Abstract
Background
Fusarium graminearum and Fusarium avenaceum are two of the most important causal agents of Fusarium head blight (FHB) of wheat. They can produce mycotoxins that accumulate in infected wheat heads, including deoxynivalenol (DON) and enniatins (ENNs), produced by F. graminearum and F. avenaceum, respectively. While the role of DON as a virulence factor in F. graminearum toward wheat is well known, ENNs in F. avenaceum has been poorly explored. Results obtained to-date indicate that ENNs may confer an advantage to F. avenaceum only on particular hosts.
Results
In this study, with the use of ENN-producing and ENN non-producing F. avenaceum strains, the role of ENNs on F. avenaceum virulence was investigated on the root, stem base and head of common wheat, and compared with the role of DON, using DON-producing and DON non-producing F. graminearum strains. The DON-producing F. graminearum strain showed a significantly higher ability to cause symptoms and colonise each of the tested tissues than the non-producing strain. On the other hand, the ability to produce ENNs increased initial symptoms of the disease and fungal biomass accumulation, measured by qPCR, only in wheat heads, and not in roots or stem bases. LC-MS/MS analysis was used to confirm the presence of ENNs and DON in the different strains, and results, both in vitro and in wheat heads, were consistent with the genetics of each strain.
Conclusion
While the key role of DON on F. graminearum virulence towards three different wheat tissues was noticeable, ENNs seemed to have a role only in influencing F. avenaceum virulence on common wheat heads probably due to an initial delay in the appearance of symptoms.
Similar content being viewed by others
Background
Wheat (Triticum spp.) is one of the most cultivated cereals in the world, with a production of about 910 million tonnes in 2021 [1]. Wheat production can be strongly compromised by fungal diseases, such as Fusarium head blight (FHB). FHB is one of the most damaging diseases affecting wheat crops around the world [2]. In addition to yield loss, FHB can adversely affect grain quality due to the accumulation of mycotoxins [3, 4].
FHB is considered a disease complex because it is caused by different species belonging to multiple Fusarium species complexes [5]. The composition of the FHB community is dynamic [6] and could be shaped by agricultural practices, susceptibility of cultivated varieties, and climatic conditions (especially during anthesis) as well as fungicide application [7,8,9,10]. Globally, Fusarium graminearum is considered the most important FHB causal agent due to its high incidence and virulence [11]. Fusarium culmorum, F. avenaceum, and F. poae are also commonly isolated from infected grains and can be found in different regions around the world [12,13,14,15,16,17,18]. The incidence of F. avenaceum has increased in recent years and is rapidly becoming one of the most common species associated with the FHB complex of wheat and barley in many cultivation areas [14, 19,20,21,22,23,24]. In addition, this species has also shown frequent co-occurrence with F. graminearum [14, 22, 25, 26].
Fusarium species associated with FHB are capable of producing a wide range of secondary metabolites that are harmful towards humans and animals [27] and can contaminate raw materials, as well as processed food and feed. Deoxynivalenol (DON), mainly biosynthesized by F. graminearum and F. culmorum, is the most common Fusarium mycotoxin detected in cereals worldwide [28,29,30].
Due to its occurrence and harmfulness, DON is the most studied and known Fusarium mycotoxin [31]. The action of DON toward plants, as well as its role in fungal virulence, has been demonstrated [32]. DON belongs to the trichothecene class of mycotoxins and its production by F. graminearum depends on the expression of trichodiene synthase, an enzyme encoded by the TRI5 gene. This enzyme catalyses the first step in trichothecene biosynthesis, the cyclization of farnesyl pyrophosphate into trichodiene [33]. It is well known that trichothecene toxicity occurs through inhibition of eukaryotic protein synthesis [34] and DON-related damage can be observed at many levels in plants, from cell metabolism, such as inhibition of cell division and mitosis [35, 36], to growth reduction of coleoptiles, roots, and shoot [37,38,39,40]. Exogenous applications of DON lead to hydrogen peroxide production, programmed cell death (PCD) as well as a defence response in wheat leaves [41]. Low concentrations of DON can inhibit PCD-disrupting biotrophic-type plant defences [42]. DON is considered a fungal virulence factor as its production is crucial for the spreading of FHB symptoms within wheat heads [43,44,45,46]. It is generally thought that the production of DON by F. graminearum increases during the switch from its short biotrophic to the necrotrophic phase [47,48,49], detectable by 24 h post-inoculation and with a significant growth increase by 96 h [50, 51]. This mycotoxin can be transported through vascular elements to the adjacent healthy spikelets, favouring disease development and the typical premature head bleaching, and sometimes associated with the appearance of water-soaked brown, dark purple to black coloured necrotic lesions on the exterior surface of the florets [47, 52, 53].
As an inhibitor of protein synthesis, DON is also dangerous for mammals. It is well-known that its ingestion can result in acute or chronic toxicity, and DON accumulation makes grains inappropriate for human and animal consumption [4, 54]. For this reason, the European Commission (EC) has set the maximum acceptable levels for DON in raw cereals and derived products for human foodstuffs [55].
In recent years, many surveys have shown an increase in the incidence of “emerging mycotoxins” which include several compounds produced by other Fusarium species [56]. Enniatins (ENNs) are the most common emerging mycotoxins detected in wheat, barley, and other cereals as well as in cereal products for human and animal consumption [57, 58]. ENNs are mainly produced by members of the Fusarium tricinctum species complex (FTSC), which includes F. avenaceum, and some members of the Fusarium sambucinum species complex (FSAMSC). Among the ENNs, enniatin A (ENA), enniatin A1 (ENA1), enniatin B (ENB) and enniatin B1 (ENB1) analogues are commonly detected in cereals globally [57, 59,60,61,62]. Despite their widespread occurrence, ENNs are presently not classified as regulated mycotoxins. This is because the knowledge about their toxicity and their impact on human and animal health is still limited [56, 63,64,65]. The European Food Safety Authority (EFSA) highlighted concern for human and animal health regarding the possible interactions of ENNs with other mycotoxins and chronic exposure from contaminated food and feed [66]. In the Fusarium genus, ENN biosynthesis is catalysed by enniatin synthetase, a non-ribosomal multifunctional enzyme encoded by the ESYN1 gene [67].
In comparison to DON, little is known about the effect of ENNs on plants or their role in fungal virulence. Early experiments in plants described an inhibition of germination and induction of wilting [68, 69]. Recent studies have demonstrated the induction of cell death, oxidative stress, as well as the reduction of shoot length in response to ENB [37, 70]. The role of ENNs in F. avenaceum virulence was preliminarily hypothesized [71] and subsequently confirmed in potato tubers [72]. By contrast, a relationship between ENNs and pathogenicity of Fusarium oxysporum f. sp. melonis on muskmelon was not detected [73]. Recently, the investigation on ENNs’ role in fungal virulence was expanded to other hosts, specifically durum wheat and pea, but again an impact of ENNs on F. avenaceum aggressiveness was only observed in potato tubers [74]. In contrast, a positive correlation between ENNs production, especially ENA1, and virulence was reported in barley seedlings and heads when comparing F. avenaceum isolates that naturally produce different levels of ENNs. This suggests that ENNs may indeed function as virulence factors in cereals [75].
Since ENN contamination of grains continues to be a problem in cereal grains around the world, a more comprehensive understanding of these compounds is needed. In this study, a F. avenaceum strain was compared to an ENN non-producing mutant (ESYN1-disrupted) and to an ESYN1 overexpression mutant to elucidate the function of ENNs on roots, stem bases, and heads of common wheat. Additionally, the role of ENNs was compared with that of DON, a known virulence factor in wheat heads [32, 43, 76]. This involved testing the disease-causing capability of a trichothecene non-producing F. graminearum mutant (TRI5-disrupted strain developed in this study) against its DON-producing wildtype strain in roots, stem bases, and heads of common wheat.
Results
In vitro production of secondary metabolites by Fusarium avenaceum and Fusarium graminearum strains
Data on the in vitro production of secondary metabolites by the F. avenaceum strains grown on autoclaved rice are shown in Table 1 (ENNs) and in Table S1 (other detected secondary metabolites). The LC-MS/MS analysis confirmed that the ESYN1-disrupted strain (FaΔesyn1) did not produce ENNs, whereas the wild type (FaWT) and the ESYN1-overexpressed strain (FaESYN1_OX) produced similar amounts of ENNs. Conversely, the other secondary metabolites were not significantly different between the three F. avenaceum strains, except for aurofusarin (Table S1).
Data on the in vitro production of secondary metabolites by the F. graminearum strains are shown in Table 2 (DON) and in Table S2 (other detected secondary metabolites). The TRI5 knockout strain (FgΔtri5) was confirmed to be a non-producer of DON and its acetylated form 15-acetyl deoxynivalenol (15-ADON). Among the other secondary metabolites, no significant differences were found in aurofusarin and fusarin C accumulation between the wild type (FgWT) and FgΔtri5. Conversely, FgΔtri5 did not produce culmorins and produced significantly lower levels of butenolide, zearalenone, and zearalenone-sulfate in comparison with FgWT.
