ae. umbellulata amphidiploids, which is a bridge material for distant hybridization between wheat and Ae. umbellulata.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- FDR:
-
First division restitution
- FISH:
-
Fluorescence in situ hybridization
- GISH:
-
Genomic in situ hybridization
- PMCs:
-
Pollen mother cells
- QTLs:
-
Quantitative trait loci
- SDM:
-
Single-division meiosis
References
Wang SW, Yin LN, Tanaka H, Tanaka K, Tsujimoto H. Wheat-Aegilops chromosome addition lines showing high iron and zinc contents in grains. Breed Sci. 2011;61:189–95.
Wang J, Wang C, Zhen SM, Li XH, Yan YM. Low molecular weight glutenin subunits from the 1U genome of Aegilops umbellulata confer superior dough rheological properties and improve breadmaking quality of bread wheat. J Agri Food Sci. 2017;98:2156–67.
Sears ER. The transfer of leaf rust resistance from Aegilops umbellulata to wheat. Brookhaven Symposia Biology. 1956;9:1–21.
Edae EA, Rouse MN. Bulked segregant analysis RNA-seq (BSR-Seq) validated a stem resistance locus in Aegilops umbellulata, a wild relative of wheat. PLoS ONE. 2019;14:e0215492.
Cakmak I, Tolay I, Özkan H, Özdemir A, Braun HJ. Variation in zinc efficiency among and within Aegilops species. J Plant Nutr Soil Sc. 1999;162:257–62.
Bansal M, Adamski NM, Toor PI, Kaur S, Molnár I, Holušová K, Vrána J, Doležel J, Valárik M, Uauy C, Chhuneja P. Aegilops umbellulata introgression carrying leaf rust and stripe rust resistance genes Lr76 and Yr70 located to 9.47-Mb region on 5DS telomeric end through a combination of chromosome sorting and sequencing. Theor Appl Genet. 2020;133:903–15.
Song ZP, Dai SF, Jia YN, Zhao L, Kang LZ, Liu DC, Wei YM, Zheng YL, Yan ZH. Development and characterization of Triticum turgidum-Aegilops umbellulata amphidiploids. Plant Genet Resour. 2019;17:24–32.
Song ZP, Zuo YY, Xiang Q, Li WJ, Li J, Liu G, Dai SF, Yan ZH. Investigation of Aegilops umbellulata for stripe rust resistance, heading date, and iron, zinc, and gluten protein content. J Integr Agr. 2023;22:1258–65.
Zhang LQ, Yen Y, Zheng YL, Liu DC. Meiotic restriction in emmer wheat is controlled by one or more nuclear genes that continue to function in derived lines. Sex Plant Reprod. 2007;20:159–66.
Peloquin SJ, Boiteux LS, Carputo D. Meiotic mutants in potato: valuable variants. Genetics. 1999;153:1493–9.
Loginova DB, Silkova OG. Mechanisms of unreduced gamete formation in flowering plants. Russ J Genet. 2017;53:741–56.
Zhang LQ, Liu DC, Zheng Y, Yan ZH, Dai SF, Li YF, Jiang Q, Ye YQ, Yen Y. Frequent occurrence of unreduced gametes in Triticum turgidum-Aegilops tauschii hybrids. Euphytica. 2010;172:285–94.
Silkova OG, Shchapova AI, Shumny VK. Meiotic restitution in amphihaploids in the tribe Triticeae. Russ J Genet. 2011;47:383–93.
Hao M, Luo JT, Zeng DY, Zhang L, Ning SZ, Yuan ZW, Zheng YL, Zhang HG, Liu DC. QTug.sau-3B is a major quantitative trait locus for wheat hexaploidization. G3-Genes. Genom Genet. 2014;4:1943–53.
Matsuoka Y, Mori N. Reproductive and genetic roles of the maternal progenitor in the origin of common wheat (Triticum aestivum L). Ecol Evol. 2020;10:13926–37.
Xu SJ, Joppa LR. First division restitution in hybrids of Langdon durum disomic substitution lines with rye and Aegilops squarrosa. Plant Breeding. 2000;119:233–41.
Silkova OG, Loginova DB. Sister chromatid separation and monopolar spindle organization in the first meiosis as two mechanisms of unreduced gametes formation in wheat–rye hybrids. Plant Reprod. 2016;29:199–213.
Tiwari VK, Rawat N, Neelam K, Randhawa GS, Singh K, Chhuneja P, Dhaliwal HS. Development of Triticum turgidum subsp. durum-Aegilops longissimia amphiploids with high iron and zinc content through unreduced gamete formation in F1 hybrids. Genome. 2008;51:757–66.