Fusarium virulence assays on different common wheat tissues
Fusarium seedling root assay
Inoculation of seedling roots did not lead to significant growth reduction of the aerial part of the plants, as no difference in the average seedling length was observed between any of the fungal treatments and the control (Figs. 1 and 2), except for FgWT, which resulted in a significant reduction (p < 0.05) of seedling length (Fig. 2b).
Length (cm) of common wheat seedlings (only the aerial part, excluding roots) at 15 days post-inoculation of the roots with (a) F. avenaceum strains FaWT, FaΔesyn1 and FaESYN1_OX and (b) F. graminearum strains FgWT and FgΔtri5. The control was mock-inoculated with sterile water. Columns represent the log-transformed average (± Standard Error) of two independent experiments each composed of nine replicates (plants) for each fungal strain. Different letters (a-b) above the columns refer to significant differences (p < 0.05) according to Tukey’s honestly significant difference multiple comparison tests
Fungal biomass accumulation in the roots was determined by DNA quantification of F. avenaceum and F. graminearum by qPCR. The R2 and the reaction efficiency calculated from the linear equation were 0.99 and 95% for F. graminearum and 0.98 and 98% for F. avenaceum (Fig. S1). The dissociation curve analysis showed specific amplification products in the presence of pure fungal DNA (standard curves) and F. avenaceum and F. graminearum DNA (samples). No target amplification was detected in the negative controls. Therefore, the Ct values used to quantify DNA were those for which the dissociation curve analysis showed the presence of specific amplification products. The reaction efficiency calculated from the common wheat roots linear equation was 97%, with R2 = 0.99 (Fig. S1). Roots inoculated with the three F. avenaceum strains did not show significant differences (p > 0.05) in their accumulation (Fig. 3a). Conversely, a significant difference was detected between the F. graminearum strains, where FgWT accumulated higher amounts of F. graminearum DNA (p < 0.05) than FgΔtri5 (Fig. 3b).
Together, these results show that ENN production by F. avenaceum did not affect seedling root rot occurrence in common wheat, but DON production by F. graminearum did increase disease severity and facilitated a higher accumulation of fungal biomass in the roots.
Fungal biomass accumulation in common wheat root tissues (pg DNA ng common wheat DNA−1) at 15 days post-inoculation with F. avenaceum strains FaWT, FaΔesyn1 and FaESYN1_OX (a) and F. graminearum strains FgWT and FgΔtri5 (b). The control was mock-inoculated with sterile water. The columns represent the log-transformed average (± Standard Error) of two independent experiments (each composed of three replicates, each composed of three roots bulked together). Different letters (a–c) above the columns refer to significant differences (p < 0.05) according to Tukey’s honestly significant difference multiple comparison tests
Fusarium seedling stem base assays
F. avenaceum strain FaESYN1_OX caused a significantly higher Disease Index (DI) (p < 0.05) in the wheat stem base when compared with that induced by FaΔesyn1; however, the DI induced by FaWT was not significantly different from those of either mutant strain (Figs. 4 and 5a). A visual comparison of symptoms caused by F. graminearum strains compared to F. avenaceum, showed more necrosis in response to F. graminearum treatments (Fig. 4). A comparison of DI between the two F. graminearum strains shows that FgWT caused significantly more disease (p < 0.05) than that induced by FgΔtri5 (Figs. 4 and 5b).
Disease Index (DI) assessed in stem base of common wheat seedlings at 15 days post-inoculation with (a) F. avenaceum strains FaWT, FaΔesyn1 and FaESYN1_OX and (b) F. graminearum strains FgWT and FgΔtri5. The control was mock-inoculated with PDA only. Columns with bars represent the log-transformed average (± Standard Error) of two independent experiments each composed of 15 replicates (plants) for each fungal strain. The letters (a–c) above the columns refer to significant differences (p < 0.05) according to Tukey’s honestly significant difference multiple comparison tests
The reaction efficiency of qPCR assays used for estimating fungal biomass accumulation in stem bases calculated from the F. avenaceum linear equation was 103% with R2 = 0.99, while for F. graminearum it was 104% with R2 = 0.99 (Fig. S2). The dissociation curve analysis showed specific amplification products and no target amplification was detected in the negative controls. The reaction efficiency calculated from the common wheat stem base linear equation was 104%, with R2 = 0.98 (Fig. S2). All three F. avenaceum strains showed a similar accumulation of fungal biomass (Fig. 6a), whereas, in the case of F. graminearum strains, FgWT inoculations resulted in more biomass accumulation in stem bases (p < 0.05) than that detected for FgΔtri5 (Fig. 6b).
Thus, the ability of F. avenaceum to produce ENNs did not appear to influence disease severity in the wheat stem base and did not affect fungal biomass accumulation in this tissue. By contrast, the ability of F. graminearum to produce DON had a significant positive impact on the disease in the stem base and also significantly increased the quantity of fungal accumulation in the same tissue.
Fungal biomass accumulation in common wheat stem base tissues (pg DNA ng common wheat DNA−1) at 15 days post-inoculation with (a) F. avenaceum strains FaWT, FaΔesyn1, and FaESYN1_OX and (b) F. graminearum strains FgWT and FgΔtri5. The control was mock-inoculated with PDA. The columns represent the log-transformed average (± Standard Error) of two independent experiments (each composed of three replicates, each composed of five stem bases bulked together). Different letters (a-b) above the columns refer to significant differences (p < 0.05) according to Tukey’s honestly significant difference multiple comparison tests
Fusarium head assay
Results of FHB symptom observation are detailed in Table S3. In particular, wheat heads inoculated with F. avenaceum and F. graminearum strains resulted in the appearance of FHB symptoms at 7 days post-inoculation (dpi). In this case, FaWT and FaESYN1_OX inoculations resulted in a small but significant increase in the percentage of symptomatic spikelets (p < 0.05) compared to FaΔesyn1 (Fig. 7a). FgWT also caused significantly higher levels (p < 0.05) of FHB symptoms than FgΔtri5 (Fig. 7b).
Fusarium head blight severity (%) on common wheat heads at 7, 14, and 21 days post-inoculation with F. avenaceum strains FaWT, FaΔesyn1 and FaESYN1_OX (a) and F. graminearum strains FgWT and FgΔtri5 (b). The control was mock-inoculated with sterile water. Columns with bars represent the log-transformed average (± Standard Error) of two independent experiments each composed of nine replicates (plants) for each fungal strain. The letters (a–c) above the columns indicate the presence of significant differences (p < 0.05) according to Tukey’s honestly significant difference multiple comparison tests
The same trend described at 7 dpi, was observed also at 14 dpi (Figs. 7 and 8) where, again, the strains unable to produce ENNs (FaΔesyn1) or DON (FgΔtri5) caused a significantly lower percentage of symptomatic spikelets (p < 0.05) than that detected in the heads inoculated with strains able to produce ENNs (FaWT and FaESYN1_OX) or DON (FgWT), respectively.
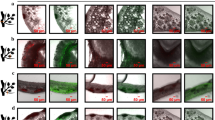
Fusarium head blight symptoms on common wheat heads at 14 (a) and 21 (b) days post-inoculation with F. avenaceum strains FaWT, FaΔesyn1 and FaESYN1_OX and F. graminearum strains FgWT and FgΔtri5. The control was mock-inoculated with sterile water. Two central spikelets, marked with a black pen, were inoculated at mid-anthesis. A picture of one representative head per thesis was taken
FHB symptoms at 21 dpi were present at a lower level on the heads inoculated with FaΔesyn1 (Fig. 7), but they were not significantly different (p < 0.05) in the heads inoculated with the three F. avenaceum strains (Figs. 7 and 8). By contrast, F. graminearum FgWT was able to maintain a higher level of severity (p < 0.05) than that detected in FgΔtri5.
qPCR assays were used to estimate fungal biomass accumulated in the head at 28 dpi (Fig. 9). R2 values calculated from the linear equations of the three standard curves were 0.99 for F. graminearum, F. avenaceum, and common wheat head. Reaction efficiencies obtained from the linear equations of the three standard curves were 104% for common wheat head and F. graminearum, and 103% for F. avenaceum. The dissociation curve analysis showed specific amplification products in the presence of pure fungal DNA (standard curves) and the presence of DNA of the two Fusarium species analyzed (samples). No target amplification was detected in negative controls (Fig. S3).