Mirzaghaderi G, Fathi N. Unreduced gamete formation in wheat: Aegilops triuncialis interspecific hybrids leads to spontaneous complete and partial amphiploids. Euphytica. 2015;206:67–75.
Fakhri Z, Mirzaghaderi G, Ahmadian S, Mason AS. Unreduced gamete formation in wheat × Aegilops spp. hybrids is genotype specific and prevented by shared homologous subgenomes. Plant Cell Rep. 2016;35:1143–54.
Zuo YY, Xiang Q, Dai SF, Song ZP, Bao TY, HaoM, Zhang LQ, Liu G, Li J, Liu DC, Wei YM, Zheng YL, Yan ZH. Development and characterization of Triticum turgidum-Aegilops comosa and T. turgidum-Ae. Markgrafii amphidiploids. Genome. 2020;63:263–73.
Zhu ZD, Zhou RH, Kong XY, Dong YC, Jia JZ. Microsatellite marker identification of a Triticum aestivum-Aegilops umbellulata substitution line with powdery mildew resistance. Euphytica. 2006;150:149–53.
Okada M, Yoshida K, Takumi S. Hybrid incompatibilities in interspecific crosses between tetraploid wheat and its wild diploid relative Aegilops umbellulata. Plant Mol Biol. 2017;95:625–45.
Okada M, Michikawa A, Yoshida K, Nagaki K, Ikeda TM, Takumi S. Phenotypic effects of the U-genome variation in nascent synthetic hexaploids derived from interspecific crosses between durum wheat and its diploid relative Aegilops umbellulata. PLoS ONE. 2020;15:e0231129.
Loureiro I, Escorial C, García-Baudin JM, Chueca MC. Spontaneous wheat-Aegilops biuncialis, ae. Geniculata and ae. Triuncialis amphiploid production, a potential way of gene transference. Span J Agric Res. 2009;7:614–20.
MatsuokaY. Evolution of polyploidy Triticum wheats under cultivation: the role of domestication, natural hybridization and allopolyploid speciation in their diversification. Plant Cell Physiol. 2011;52:750–64.
Cai X, Xu SS. Meiosis-driven genome variation in plants. Curr Genomics. 2007;8:151–61.
Jauhar PP. Meiotic restitution in wheat polyhaploid (amphihaploids): a potent evolutionary force. J Hered. 2007;98:188–93.
Ramsey J. Unreduced gametes and neopolyploids in natural populations of Achillea borealis. Heredity. 2007;98:143–50.
Ramsey J, Schemske DW. Pathways, mechanisms and rates of polyploidy formation in the flowering plants. Annu Rev Ecol Syst. 1998;29:267–501.
Wang CJ, Zhang LQ, Dai SF, Zheng YL, Zhang HG, Liu DC. Formation of unreduced gametes is impeded by homologous chromosome pairing in tetraploid Triticum turgidum×Aegilops tauschii hybrids. Euphytica. 2010;175:323–9.
Lim K, Shen T, Barba-Gonzalez R, Ramanna MS, Van Tuyl JM. Occurrence of SDR 2n-gamete in Lilium hybrids. Breed Sci. 2004;54:13–8.
Matsuoka Y, Nasuda S. Durum wheat as a candidate for the unknown female progenitor of bread wheat: an empirical study with a highly fertile F1 hybrid with Aegilops tauschii Coss. Theor Appl Genet. 2004;109:1710–7.
Gaeta RT, Pires JC. Homoeologous recombination in allopolyploids: the polyploid ratchet. New Phytol. 2010;186:18–28.
Xiong ZY, Gaeta RT, Pires JC. Homoeologous shuffling and chromosome compensation maintain genome balance in resynthesized allopolyploid Brassica napus. Proc Natl Acad Sci USA. 2011;108:7908–13.
Chester M, Gallagher JP, Symonds VV, Silva AVC, Mavrodiev EV, Leitch AR, Soltis PS, Soltis DE. Extensive chromosomal variation in a recently formed natural allopolyploid species, Tragopogon miscellus (Asteraceae). Proc Natl Acad Sci USA. 2012;109:1176–81.
Zhang HK, Bian Y, Gou XW, Zhu B, Xu CM, Qi B, Li N, Rustgi S, Zhou H, Han FP, Jiang JM, von Wettstein D, Liu B. Persistent whole-chromosome aneuploidy is generally associated with nascent allohexaploid wheat. Proc. Natl Acad Sci USA. 2013;110:3447–3452.
Mestiri I, Chagué V, Tanguy A, Huneau C, Huteau V, Belcram H, Coriton O, Chalhoub B, Jahier J. Newly synthesized wheat allohexaploids display progenitordependent meiotic stability and aneuploidy but structural genomic additivity. New Phytol. 2010;186:86–101.