The two species analysed by qPCR were detectable through the presence of DNA accumulation on heads harvested at 28 dpi. Focusing on F. avenaceum strains (Fig. 9a), FaWT accumulated in the head tissue in higher abundance than FaΔesyn1, where significantly higher biomass (p < 0.05) was observed in the FaWT inoculated heads. Meanwhile, the biomass of FaESYN1_OX did not differ significantly (p < 0.05) from either the FaWT or FaΔesyn1. Regarding F. graminearum strains (Fig. 9b), FgWT showed a significantly higher amount of biomass (p < 0.05) in comparison to FgΔtri5.
In summary, ENN production by F. avenaceum increased disease symptoms, and while the difference in the percentage of diseased spikelets was no longer significant by 21 dpi, the amount of fungal biomass accumulation remained lower in the heads inoculated with FaΔesyn1 when compared to the FaWT but not when compared with FaESYN1_OX by 28 dpi.
Fungal biomass accumulation in common wheat heads (pg DNA ng common wheat DNA−1) at 28 days post-inoculation with (a) F. avenaceum strains FaWT, FaΔesyn1, and FaESYN1_OX and (b) F. graminearum strains FgWT and FgΔtri5. The control was mock-inoculated with PDA. The columns represent the log-transformed average (± Standard Error) of two independent experiments (each composed of three replicates, in turn, composed of three heads bulked together). Different letters (a-b) above the columns refer to significant differences (p < 0.05) according to Tukey’s honestly significant difference multiple comparison tests
The amount of ENNs detected in common wheat heads at 28 dpi with the three F. avenaceum strains is shown in Table 3. As expected, no ENNs were detected in the heads inoculated with FaΔesyn1, but they did accumulate in the heads inoculated with either FaWT and FaESYN1_OX (Table S4). Accumulation of aurofusarin and chrysogin in inoculated heads was the same among the three F. avenaceum strains (p > 0.05). Moniliformin also accumulated to a similar amount (p > 0.05) in both FaWT and FaESYN1_OX, showing significantly higher levels (p < 0.05) compared to FaΔesyn1.
In the head tissue inoculated with F. graminearum strain FgΔtri5, the trichothecene DON and its acetylated derivates were not detected (Table 4), and, similarly, DON-3-glucoside was absent (Table S5). Meanwhile, the head tissues inoculated with FgWT resulted in DON and acetylated-DON (3-ADON and 15-ADON) accumulation (Table 4). Among the other secondary metabolites, similar levels (p > 0.05) of aurofusarin and chrysogin were detected in the heads inoculated with either FgWT and FgΔtri5. Finally, 15-hydroxyculmorin, 5-hydroxyculmorin, 15-hydroxyculmorun, culmorin and butenolide were mainly produced (p < 0.05) by FgWT (Table S5).
Discussion
FHB is a disease affecting wheat around the world. The most challenging problem associated with this disease is mycotoxin accumulation that occurs in grains of infected heads and that compromises the quality and safety of raw materials, as well as processed food and feed, for human and animal consumption [77]. Mycotoxins are secondary metabolites and are therefore not required for the growth and development of fungi; however, these molecules can influence the fitness of the producing organism in some environments. For example, certain mycotoxins, such as DON, have been shown to enhance fungal virulence [78, 79], as observed for FHB in the F. graminearum-wheat interaction [76]. The significance of DON was initially demonstrated by generating and studying a trichothecene non-producing mutant strain, with several subsequent studies reinforcing these findings [32]. In contrast, despite their frequent occurrence, little is known about other Fusarium secondary metabolites such as ENNs [80]. For this reason, in the present study, the role of ENNs in F. avenaceum virulence was investigated on three different common wheat tissues and compared to that of DON in F. graminearum.
This study highlights the importance of DON in F. graminearum virulence in common wheat, regardless of the infected tissue. In wheat roots, the DON-producing F. graminearum wild-type strain (FgWT) stunted seedling development and accumulated in higher abundance than in roots inoculated with the DON non-producing mutant (FgΔtri5) (Figs. 2b and 3b). The involvement of DON in F. graminearum virulence in seedling blight and root rot has previously been demonstrated in wheat, barley and triticale [81]. Conversely, it has been demonstrated that the capability of F. graminearum and F. culmorum to produce trichothecenes adversely affects the early stages of wheat root colonization [82]. This suggests the hypothesis that tissues lacking pigments, such as roots, might be less affected by the trichothecene mycotoxins than green tissues. The different outcomes reported among these studies might be explained by differences in cultivars or methodologies and demonstrate the need for further investigation on DON virulence in tissues other than the wheat head.
The present study also showed that DON acts as a virulence factor for F. graminearum in the seedling stem base of common wheat. In fact, DON-producing FgWT provoked higher symptom severity and resulted in a higher accumulation of fungal biomass in the seedling stem base when compared to the DON non-producing strain, FgΔtri5 (Figs. 5b and 6b). These findings are consistent with previous reports in which DON acts as a virulence factor in stem base infection of wheat [76, 82, 83]. Interestingly, in one study investigating Fusarium crown rot, DON production in F. graminearum did not contribute to virulence in the wheat stem base, but did contribute to stem colonization [84].
Similarly to the results observed in this study in roots and stem bases of wheat seedlings, an increased virulence was observed in wheat heads infected with the DON-producing F. graminearum strain. A higher degree of disease symptom severity was observed, coupled with a higher accumulation of fungal biomass, in the wheat heads infected with FgWT compared to FgΔtri5 (Figs. 7b and 9b). The role of DON as a virulence factor in FHB disease has been described in detail in previous studies [32, 43, 45, 76, 85]. Restricted growth of a TRI5-disrupted mutant, where the fungus was retained in the infected spikelets, was observed [43], indicating that DON may facilitate the spread of F. graminearum throughout the wheat head. Results of the present research are in alignment with previous results, as disease spread was not observed in FgΔtri5 infected heads of common wheat.
Interestingly, the role of trichothecene mycotoxins in virulence is host- and chemotype-specific [86], where chemotype refers to the chemical profile, in this case the trichothecene profile, of the fungus [87]. For example, while DON non-producing (TRI5-disrupted) mutants have reduced virulence in wheat heads, the same is not true for NIV non-producing mutants compared with their NIV producing wild-type strains [86], which are generally less aggressive than DON chemotypes [4]. By contrast, NIV appears to contribute to virulence in maize [86]. Furthermore, TRI5-disruption of DON-producing strains does not affect disease spread in barley heads as it does in wheat [86].
ENNs are a different class of mycotoxins than trichothecenes and have a different mode of action which is thought to be via their interaction with cell membranes and subsequent creation of cation-selective pores [65]. Their role in F. avenaceum virulence in common wheat appears to be less straightforward than that of the DON in the F. graminearum-wheat interaction. Using an ESYN1-disrupted strain (FaΔesyn1) and an ESYN1-overexpressed strain (FaESYN1_OX) of F. avenaceum (FaWT), the role of ENNs was investigated in the same three tissues comparing their effects to those of DON.
Generally, the results indicate that ENNs are not crucial for F. avenaceum virulence in common wheat, but a possible tissue-dependent interaction was observed. ENNs did not act as a virulence factor for F. avenaceum in common wheat roots, as observed in our disease assay and also as shown by fungal biomass accumulation (Figs. 2a and 3a). Similarly, in a previous study with the same F. avenaceum strains (WT and mutants) but on pea roots, ENNs did not appear to influence F. avenaceum virulence in this host/tissue [74]. Using these same strains, ENNs were shown to affect F. avenaceum virulence in potato tubers [74], confirming the previously reported role of ENN production, and influencing virulence in potato tuber necrosis [71, 72]. Given F. avenaceum polyphagia, further studies on other hosts are necessary to test and better define the role of ENNs on virulence in roots and other hypogeal organs.
Similarly to what was observed for wheat roots, no differences were observed among the F. avenaceum isolates, FaWT, FaΔesyn1, and FaESYN1_OX, in terms of virulence or biomass accumulation during stem base infection of common wheat seedlings (Figs. 5a and 6a). These results are consistent with earlier studies suggesting that ENNs do not appear to contribute to virulence in wheat and rye seedlings [88]. Conversely, in a recent study, a correlation was reported between the virulence of F. avenaceum and ENA1 production in planta [75]. In that study, the authors inoculated malting barley plants by growing seeds in soil amended with macerated mycelium and compared the effect of nine F. avenaceum isolates, all of which produced ENN, but those producing ENA1 in planta were the most aggressive in their ability to cause seedling blight. For this reason, the authors proposed ENNs, and in particular ENA1, as potential virulence factors for F. avenaceum in cereals. Based on these observations, the role of ENNs in F. avenaceum virulence in the stem base of cereal seedlings is something to be further investigated. In fact, given the lower virulence of FaΔesyn1 in comparison to FaESYN1_OX in the present study, but not to FaWT, it would be interesting to determine whether there might be any differences in ENN production between the two ENN-producing strains in their interaction with the stem base of common wheat, and, if so, this might suggest a quantitative effect of these compounds in virulence. While in our work an increase in ENN accumulation was not observed for the FaESYN1_OX strain grown in culture, this strain was previously found to show higher ENN accumulation in both axenic cultures and in pea roots [74]. These results demonstrate that ENN production can be higher in the FaESYN1_OX strain under certain conditions and/or host interactions, although it is not clear whether such a difference may occur in the wheat stem base.
In the present work, a possible contribution of ENNs to fungal virulence was observed in the wheat head. Inoculation with FaΔesyn1 resulted in a reduction of FHB symptoms at 7 and 14 dpi, compared to inoculation with either of the ENN-producing strains (FaWT and FaESYN1_OX). This effect was no longer significant at 21 dpi (Fig. 7a), suggesting that the lack of ENN production can cause an initial delay in FHB symptoms. Importantly, while the percentage of diseased spikelets (considered here as disease severity) is similar among the three treatments at 21 dpi, the visual symptoms observed in the diseased spikelets appear to be less severe (Fig. 8). This result is partially supported by the lower fungal biomass accumulation of the FaΔesyn1 in comparison to FaWT observed at 28 dpi (Fig. 9a). While, a slight difference, even if not significant, was observed between FaΔesyn1 and FaESYN1_OX fungal biomass.
It should be noted that the role of ENNs for F. avenaceum in FHB virulence is less impactful than the role of DON for F. graminearum. In fact, the reduction in biomass accumulation in the absence of DON (FgWT vs. FgΔtri5) is around 60%, whereas in the absence of ENNs (FaWT vs. FaΔesyn1) is around 40%.
F. graminearum is itself more aggressive than F. avenaceum, and will spread easily from spikelet to spikelet, except in the most highly resistant cultivars [89, 90]. In the absence of trichothecene production in F. graminearum, the fungus is restricted to the inoculated spikelet of wheat. In the case of F. avenaceum, point inoculation of an individual spikelet in common wheat rarely results in disease spread from the inoculated spikelet (Table S3; [74]). As such, the effect of ENN disruption is less evident than that of DON. The same isolates were previously used to evaluate the role of ENNs in virulence in durum wheat, where disease spread frequently occurs, but no difference was observed in disease severity among the ESYN1-disruption and ENN-producing strains [74]. In durum wheat, biomass accumulation was not evaluated and it is unknown whether the difference in the percentage of diseased spikelets per head was representative of biomass accumulation within the head [74]. In the present work, a delay in the onset of symptoms was observed in response to FaΔesyn1 in common wheat, as recorded at 7 and 14 days. In the previous work [74], disease spread was already observed at 14 dpi in durum wheat and it would have been interesting to determine whether an earlier evaluation date in durum wheat would have resulted in a different outcome. In barley, it was shown that, similarly to what the same authors observed in barley seedlings (described above), F. avenaceum isolates that produced ENA1 in planta caused more FHB disease than those that did not, suggesting that ENA1 might be an important player in F. avenaceum virulence [75]. Taken together, the results indicate that ENNs could contribute to virulence in cereals, though the effect is not as prominent or widespread as the role of DON.
In this study, ENNs appeared to confer a specific advantage only on a particular tissue of common wheat. Transcriptomic analysis of Brachypodium distachyon in response to infection of head and root tissues by F. graminearum exhibits many tissue-specific differences and among these, some are related to phytohormones, or the expression of genes associated with ROS and antioxidant production [91]. While in the case of common wheat DON appears to contribute to virulence regardless of tissue type, a different defense response mounted by different tissues within a host plant might readily explain differences in resistance to infection of those tissues. ENNs play an important role in ROS production [65] and could also influence hormonal defence pathways. Preliminary results from our group indicate that ENNs, in particular ENB, may also affect the expression of salicylate and jasmonate-related genes in a concentration-dependent manner (unpublished data). It would be interesting to determine whether ENN accumulation differs in different wheat tissues during F. avenaceum infection, and if so, whether this may result in different host-responses, such as those regulated by plant hormones.
Conclusions
The present study confirms the role of DON as a crucial virulence factor for F. graminearum infection of common wheat, regardless of the infected tissue, whereas the role of ENNs in F. avenaceum virulence was reduced compared to DON and appeared to be tissue-dependent. Our results suggest that ENNs, in addition to being able to confer an advantage to F. avenaceum only on particular hosts [71, 72, 74, 75, 88], could also confer an advantage only on particular tissues in a given host. It is currently unknown what factor is responsible for the tissue-specific role of ENNs in F. avenaceum and why ENNs appear only to affect virulence in wheat heads and not in seedling roots and stem bases.
Materials and methods
Fungal material
To explore the role of ENNs in fungal virulence on the three different tissues of common wheat, F. avenaceum DAOM242378 (FaLH27), its ENN non-producing mutant (ESYN1-disrupted) FaLH27Δesyn1_8, and in locus ENN over-expression mutant FaLH27ESYN1_OX6, previously developed [74], were used. The F. avenaceum strains are described here in this paper as FaWT (wild type), FaESYN1_OX (ESYN1-overexpressed), and FaΔesyn1 (ESYN1-disrupted) (Table 5).
To compare the role of ENNs in virulence with that of DON, a F. graminearum wild-type strain and its TRI5 deletion mutant were investigated. The F. graminearum TRI5-disruption (AB123 described here as FgΔtri5) was created in the wild-type CS3005 background (FgWT) (Table 5). The FgΔtri5 strain was generated via protoplast-mediated transformation of F. graminearum isolate CS3005, as previously described [41], using a synthesised DNA fragment ordered from GenScript (Piscataway, NJ, USA) that contained 1000 bp of sequence immediately upstream of the TRI5 start codon, a short barcoding sequence (Table S6), an Aspergillus nidulans TRPC promoter nourseothricin acetyl transferase cassette corresponding to nucleotide positions 437–1387 of GenBank accession AY631958.2, and 993 bp of sequence immediately downstream of the TRI5 stop codon. Transformants were selected on 50 mg L− 1 nourseothricin sulfate (Werner Bioagents, Jena, Thuringia, Germany). Transformants were screened for successful knockout of the TRI5 gene using a three-primer PCR reaction (Table S6) designed such that the presence of the transforming DNA and absence of the DNA deleted from the genome was confirmed in a single PCR reaction as previously described [92, 93].
Secondary metabolite production by F. avenaceum and F. graminearum strains in rice cultures
To characterize the in vitro secondary metabolite production of the F. avenaceum and F. graminearum strains used in the present work, the protocol previously described was followed [16]. Briefly, 20 g of rice were added to 10 mL of deionized water in 100-mL glass flasks and autoclaved twice at 24 h intervals. Autoclaved flasks were inoculated with mycelial plugs (0.5 cm in diameter) taken from pure cultures of FaWT, FaΔesyn1, FaESYN1_OX, FgWT, and FgΔtri5 grown for one week on potato dextrose agar (PDA, Biolife Italiana, Milan, Italy) at 22°C in the dark. Control flasks were inoculated with PDA plugs from clean plates without fungus. Once inoculated, flasks were incubated for 4 weeks in the dark at 22°C. The fungal cultures were subsequently placed into 50-mL plastic tubes (Thermo Fisher Scientific, Waltham, MA, USA) and, after 2 days at −80°C, were freeze-dried with a Heto Powder Dry LL3000 (Thermo Fisher Scientific) lyophilizer, finely ground with a Mixer Mill 400 (Retsch, Haan, Germany) for 6 min at 25 Hz, and stored at −80°C.
Five grams of each milled sample was used for secondary metabolite extraction and quantification as previously described [96]. Extraction was performed in 50-mL polypropylene tubes (Sarstedt, Nümbrecht, Germany) using 20 mL of extraction solvent (acetonitrile/water/acetic acid 79:20:1, v/v/v). After 90 min of shaking on a GFL 3017 rotary shaker (GFL, Burgwedel, Germany), 500 µL of the raw extract were transferred to vials and diluted with an equal volume of the dilution solvent (acetonitrile/water/acetic acid 20:79:1, v/v/v). In cases where the metabolite concentration exceeds the highest calibration standard, extracts were diluted 1:50 and/or 1:1000 (v/v), and re-analyzed.
Metabolite detection and quantification was performed using a qTrap 5500 MS/MS system (Sciex, Foster City, CA, USA) equipped with a TurboV electrospray ionisation (ESI) source, coupled to a 1290 series UHPLC system (Agilent Technologies, Waldbronn, Germany). Chromatographic separation was performed at 25°C on a Gemini C18-column, 150 × 4.6 mm i.d., 5 μm particle size, equipped with a C18 security guard cartridge, 4 × 3 mm i.d. (both Phenomenex, Torrance, CA, USA). Two MS/MS transitions were acquired per analyte except for moniliformin and 3-nitroropionic acid that each yield only one product ion. For confirmation of identification, the ion ratio had to agree with the relative values of the related authentic standard within 30% and an in-house retention time criterion of ± 0.03 min was applied. Quantification was based on external calibration using a serial dilution of a multi-analyte stock solution. The accuracy of the method is verified on a routine basis by participation in a proficiency testing scheme organized by BIPEA (Gennevilliers, France) with a rate of satisfactory z-scores of −2 < z < 2 of 96% for the > 2000 results submitted to date. LODs and LOQs for DON and ENNs are reported in Table S7.