Zhao LB, Xie D, Fan CL, Zhang SJ, Huang L, Ning SZ, Jiang B, Zhang LQ, Yuan ZW, Liu DC, Hao M. Chromosome stability of synthetic-natural wheat hybrids. Front Plant Sci. 2021;112:654382.
Hao M, Zhang Q, Zhao LB, Dai SF, Li AL, Yang WY, Xie D, Li QC, Ning SZ, Yan ZH, Wu BH, Lan XJ, Yuan ZW, Huang L, Wang JR, Zheng K, Chen WS, Yu M, Chen XJ, Chen MP, Wei YM, Zhang HG, Kishii M, Hawkesford MJ, Mao L, Zheng YL, Liu DC. A breeding strategy targeting the secondary gene pool of bread wheat: introgression from a synthetic hexaploid wheat. Theor Appl Genet. 2019;132:2285–94.
Zhao N, Xu L, Zhu B, Li M, Zhang H, Qi B, Xu CM, Han FP, Liu B. Chromosomal and genome-wide molecular changes associated with initial stages of allohexaploidization in wheat can be transit and incidental. Genome. 2011;54:692–9.
Zeng DY, Guan JT, Luo JT, Zhao LB, Li YZ, Chen WS, Zhang LQ, Ning SZ, Yuan ZW, Li AL, Zheng YL, Mao L, Liu DC, Hao M. A transcriptomic view of the ability of nascent hexaploid wheat to tolerate aneuploidy. BMC Plant Biol. 2020;20:97.
Sears ER. Misdivision of univalents in common wheat. Chromosoma. 1952;4:535–50.
Zohary D, Feldman M. Hybridization between amphidiploids and the evolution of polyploids in the wheat (Aegilops-Triticum) group. Evolution. 1962;16:44–61.
Zhang LQ, Yan ZH, Dai SF, Chen QJ, Yuan ZW, Zheng YL, Liu DC. The crossability of Triticum turgidum with Aegilops tauschii. Cereal Res Comm. 2008;36:417–27.
Hao M, Luo JT, Yang M, Zhang LQ, Yan ZH, Yuan ZW, Zheng YL, Zhang HG, Liu DC. Comparison of homoeologous chromosome pairing between hybrids of wheat genotypes Chinese spring ph1b and Kaixian-Luohanmai with rye. Genome. 2011;54:959–64.
Zhao LB, Ning SZ, Yu JJ, Hao M, Zhang LQ, Yuan ZW, Zheng YL, Liu DC. Cytological identification of an Aegilops variabilis chromosome carrying stripe rust resistance in wheat. Breed Sci. 2016;66:522–9.
Tang ZX, Yang ZJ, Fu SL. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J Appl Genet. 2014;55:313–8.
Song ZP, Da SF, Bao TY, Zuo YY, Xiang Q, Li J, Liu G, Yan ZH. Analysis of structural genomic diversity in Aegilops umbellulata, Ae. Markgrafii, ae. Comosa, and ae. Uniaristata by fluorescence in situ hybridization karyotyping. Front Plant Sci. 2020;11:710.
Kidwell KK, Osborn TC. Simple plant DNA isolation procedures. In: Beckmann JS, Osborn TC, editors. Plant genomes: methods for genetic and physical mapping. Dordrecht: Springer; 1992. pp. 1–13.
Zhao LB, Nin SZ, Yi YJ, Zhang LQ, Yuan ZW, Wang JR, Zheng YL, Hao M, Liu DC. Fluorescence in situ hybridization karyotyping reveals the presence of two distinct genomes in the taxon Aegilops tauschii. BMC Genomics. 2018;19:3.
Acknowledgements
Not applicable.
Funding
This research was funded by the grants from the National Natural Science Foundation of China (U23A20187), the Sichuan Science and Technology Program, China (Nos. 2022YFH0105, 2022ZDZX0014), the open topic of Environment-friendly Crop Germplasm Innovation and Genetic Improvement Key Laboratory of Sichuan Province (2022LYFK01), the Key Research and Development Program of Sichuan Province, China (2021YFYZ0002).
Author information
Authors and Affiliations
Contributions
ZY and ZS designed the research. ZS performed the research and draft the article. YZ and WL analyzed the data. GL and SD contributed to the development of the material. ZY, and ZP edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ae. umbellulata and T. turgidum ssp. dicoccum accessions were clollected and permited from the USDA-AGRS germplasm bank (https://www.ars-grin.gov/).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, Z., Zuo, Y., Li, W. et al. Chromosome stability of synthetic Triticum turgidum–Aegilops umbellulata hybrids. BMC Plant Biol 24, 391 (2024). https://doi.org/10.1186/s12870-024-05110-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05110-8