Plant growth conditions
Briefly, common wheat seeds (cv. A416, with known susceptibility to FHB) were surface sterilized in a water: sodium hypochlorite (7%; Carlo Erba Reagents, Milan, Italy) sterile solution [90:10 (v/v)] and rinsed three times with sterile deionized water. Seeds were placed in 14-cm Petri dishes (Nuova Aptaca, Asti, Italy) containing three filter papers soaked with 15 mL of sterile water. To promote the germination process, the Petri dishes were incubated in the dark at 4°C for 3 days and then at 22°C for 2 days. The germinated seeds were transplanted either into 24-well plates (Dominique Duthscher Group, Bernolsheim, France) in water-agar (1% w/v; Biolife) or pots filled with “traysubstrat” soil (Klasmann-Deilmann GmbH, Geeste, Germany). Plants grown in plates or pots were reared in a Conviron® growth chamber (Controlled Environments Limited, Winnipeg, Manitoba, Canada) at 22°C and 80% relative humidity, with a 16 h photoperiod (light intensity starting at 10% and increasing hourly by 10% for 4 h where it was maintained at 50% for 8 h and then was decreased hourly in 10% increments until 16 h), or in a greenhouse at the Department of Agricultural, Food and Environmental Sciences (University of Perugia, Perugia, Italy) at 22 ± 4°C and 12 h photoperiod (supplied with artificial light in seasons when natural light was not sufficient) as specified and described in the virulence assay sections.
Fusarium virulence assays on different tissues of common wheat
To investigate the role of ENNs and DON on fungal virulence, three assays were carried out on common wheat (cv. A416) to compare the virulence of F. avenaceum and F. graminearum wild types and mutant strains on three different host tissues: seedling roots, seedling stem bases, and heads.
Fusarium seedling root assay
F. avenaceum strains (FaWT, FaΔesyn1, and FaESYN1_OX) and F. graminearum strains (FgWT and FgΔtri5) were cultured in the dark at 22°C for 7 days in 9-cm Petri dishes with PDA for producing mycelium. Mung bean broth was prepared by adding 40 g of mung bean to 1 L of sterile water and boiling for 10 min. The beans were then separated from the broth through filtration with cheesecloth and the broth was autoclaved. One liter of mung bean broth in 2-L flasks was inoculated with a mycelial plug of the different strains and aerated with forced sterile air for 5 days at room temperature. All resulting conidial suspensions were filtered through Miracloth (EMD Millipore Corporation, Billerica, MA, USA) and the conidia were collected by centrifugation at 3000 rpm for 15 min at 4°C in a 5804 R centrifuge (Eppendorf, Hamburg, Germany). Finally, the concentration of conidia suspension was adjusted to 5 × 106 macroconidia mL−1 using a hemocytometer.
The seedling root assay was based on a previously described method [97], with some modifications. Common wheat seedlings were grown in 24-well plates and incubated in the growth chamber. At the development stage first leaf unfolded (BBCH11) [98], the seedlings were moved from water-agar, wrapped at stem level with aluminium foil and placed in 50-mL tubes (Thermo Fisher Scientific), immersing only the roots in 10 mL of conidial suspension (1 × 106 macroconidia mL−1) of the different strains. The roots of the control seedling were placed in distilled sterile water only. Tubes were placed at 180 rpm on a rotary shaker (Lab-line Instruments, Melrose Park, IL, USA) at room temperature for 24 h. Afterwards, the seedlings were individually transplanted into 5 × 5 × 3 cm plastic pots filled with “traysubstrat” soil and then returned to the Conviron® for 15 days. Fifteen dpi seedlings were removed from the soil and thoroughly rinsed, taking care to keep the root system intact. The effect of the different Fusarium strains was subsequently assessed by measuring seedling (only aerial part) length (cm). Afterwards, the roots were cut off from the seedlings, freeze-dried by Heto Powder Dry LL3000 (Thermo Fisher Scientific), finely grounded with a Mixer Mill 400 (Retsch; 6 min, 25 Hz), and stored at −80°C for fungal biomass quantification as described in the ‘DNA extraction and Fusarium quantification by qPCR’ section The experiment was repeated twice and for each of the two experiments, nine independent seedlings, considered as replicates, per strain (or control) were carried out for a total of 54 seedlings. For qPCR analysis, three independent seedlings per strain or control were bulked into one replicate for DNA extraction, for a total of three replicates per strain or control.
Fusarium seedling stem base assay
Virulence assays on stem bases of common wheat seedlings were carried out according to a method previously reported [99] with several modifications [100]. Common wheat plants were grown in 6 × 8 × 8 cm plastic pots (5 seedlings per pot) in a growth chamber as described in the section on plant growth conditions. A 3 cm-long PVC collar (3 mm of internal diameter) was placed around the emerging coleoptiles. Seedlings were inoculated, at the fully extended second leaf stage (BBCH12 [98]). Inoculum was obtained by culturing the five Fusarium strains for one week at 22°C on 9-cm Petri dishes containing PDA (2 Petri dishes per strain). Subsequently, the fungal colony (together with the PDA) was homogenized in the Mixer Mill 400 (Retsch) with 12 mL of sterile water to obtain a homogenate of pipettable consistency. For control treatments, uninoculated PDA and sterile water were homogenised as described for the inoculum. Seedlings were inoculated by pipetting 700 µL of homogenate into the PVC collar. Seedlings were removed from the soil 15 dpi and rinsed thoroughly. Stem base infections were evaluated by measuring the length (cm) of the necrotic area and the presence of necrosis across leaf sheaths composing the stem base, using a 0–14 arbitrary scale (0 = clean; 1–2 = coleoptile; 3-4-5 = 1st leaf; 6-7-8 = 2nd leaf; 9-10-11 = 3rd leaf; 12-13-14 = 4th leaf) [99]. DI was calculated as the product between the average value (0–14) of necrosis across leaf sheaths and the average length (cm) of the necrotic area in the stem bases. Upon completion of disease evaluations, the first 5 cm of the stem base was cut, freeze-dried and finely ground as described in the Fusarium seedling root assay section, and stored at −80°C for DNA extractions and fungal biomass quantification. The experiment was repeated twice, each carried out with 15 independent seedlings (replicates) per strain, or control, for a total of 90 seedlings. For qPCR analysis, five independent seedlings per strain, or control, were bulked in one replicate for a total of three replicates.
Fusarium head assay
The head assay was performed as previously described [100] with several modifications. Common wheat plants were grown in the greenhouse in 9 × 9 × 13 cm pots (one seedling per pot) as described in the plant growth conditions section. Plants were watered regularly and fertilized at tillering (BBCH20 [98]), (using 3 g per pot of Nitrophoska® 13-10-20, EuroChem Agro SpA, Cesano Maderno, Italy). When the plants reached the beginning of the flowering stage (BBCH61) the main head was point inoculated with conidial suspensions of the different Fusarium strains, as previously described [101]. Inoculum was prepared using the same method described for the seedling root assay. Fifteen microliters of conidial suspension (1 × 106 macroconidia mL−1) were injected between the palea and lemma of two adjacent spikelets near the center of the head. Inoculated spikelets were previously marked with a non-toxic permanent black marker to recognize them during the symptoms score. Immediately after the inoculation, the inoculated heads were covered for 48 h in clear plastic bags previously sprayed with water to maintain high humidity. After inoculation, plants were placed in a climatic chamber (F.lli Bertagnin, Bologna, Italy) at 22°C with a 16 h photoperiod and regularly watered. Mock-inoculated heads were used as controls.
FHB symptoms were evaluated at 7, 14, and 21 dpi, and assessed as the number of symptomatic spikelets out of the total number of spikelets per head, expressed as the percentage of the severity of symptoms (%). Wheat heads were hand-harvested at 28 dpi, freeze-dried by Heto Powder Dry LL3000 (Thermo Fisher Scientific) and finely ground by Mixer Mill 400 (Retsch). Three heads inoculated by each strain (or control) were bulked together and split into two subsamples. The first subsample was used to determine fungal biomass by qPCR, whereas the second one was used to quantify Fusarium secondary metabolites by LC-MS/MS, as mentioned before. The experiment was performed two times, with nine independent heads per strain or control, for a total of 54 heads each time. For qPCR analysis and LC-MS/MS analysis, three independent heads per strain or control were bulked in one replicate, resulting in three replicates per strain or control.
DNA extraction and Fusarium quantification by qPCR
Total DNA was extracted from 20 mg of ground tissues collected from Fusarium virulence assay section using the CTAB method as previously described [102] with modifications [103]. DNA was quantified with a NanoDrop One (Thermo Fisher Scientific) and the concentration of each sample was adjusted to 25 ng µL−1. The qPCR assay was performed as described [103], using a CFX96 real-time PCR detection system (Bio-Rad, Hercules, California, USA). Species-specific primers (Table S6) were used for the quantification of F. avenaceum and of F. graminearum [104], whereas translation elongation factor 1α (tef1α) primers (Table S6) [102] were used for the quantification of wheat DNA (roots, stem bases, and heads). For standard curves, DNA was extracted from wild-type F. avenaceum and F. graminearum colonies (FaWT and FgWT, respectively) and healthy tissues (roots, stem base, and heads) of common wheat. Standard curves were generated as previously described [16, 104, 105] using a 10-fold dilution series of DNA ranging from 90 ng to 90 pg for wheat and 25 ng to 25 pg for F. avenaceum and F. graminearum in the seedling root assay. For the seedling stem base and head assays, the DNA ranging from 25 ng to 2.5 pg for F. avenaceum and F. graminearum and from 125 ng to 12.5 pg for wheat. The curves were generated by plotting the logarithmic values of known DNA quantities versus the corresponding cycle threshold (Ct) values and were performed for each assay. The Fusarium DNA in roots, stem bases, and heads were expressed as the ratio of the Fusarium DNA (pg) to the wheat DNA (ng).
Statistical analysis
In vitro LC-MS/MS analysis was set up in a single experiment with three biological replicates. Virulence assays, for each of the two experiments, were set up in 9 (seedling length in root assay), 15 (DI in stem base assay), 9 (FHB symptoms in head assay), or 3 (qPCR from the root, stem bases, and heads; LC-MS/MS from heads) biological replicates. For the analysis of each of the virulence assays, two experiments were considered together and, except for in planta LC-MS/MS data, were log-transformed to normalize their distribution and homogenize the variances. As a consequence, the biological replicates increased to 18 (seedling length in root assay), 30 (DI in stem base assay), 18 (FHB symptoms in head assay), or 6 (qPCR from the root, stem bases and heads; LC-MS/MS from heads). Data are shown as their log-transformed average (two-experiment data except in planta LC-MS/MS data) or average (single-experiment data and in planta LC-MS/MS data) (± Standard Error). All data (log-transformed or not) were subject to one-way analysis of variance (ANOVA) by considering each “Fusarium strain” as the experimental factor and the different evaluated parameters as the variable. Finally, in the case of ANOVA significance (p < 0.05), to test pairwise contrasts, Tukey’s honestly significant difference multiple comparison tests were performed. All statistical analyses were performed using the Macro Excel® “DSAASTAT” (version 1.0192; [106]).
Data availability
Data is provided within the manuscript or supplementary information files.
References
FAOSTAT. 2022, https://www.fao.org/faostat/en/#home accessed on 11/01/2023.
Gilbert J, Haber S. Overview of some recent research developments in Fusarium head blight of wheat. Can J Plant Pathol. 2013;35:37–41.
Bottalico A, Perrone G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur J Plant Pathol. 2002;108:611–24.
Foroud NA, Baines D, Gagkaeva T, Thakor N, Badea A, Steiner B, et al. Trichothecenes in cereal grains – an update. Toxins. 2019;11:634.
O’Donnell K, Rooney AP, Proctor RH, Brown DW, McCormick SP, Ward TJ, et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet Biol. 2013;52:20–31.
Kohl J, De Haas BH, Kastlein P, Burgers S, Waalwijk C. Population dynamics of Fusarium spp. and Microdochium nivale in crops and crop residues of winter wheat. Phytopathol. 2007;97:971–78.
Tini F, Covarelli L, Cowger C, Sulyok M, Benincasa P, Beccari G. Infection timing affects Fusarium poae colonization of bread wheat spikes and mycotoxin contamination in the grain. J Sci Food Agric. 2022;102:6358–72.
Tini F, Beccari G, Onofri A, Ciavatta E, Gardiner DM, Covarelli L. Fungicides may have differential efficacies towards the main causal agents of Fusarium head blight of wheat. Pest Manag Sci. 2020;76:3738–48.
Scala V, Aureli G, Cesarano G, Incerti G, Fanelli C, Scala F, et al. Climate, soil management, and cultivar affect Fusarium head blight incidence and deoxynivalenol accumulation in durum wheat of Southern Italy. Front Microbiol. 2016;7:1–10.
Xu X. Effects of environmental conditions on the development of Fusarium ear blight. Eur J Plant Pathol. 2003;109:683–89.
Amarisinghe C, Sharanowski B, Dilanta Fernando WG. Molecular phylogenetic relationship, trichothecene chemotype diversity and aggressiveness of strains in a global collection of Fusarium graminearum species. Toxins. 2019;11:263.
Beccari G, Prodi A, Senatore MT, Balmas V, Tini F, Onofri A, et al. Cultivation area affects the presence of fungal communities and secondary metabolites in Italian durum wheat grains. Toxins. 2020;12:97.
Cowger C, Ward TJ, Nilsson K, Arellano C, McCormick SP, Busman M. Regional and field-specific differences in Fusarium species and mycotoxins associated with blighted North Carolina wheat. Int J Food Microbiol. 2020;323:108594.
Beccari G, Colasante V, Tini F, Senatore MT, Prodi A, Sulyok M, et al. Causal agents of Fusarium head blight of durum wheat (Triticum durum Desf.) in central Italy and their in vitro biosynthesis of secondary metabolites. Food Microbiol. 2018;70:17–27.
Balmas V, Scherm B, Marcello A, Beyer M, Hoffmann L, Migheli Q, et al. Fusarium species and chemotypes associated with Fusarium head blight and fusarium root rot on wheat in Sardinia. Plant Pathol. 2015;64:972–79.
Covarelli L, Beccari G, Prodi A, Generotti S, Etruschi F, Juan C, et al. Fusarium species, chemotype characterisation and trichothecene contamination of durum and soft wheat in an area of central Italy. J Sci Food Agric. 2015;95:540–51.
Dinolfo M, Stenglein SA. Fusarium poae and mycotoxins: potential risk for consumers. Bol Soc Argent Bot. 2014;49:5–20.
Scherm B, Balmas V, Spanu F, Pani G, Delogu G, Pasquali M, et al. Fusarium culmorum: causal agent of foot and root rot and head blight on wheat. Mol Plant Pathol. 2013;14:323–41.
Ceron-Bustamante M, Ward TJ, Kelly A, Vaughan MM, McCormick SP, Cowger C, et al. Regional differences in the composition of Fusarium head blight pathogens and mycotoxins associated with wheat in Mexico. Int J Food Microbiol. 2018;273:11–9.
Karlsson I, Edel-Hermann V, Gautheron N, Durling MB, Kolseth A, Steinberg C, et al. Genus-specific primers for study of Fusarium communities in field samples. Appl Environ Microbiol. 2016;82:491–501.
Karlsson I, Frieberg H, Kolseth A, Steinberg C, Persson P. Agricultural factors affecting Fusarium communities in wheat kernels. Int J Food Microbiol. 2017;252:53–60.
Beccari G, Caproni L, Tini F, Uhlig S, Covarelli L. Presence of Fusarium species and other toxigenic fungi in malting barley and multi-mycotoxin analysis by liquid chromatography-high-resolution mass spectrometry. J Agric Food Chem. 2016;64:4390–99.
Tittlemeier SA, Roscoe M, Trelka R, Gaba D, Chan JM, Patrick SK, et al. Fusarium damage in small cereal grains from Western Canada. 2. Occurrence of Fusarium toxins and their source organisms in durum wheat harvested in 2010. J Agric Food Chem. 2013;61:5438–48.
Lindblad M, Gidlund A, Sulyok M, Borjesson T, Krska R, Olsen M, et al. Deoxynivalenol and other selected Fusarium toxins in Swedish wheat – occurrence and correlation to specific Fusarium species. Int J Food Microbiol. 2013;167:284–91.
Senatore MT, Ward TJ, Cappelletti E, Beccari G, McCormick SP, Busman M, et al. Species diversity and mycotoxin production by members of the Fusarium tricinctum species complex associated with Fusarium head blight of wheat and barley in Italy. Int J Food Microbiol. 2021;358:109298.
Beccari G, Prodi A, Tini F, Bonciarelli U, Onofri A, Oueslati S, et al. Changes in the Fusarium head blight complex of malting barley in a three-year field experiment in Italy. Toxins. 2017;9:120.
Bryla M, Pierzgalski A, Zapasnik A, Uwineza PA, Ksieniewicz-Wozniak E, et al. Recent research on Fusarium mycotoxins in maize – a review. Foods. 2022;11:3465.
Kamle M, Mahato DK, Gupta A, Pandhi S, Sharma B, Dhawan K, et al. Deoxynivalenol: an overview on occurrence, chemistry, biosynthesis, health effects and its detection, management, and control strategies in food and feed. Microbiol Res. 2022;13:292–314.
Chen Y, Kistler HC, Ma Z. Fusarium graminearum trichothecene mycotoxins: biosynthesis, regulation, and management. Annu Rev Phytopathol. 2019;13:3.
Khaneghah AM, Martins LM, von Hertwig AM, Bertoldo R, Sant’Ana AS. Deoxynivalenol and its masked forms: characteristics, incidence, control and fate during wheat and wheat-based products processing – a review. Trends Food Sci Technol. 2018;71:13–24.
Pinto ACSM, De Pierri CR, Evangelista AG, Gomes ASLPB, Luciano FB. Deoxynivalenol: toxicology, degradation by bacteria, and phylogenetic analysis. Toxins. 2022;14:90.
Audenaert K, Vanheule A, Höfte M, Haesaert G. Deoxynivalenol: a major player in the multifaceted response of Fusarium to its environment. Toxins. 2013;6:1–19.
Hohn TM, Beremand PD. Isolation and nucleotide sequence of a sesquiterpene cyclase gene from the trichothecene-producing fungus Fusarium sporotrichioides. Gene. 1989;79:131–38.
Ueno Y, Hosoya M, Morita Y, Ueno I, Tatsuno T. Inhibition of the protein synthesis in rabbit reticulocyte by nivalenol, a toxic principle isolated from Fusarium nivale-growing rice. J Biochem. 1968;64:479–85.
Masuda D, Ishida M, Yamaguchi K, Yamaguchi I, Kimura M, Nishiuchi T. Phytotoxic effects of trichothecenes on the growth and morphology of Arabidopsis thaliana. J Exp Bot. 2007;58:1617–26.
Packa D. Cytogenetic changes in plant cells as influenced by mycotoxins. Mycotoxin Res. 1991;7:150–55.
Ederli L, Beccari G, Tini F, Bergamini I, Bellezza I, Romani R, et al. Enniatin B and deoxynivalenol activity on bread wheat and on Fusarium species development. Toxins. 2021;13:728.
Wang Y, Yan H, Wang Q, Zheng R, Xia K, Liu Y. Regulation of the phytotoxic response of Arabidopsis thaliana to the Fusarium mycotoxin deoxynivalenol. J Integr Agric. 2020;19:759–67.
Bruins MBM, Karsaï I, Schepers J, Snijders CHA. Phytotoxicity of deoxynivalenol to wheat tissue with regard to in vitro selection for Fusarium head blight resistance. Plant Sci. 1993;94:195–206.
Shimada T, Otani M. Effects of Fusarium mycotoxins on the growth of shoots and roots at germination in some Japanese wheat cultivars. Cereal Res Commun. 1990;18:229–32.
Desmond OJ, Manners JM, Stephens AE, Maclean DJ, Schenk PM, Gardiner DM, et al. The Fusarium mycotoxin deoxynivalenol elicitis hydrogen peroxide production, programmed cell death and defence responses in wheat. Mol Plant Pathol. 2008;9:435–45.
Diamond M, Reape TJ, Rocha O, Doyle SM, Kacprzyk J, Doohan FM, et al. The Fusarium mycotoxin deoxynivalenol can inhibit plant apoptosis-like programmed cell death. PLoS ONE. 2013;8:e69542.
Jansen C, von Wettstein D, Schafer W, Kogel KH, Felk A, Maier FJ. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc Nati Acad Sci. 2005;102:16892–97.
Langevin F, Eudes F, Comeau A. Effect of trichothecenes produced by Fusarium graminearum during Fusarium head blight development in six cereals species. Eur J Plant Pathol. 2004;110:735–46.
Bai GH, Desjardins AE, Plattner RD. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia. 2002;153:91–8.
Desjardins AE, Proctor RH, Bai GH, McCormick SP, Shaner G, Buechley G, et al. Reduced virulence of trichothecene-nonproducing mutants of Gibberella zeae in wheat field tests. Mol Plant Microbe Interact. 1996;9:775–81.
Gunupuru LR, Perchon A, Doohan FM. Deoxynivalnol resistance as a component of FHB resistance. Trop Plant Pathol. 2017;42:175–83.
Walter S, Nicholson P, Doohan FM. Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol. 2010;185:54–66.
Boddu J, Cho S, Kruger WM, Muehlbauer GJ. Transcriptome analysis of the barley Fusarium graminearum interaction. Mol Plant Microbe Interact. 2006;19:407–17.
Savard ME, Sinha RC, Lloyd Seaman W, Fedak G. Sequential distribution of the mycotoxin deoxynivalenol in wheat spikes after inoculation with Fusarium graminearum. Can J Plant Pathol. 2000;22:280–85.
Chen L, Song Y, Xu Y. Variation in the concentrations of deoxynivalenol in the spikes of winter wheat infected by Fusarium graminearum Schw. Acta Phys Sinica. 1995;26:25–8.
Lemmens M, Scholz U, Berthiller F, Dall’asta C, Koutnik A, Schuhmacher R, et al. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol Plant Microbe Interact. 2005;18:1318–24.
Kang Z, Buchenauer H. Immunocytochemical localization of Fusarium toxins in infected wheat spikes by Fusarium culmorum. Physiol Mol Plant Pathol. 1999;55:275–88.
EFSA. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017;15:4718.
European Commission. Commission Regulation (EC) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing regulation (EC) 1881/2006. Off J Eur Un. 2023;L119:112.
Gruber-Dorninger C, Novak B, Nagl V, Berthiller F. Emerging mycotoxins: beyond traditionally determined food contaminants. J Agric Food Chem. 2017;65:7052–70.
Gautier C, Pinson-Gadais L, Richard-Forget F. Fusarium mycotoxins enniatins: an updated review of their occurrence, the producing Fusarium species, and the abiotic determinants of their accumulation in crop harvest. J Agric Food Chem. 2020;68:4788–98.
Urbaniak M, Waskiewicz A, Stepien L. Fusarium cyclodepsipeptide mycotoxins: chemistry, biosynthesis, and occurrence. Toxins. 2020;12:765.
Andrè A, Müller N, Chetschik I. Occurrence of zearalenone and enniatin b in Swiss wheat grains and wheat flours. Appl Sci. 2022;12:10566.
Fusilier K, Chilvers MI, Limay-Rios V, Singh MP. Mycotoxin co-occurrence in Michigan harvested maize grain. Toxins. 2022;14:431.
Novak B, Rainer V, Sulyok M, Haltrich D, Schatzmayr G, Mayer E. Twenty-eight fungal secondary metabolites detected in pig feed samples: their occurrence, relevance and cytotoxic effects in vitro. Toxins. 2019;11:537.
Reisinger N, Schurer-Waldheim S, Mayer E, Debevere S, Antonissen G, Sulyok M, et al. Mycotoxin occurrence in maize silage – a neglected risk for bovine gut health? Toxins. 2019;11:577.
Křížová L, Dadáková K, Dvořáčková M, Kašparovský T. Feedborne mycotoxins beauvericin and enniatins and livestock animals. Toxins. 2021;13:32.
Bertero A, Fossati P, Tedesco DEA, Caloni F. Beauvericin and enniatins: in vitro intestinal effects. Toxins. 2020;12:686.
Prosperini A, Berrada H, Riuz MJ, Caloni F, Coccini T, Spicer LJ, et al. A review of the mycotoxin enniatin B. Public Health Front. 2017;5:304.
EFSA. Scientific opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA J. 2014;12:3802.
Liuzzi VC, Mirabelli V, Cimmarusti MT, Haidukowski M, Leslie JF, Logrieco AF, et al. Enniatin and beauvericin biosynthesis in Fusarium species: production profiles and structural determinant prediction. Toxins. 2017;9:45.
Burmeister H, Plattner R. Enniatin production by Fusarium tricinctum and its effect on germinating wheat seeds. Phytopathol. 1987;77:1483–87.
Gäumann E, Roth S, Ettlinger L, Plattner PA, Nager U. Enniatin, ein neues, gegen mykobakterien wirksames antibiotikum. [Enniatin, a new antibiotic that works against mycobacteria]. Experientia. 1947;3:202–03.
Serra V, Salvatori G, Pastorelli G. Pilot study: does contamination with enniatin B and beauvericin affect the antioxidant capacity of cereals commonly used in animal feeding? Plants. 2021;10:1835.
Herrmann M, Zocher R, Haese A. Enniatin production by Fusarium strains and its effect on potato tuber tissue. Appl Environ Microbiol. 1996;62:393–98.
Herrmann M, Zocher R, Haese A. Effect of the enniatin synthetase gene on the virulence of Fusarium avenaceum. Mol Plant Microbe Interact. 1996;9:226–32.
Moretti A, Belisario A, Tafuri A, Ritieni A, Corazza L, Logrieco A. Production of beauvericin by different races of Fusarium oxysporum f. sp. melonis, the Fusarium wilt agent of muskmelon. Eur J Plant Pathol. 2002;108:661–66.
Eranthodi A, Schneiderman D, Harris LJ, Witte TE, Sproule A, Hermans A, et al. Enniatin production influences Fusarium avenaceum virulence on potato tubers, but not on durum wheat or peas. Pathogens. 2020;9:75.
Inbaia S, Farooqi A, Ray RV. Aggressiveness and mycotoxin profile of Fusarium avenaceum isolates causing Fusarium seedling blight and Fusarium head blight in UK malting barley. Front Plant Sci. 2023;14:1121553.
Proctor RH, Hohn TM, McCormick SP. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant Microbe Interact. 1995;8:593–01.
Alissaac E, Mahlein AK. Fusarium head blight on wheat: biology, modern detection and diagnosis and integrated disease management. Toxins. 2023;15:192.
Venkatesh N, Keller NP. Mycotoxins in conversation with bacteria and fungi. Front Microbiol. 2019;10:403.
López-Díaz C, Rahjoo V, Sulyok M, Ghionna V, Martín-Vicente A, Capilla J, et al. Fusaric acid contributes to virulence of Fusarium oxysporum on plant and mammalian hosts. Mol Plant Pathol. 2017;19:440–53.
Valenti I, Tini F, Sevarika M, Agazzi A, Beccari G, Bellezza I, et al. Impact of enniatin and deoxynivalenol co-occurrence on plant, microbial, insect, animal and human systems: current knowledge and future perspectives. Toxins. 2023;15:271.
Wang H, Hwang SF, Eudes F, Chang KF, Howard RJ, Turnbull GD. Trichothecenes and aggressiveness of Fusarium graminearum causing seedling blight and root rot in cereals. Plant Pathol. 2006;55:224–30.
Winter M, Samuels PL, Dong Y, Dill-Macky R. Trichothecene production is detrimental to early root colonization by Fusarium culmorum and F. graminearum in Fusarium crown and root rot of wheat. Plant Pathol. 2019;68:185–95.
Dehghanpour-Farashah S, Taheri P, Falahati-Rastegar M. Virulence factors of Fusarium spp., causing wheat crown and root rot in Iran. Phytopathol Mediterr. 2019;58:115–25.
Mudge AM, Dill-Macky R, Dong Y, Gardiner DM, White RG, Manners JM. A role of the mycotoxin deoxynivalenol in stem base colonisation during crown rot disease of wheat caused by Fusarium graminearum and Fusarium pseudograminearum. Physiol Mol Plant Pathol. 2006;69:73–85.
Xu M, Wang Q, Wang G, Zhang X, Liu H, Jiang C. Combatting Fusarium head blight: advances in molecular interactions between Fusarium graminearum and wheat. Phytopathol Res. 2022;4:37.
Maier FJ, Miedaner T, Hadeler B, Felk A, Salomon S, Lemmens M. Involvement of trichothecenes in fusarioses of wheat, barley and maize evaluated by gene disruption of the trichodiene synthase (Tri5) gene in three field isolates of different chemotype and virulence. Mol Plant Pathol. 2006;7:449–61.
Desjardins AE. Natural product chemistry meets genetics: when is a genotype a chemotype? J Agric Food Chem. 2008;56:7587–92.
Xu X, Nicholson P. Community ecology of fungal pathogens causing wheat head blight. Annu Rev Phytopathol. 2009;47:83–103.
Bai G, Su Z, Cai J. Wheat resistance to Fusarium head blight. Can J Plant Pathol. 2018;40:336–46.
Foroud NA, Oullet T, Laroche A, Oosterveen B, Jordan MC, Ellis BE, et al. Differential transcriptome analyses of three wheat genotypes reveal host response pathways associated with Fusarium head blight and trichothecene resistance. Plant Pathol. 2012;61:296–314.
Haidoulis JF, Nicholson P. Tissue-specific transcriptome responses to Fusarium head blight and fusarium root rot. Front Plant Sci. 2022;24:131025161.
Gardiner DM, McDonald M, Covarelli L, Solomon PS, Rusu AG, Marshall M, et al. Comparative pathogenomics reveals horizontally acquired novel virulence genes in fungi infecting cereal hosts. Plos Path. 2012;9:e1002952.
Carere J, Benfield AH, Ollivier M, Liu CJ, Kazan K, Gardnier DM. A tomatinase-like enzyme acts as a virulence factor in wheat pathogen Fusarium graminearum. Fungal Genet Biol. 2017;100:33–41.
Lysøe E, Harris LJ, Walkowiak S, Subramaniam R, Divon HH, Riiser ES, et al. The genome of the generalist plant pathogen Fusarium avenaceum is enriched with genes involved in redox signaling and secondary metabolism. PLoS ONE. 2014;9:e112703.
Gardiner DM, Stiller J, Kazan K. Genome sequence of Fusarium graminearum isolate CS3005. Genome Announc. 2014;17:e200227–14.
Sulyok M, Stadler D, Steiner D, Krska R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of > 500 mycotoxins and other secondary metabolites in food crops: challenges and solutions. Anal Bional Chem. 2020;412:2607–20.
Wang Q, Gottwald S. Wheat rot-dip inoculation with Fusarium graminearum and assessment of root rot disease severity. Bio Protoc. 2017, 7.
Meier U. Growth stages of mono-and dicotyledonous plants. BBCH monograph. EdBy Meier U. Federal Biological Research Centre for Agriculture and Forestry, Quedlinburg (Germany). 2001; p. 158.
Simpson DR, Rezanoor HN, Parry DW, Nicholson P. Evidence for differential host preference in Michrodochium nivale var. majus, M. nivale var. nivale. Plant Pathol. 2000; 49:261 – 68.
Tini F, Beccari G, Benfield AH, Gardiner DM, Covarelli L. Role of the XylA gene, encoding a cell wall degrading enzyme, during common wheat, durum wheat and barley colonization by Fusarium graminearum. Fungal Genet Biol. 2020;136:103318.
Miedaner T, Moldovan M, Ittu M. Comparison of spray and point inoculation to assess resistance to Fusarium head blight in a multienvironment wheat trial. Phytopathology. 2003;93:1068–72.
Parry DW, Nicholson P. Development of a PCR assay to detect Fusarium poae in wheat. Plant Pathol. 1996;45:383–91.
Beccari G, Arellano C, Covarelli L, Tini F, Sulyok M, Cowger C. Effect of wheat infection timing on Fusarium head blight causal agents and secondary metabolites in grain. Int J Food Microbiol. 2019;290:214–25.
Nicolaisen M, Supronien S, Nielsen LK, Lazzaro I, Spliid NH, Justesen AF. Real-time PCR for quantification of eleven individual Fusarium species in cereals. J Microbiol Meth. 2009;76:234–40.
Brandfass C, Karlovsky P. Upscaled CTAB-Based DNA extraction and real-time PCR assays for Fusarium culmorum and F. graminearum DNA in plant material with reduced sampling error. Int J Mol Sci. 2008;9:2306–21.
Onofri A, Pannacci E. Spreadsheet tools for biometry classes in crop science programs. Commun Biometry Crop Sci. 2014;9:43–53.
Acknowledgements
Not applicable.
Funding
This research was funded by the Italian Ministry of University and Research (MUR), PRIN2020 project “Role of enniatins as emerging mycotoxins and their association with deoxynivalenol in plant, insect, animal and human systems (MYCENDEA)”, grant number 2020ZAYHK.
Author information
Authors and Affiliations
Contributions
G.B., F.T., N.A.F. and L.E.: conceptualization. G.B., F.T. and N.A.F.: data curation. G.B., F.T., L.E. and M.S. formal analysis. G.B., F.T., N.A.F., L.E., D.M.G., A.H.B., L.J.H. and M.S.: investigations. G.B., L.E., R.R., I.B. and L.C.: resources. N.A.F. and L.C.: supervision. G.B., F.T. and N.A.F.: visualization; G.B and F.T.: writing - original draft. N.A.F., L.E., D.M.G., A.H.B., L.J.H., R.R., I.B. and L.C.: writing – review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval, guidelines and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Beccari, G., Tini, F., Foroud, N.A. et al. A comparison between the role of enniatins and deoxynivalenol in Fusarium virulence on different tissues of common wheat. BMC Plant Biol 24, 463 (2024). https://doi.org/10.1186/s12870-024-04945-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-04945-5